Abstract
Culturing of primary hepatocytes and stem cell-derived hepatocytes faces a major issue of dedifferentiation due to absence of cell–cell adhesion and 3D structures. One of the possible ways to eliminate the problem of dedifferentiation is mimicking the expression pattern of adhesion proteins during the normal developmental process of liver cells. The purpose of this study was to evaluate the expression pattern of some key adhesion proteins, namely, E-cadherin, N-cadherin, epithelial CAM (EpCAM), intracellular CAM (ICAM), collagen 1α1, α-actinin, β-catenin and vimentin, in the liver tissue during prenatal and postnatal stages. Furthermore, differences in their expression between prenatal, early postnatal and adult stages were highlighted. Wistar rats were used to isolate livers at prenatal Day 14 and 17 as well as on postnatal Day 1, 3, 7 and 14. The liver from adult rats was used as control. Both conventional and real-time quantitative polymerase chain reactions (PCRs) were performed. For most of the adhesion proteins such as E-cadherin, N-cadherin, EpCAM, ICAM, collagen 1α1 and α-actinin, low expression was observed around prenatal Day 14 and an increasing expression was observed in the postnatal period. Moreover, β-catenin and vimentin showed higher expression in the early prenatal period, which decreased gradually in the postnatal period, but still this low expression was considerably higher than that in the adult control rats. This basic knowledge of the regulation of expression of adhesion proteins during different developmental stages indicates their vital role in liver development. This pattern can be further studied and imitated under in vitro conditions to achieve better cell–cell interactions.
Introduction
Several liver diseases such as viral hepatitis, fatty liver disease, hepatic carcinoma and liver cirrhosis can cause chronic or acute liver failure. Surgical complications associated with liver transplantation and limited donor availability have shifted the major therapeutic approach toward the use of stem cells.Citation1 Stem cells have potential for a number of applications in the biomedical field, including cell replacement for repair of damaged organs, as an in vitro source of drug testing and as a tool for studying genetic defects.Citation2 Stem cells are a very attractive source for cell replacement therapy in liver diseases as they can differentiate into hepatocyte-like cells (HLCs) in vitro.Citation3 HLCs can be derived from induced pluripotent stem cells (iPSCs),Citation4 hepatic progenitor cells (HPCs),Citation5 embryonic stem cells (ESCs)Citation6 and mesenchymal stem cells (MSCs).Citation7
A challenge that confronts all primary and stem cell-derived cell cultures is maintaining a mature cell phenotype. The life span of HLCs and primary hepatocytes is usually limited, and they progressively lose their liver-specific functions in cultures, a process known as dedifferentiation.Citation8,Citation9 Moreover, liver perfusion and culturing practices destroy the normal tissue architecture and cell junctions between adjacent cells of hepatocytes, as well as resulting in loss of normal cell polarity.Citation10 This renders these cultures ineffective for various protocols such as drug testing, because for these applications, a stable liver-specific culture comparable to the in vivo conditions is needed. The main reason behind this problem is the absence of both cell–cell adhesion and 3D matrices to which cells can adhere. Conventional approaches to overcome this difficulty include the addition of differentiation-promoting compounds to the culture medium, reintroduction of the extracellular matrix (ECM) or cocultivation of hepatocytes with other cell types.Citation11 Currently, different biomaterials, such as collagen and Matrigel layers,Citation12 fibroblast feeder layer cultures and nanofiber scaffolds,Citation7 are used to maintain stable cell adhesive properties. Despite their advantages, these methods cannot provide complex cell adhesions present in vivo and exhibit low cell density/functional capacity per unit volume.Citation13 Therefore, there is a need to analyze the temporal and spatial expression patterns of cell adhesion proteins during embryonic liver development to mimic them in the early stages of hepatic differentiation during in vitro culture.
Cell–cell interactions among different cell types of fetal liver are essential for the development of functional lob-ules.Citation14 CAMs are specialized integral membrane proteins for cell-cell adhesion in tissues. CAMs can be organized in the form of dense clusters to create specialized junctions such as adherens, tight and gap junctions.Citation15 The most important class of CAMs is the cadherins, which are Ca2+-dependent transmembrane proteins involved in hemophilic cell–cell adhesion. The other important class of CAMs is the immunoglobulin (Ig)-like superfamily, which provides Ca2+-independent cell–cell adhesion. It includes intracellular CAMs (ICAMs), which are mainly present on the endothelial cell surface and function as signaling molecules for the attachment of cytotoxic T-lymphocytes (CTLs) and natural killer (NK) cells, in addition to providing leukocyte–endothelial cell adhesion.Citation16 All these adhesion molecules are collectively responsible for the smooth control of cell motility, cell shape and tissue organization. The timing of expression of these molecules in the prenatal and postnatal phases is important, as many of these proteins also regulate signaling functions during developmental stages.Citation10
The aim of the current study is to evaluate the regulation of expression of key adhesion proteins and their differences in the prenatal, early postnatal and adult stages. This may provide key information about when and how much of these proteins can be used in HLC cultures to imitate the early developmental process and obtain a stable culture.
Animals and methods
Animals
The current study was carried out on almost 6 months old Wistar rats. The animals were kept in an animal house under pathogen-free controlled climate conditions (temperature 22°C) and 12-hour light/dark cycle. Ad libitum access to standard rodent chow and water was provided. All the experimental procedures were approved by the Committee of Animal Care, National Center of Excellence in Molecular Biology, University of the Punjab, Lahore, Pakistan. The investigation conforms to the Guidelines for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No 85–23, revised 1985).
Experimental design
Liver sample collection was done at two phases, namely, the prenatal phase and postnatal phase. Prenatal sampling was done at Day 14 and Day 17 of gestation. Postnatal sampling was done at days 1, 3, 7 and 14 after birth. The detailed scheme is shown in .
Table 1 Experimental groups indicated by days and abbreviations
Liver isolation at prenatal and postnatal stages
Male and female rats were paired in the evening and on the next morning, female rats were observed for vaginal plugs. The presence of the plug indicated mating, and that day was designated as the 0.5th day postcoitum (dpc). On the specific days of liver isolation, female rats were euthanized, and the fetuses were removed by cutting the uterus lining. The fetus was further dissected under a microscope using a 10× objective lens to isolate the liver. For the isolation of the postnatal liver, rat pups were dissected. Excised livers were washed with PBS (Thermo Fisher Scientific,, Waltham, MA, USA) twice to remove extra blood. A part of the liver (almost 2 mg) was taken in 1 mL TRIzol® (Thermo Fisher Scientific) and used for RNA extraction.
Gene expression profiling
After careful isolation of the rat livers on the specific prenatal and postnatal days, gene expression profiling for the adhesive proteins was performed using both conventional polymerase chain reaction (PCR) and real-time quantitative PCR (qPCR).
Total RNA was extracted from all experimental groups by using TRIzol reagent according to the manufacturer’s instructions. RNA was quantified using a spectrophotometer (ND-1000; Nanodrop Technologies, Wilmington, DE, USA). The extracted RNA was stored at −70°C until further use. cDNA was synthesized from total RNA by using the Moloney murine leukemia virus (M-MLV) reverse transcriptase cDNA synthesis kit (Thermo Fisher Scientific) and stored at −20°C. Reverse transcription (RT)-PCR was performed for various adhesion proteins. cDNA was amplified against specific genes by using Taq polymerase kit (Thermo Fisher Scientific). Amplified products were electrophoresed on 2% agarose gel (Sigma-Aldrich, St Louis, MO, USA) containing 0.5 µg/mL ethidium bromide (Sigma-Aldrich). Gel bands were analyzed using the GelDoc system (Benchtop UV Transilluminator, Syngene Ingenius, UK).
Furthermore, real-time RT-PCR was carried out for the quantitative analysis of gene expression using Maxima SYBR Green/ROX qPCR Master Mix (2×) (Thermo Fisher Scientific). Samples were run in triplicate. Data acquisition was performed during the extension step. Comparative cycle threshold method (ΔCt value) was used for the relative quantification of target genes. β-actin was used as an internal control. The primer sequences used for both qualitative and quantitative expression analyses of the relevant genes, along with their annealing temperatures, are given in .
Table 2 Primer sequences and optimized annealing temperatures for the genes used for expression analysis
Statistical analysis
All values are presented as mean value ± SD. Significant differences between the experimental groups were determined by using repeated-measures (RM) one-way analysis of variance (ANOVA) using GraphPad Prism software. Statistical significance was considered at P≤0.05.
Results and discussion
The assembly of different types of cells into tissues leading to organs requires molecular interactions at the cellular level. These molecular interactions are achieved by a wide array of temporally and spatially regulated adhesion proteins. These adhesion proteins also play a decisive role in hepatic morphogenesis during development.Citation17 In the present study, we aimed to evaluate the expression of important adhesion proteins in the liver at the prenatal stages as well as in the early postnatal period in rats.
The most crucial CAMs responsible for cell–cell adhesion are the cadherins. E-cadherin and N-cadherin showed similar expression patterns. Expression was low in the early prenatal stage F14, which gradually increased until the late postnatal stage N14. Reduced expression could be observed in the adult control (A) for both genes (). E-cadherin is a transmembrane protein, majorly involved in Ca2+-dependent cell–cell adhesions. It is found most abundantly in adherens junctions and it ensures the integrity of epithelial tissues in the liver.Citation18 In the present study, low expression of E-cadherin was observed on prenatal Day 14 (0.5±0.14 fold), which doubled on the prenatal Day 17 (). Shiojiri and SugiyamaCitation19 previously reported high expression of E-cadherin in biliary epithelial cells (BECs). This justifies the considerably low expression observed at prenatal Day 14, around which time the hepatoblasts are reported to start differentiating into hepatocytes and BECs.Citation17 As the population of BECs and hepatocytes increases, an increase in E-cadherin expression could be observed. Expression of E-cadherin increased steadily throughout the postnatal period and reached 1.6±0.11 fold level at postnatal Day 14. As the postnatal period is the phase of extensive liver growth, this increase in expression indicates the active role of E-cadherin in the proliferation of hepatocytes and maintenance of the tissue architecture.
Figure 1 Expression of selected genes at different prenatal and postnatal stages observed through gel electrophoresis.
Abbreviations: E-cad, E-cadherin, ICAM, intracellular CAM; N-cad, N-cadherin.
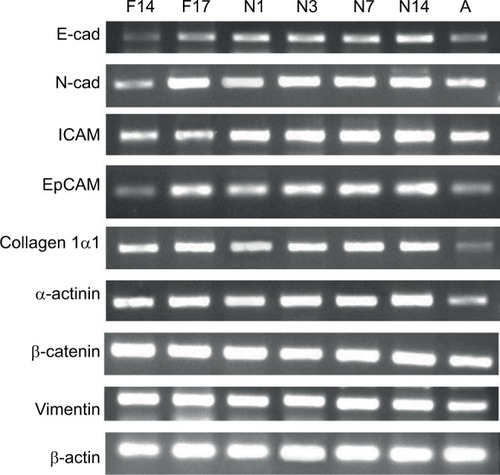
Figure 2 Real-time PCR analysis of selected genes at different prenatal and postnatal stages.
Abbreviations: ICAM, intracellular CAM; PCR, polymerase chain reaction.
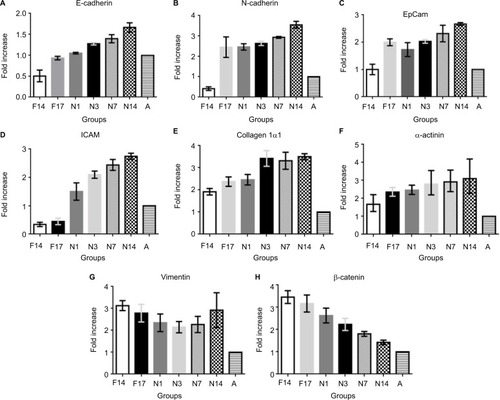
Neural or N-cadherin is another Ca2+-dependent cadherin, involved in heterotypic cell–cell adhesion.Citation20 It was originally identified as a CAM in the neural tissues, but it has been shown to be expressed in various nonneural tissues including the liver.Citation21 N-cadherin participates in multiple signal transduction events, including cell survival, cell motility and morphological transformation.Citation22 N-cadherin showed a similar expression pattern as E-cadherin in different groups. N-cadherin expression on the prenatal Day 14 was 0.4±0.09 fold less than that in the adult control. Afterward, the expression increased significantly on prenatal Day 17, ie, by 2.4±0.50 fold, and almost remained the same in the postnatal period, up to Day 3. Later groups showed further increase in the expression, reaching 3.5±0.16 fold at postnatal Day 14 (). The expression abruptly increased between F14 and F17, indicating the role of N-cadherin in the later stages of embryonic liver development. In previous studies, expression of N-cadherin has also been reported to be important for the differentiation of hepatoblasts into hepatocytes and BECs.Citation21 Throughout the postnatal period, an increase in the expression of N-cadherin was observed, indicating its key role in liver growth. It was also observed that expression of N-cadherin was significantly higher than the expression of E-cadherin. It is possible that both these cadherins are expressed in separate compartments or cells of the liver tissue, but as we have taken the whole tissue sample for qPCR, this could not be determined without immunostaining of the liver tissue sections.
ICAM is expressed on the endothelial cells and is responsible for inflammatory cell recruitment and activation by mediating leukocyte–endothelial cell adhesion.Citation23 The expression of ICAM on the prenatal days 14 and 17 was 0.34±0.07 and 0.44±0.11 fold lower than that in the adult control. However, its expression markedly increased readily after birth on postnatal Day 1. The expression gradually increased in the postnatal period, and the highest expression was observed on postnatal Day 14, ie, 2.74±0.11 fold higher than that in the adult control (). Opposite of this expression pattern was observed by Sugiyama et al,Citation24 wherein peak expression was seen at embryonic Day 12 and no expression was seen in the postnatal stage. However, Attar et alCitation25 reported the same pattern of change of expression, namely, lower in early development and higher in neonatal stage, during the lung development. ICAM is expressed constitutively on all endothelial cells; therefore, as the mass of the liver increases, a surge in the expression of ICAM can be observed. It has also been reported that in young fetal mouse livers, the major cells responsible for ICAM expression are hepatoblasts, whose expression of both cell adhesion molecules is in good agreement with the timing of hepatic hemopoiesis, suggesting that ICAM may be involved in some step of hepatic hemopoiesis.Citation26,Citation27
Expression analysis was also carried out for epithelial CAM (EpCAM), a type 1 transmembrane glycoprotein, reported to be involved in cell adhesion by binding α-actinin.Citation28 EpCAM gene showed same expression at the prenatal Day 14 as the adult control (1.00±0.19), which doubled at the prenatal Day 17 (1.99±0.12). A slight decrease in the expression was observed after birth at postnatal Day 1, which gradually increased in later postnatal days. The highest expression of 2.66±0.05 fold was observed in postnatal Day 14 (). Studies have shown that EpCAM is majorly expressed in the epithelia of bile ducts (novel hepatic). This explains the increasing expression of EpCAM after prenatal Day 14 because of the biliary commitment of some hepatoblasts and formation of intrahepatic bile ducts. However, the finding of EpCAM’s considerable expression at prenatal Day 14 also confirms that hepatoblasts also express EpCAM. No study explains the behavior of EpCAM in the postnatal phase. It can be observed from the qPCR results that EpCAM expression gradually increases in the postnatal days. Although no concrete conclusion can be drawn from this observation, the possible role of EpCAM in hepatic growth and maturation can be speculated. Moreover, in the adult liver, reduced expression of EpCAM was observed. EpCAM is involved in oval cell migration and is considered an early biomarker of hepatocellular carcinoma (HCC); the low expression in the normal adult liver is expected unless acted upon by liver injury.Citation29
Collagen Iα1 is a fibrillar type of collagen that provides mechanical strength and resilience to the ECM.Citation30 Our data indicate that collagen Iα1 is expressed very early in the embryonic development of liver. Liver samples at prenatal Day 14 showed high expression of collagen Iα1, which further increased up to prenatal Day 17. Expression of collagen 1α1 was almost 1.91±0.14 fold higher on the prenatal Day 14 compared to that in the adult control. The expression further increased up to prenatal Day 17 and remained almost the same after birth at postnatal Day 1. The expression markedly increased on postnatal Day 3 (3.42±0.35 fold) and remained high throughout the later postnatal days (). As collagen is the most abundant protein of the ECM, its higher concentration is expected during development for the establishment of tissue architecture. This higher expression was retained after birth and it peaked on the postnatal Day 3, indicating the need for collagen Iα1 during the increase in the liver size to provide scaffold for the cells. However, once the proper liver size is achieved, as in adult control, the collagen Iα1 expression markedly reduces. There is no need for the highly active mRNA expression of collagen Iα1 in adults, unless faced by liver injury or fibrosis.
α-actinin is an actin-binding protein of the cytoskeleton that maintains the cell shape and provides mechanical stability to the cell.Citation31 A gradual increase in the α-actinin expression was observed with the increasing time of the developmental stages. The lowest expression was observed at the prenatal Day 14 and the highest was at the postnatal Day 14, ie, 1.68±0.18 fold and 3.11±0.39 fold, respectively (). Because of the role of α-actinin in tissue structure formation, its expression initiates with the start of embryonic liver development. With increasing size of liver bud and liver mass, increased α-actinin expression is observed. However, in adult liver, it is reduced because the liver has achieved its normal size, and active mRNAs of α-actinin are not present in abundance. There is no previous report available describing the change of expression of α-actinin in the postnatal period. Therefore, there is need for further detailed analysis of α-actinin expression at the cellular level in the postnatal phase.
Vimentin is also a tissue architectural protein, a member of type III intermediate filaments, involved in maintenance of cell shape and motility.Citation32 In the present study, vimentin gene expression was 3.13±0.22 fold on the prenatal Day 14 and it decreased gradually to 2.14±0.25 fold on postnatal Day 3. Later on, the expression again increased up to 2.92±0.79 fold, compared to the control, on the postnatal Day 14 (). Even this reduced expression was considerably high compared to that in the adult control rats. It has also been reported that vimentin expression starts commencing around embryonic Day 8.5, whereas in adults, the vimentin expression is limited and temporal.Citation33 This study has revealed a similar pattern of vimentin expression during postnatal liver development. Further investigations to explain this pattern of expression are required. However, this high expression throughout the developmental process clearly indicates that vimentin is a key regulator in early tissue formation and maintenance of architecture in late postnatal stages.
A very high expression of β-catenin, a cytosolic cell adhesion protein, was observed at the prenatal Day 14 (3.45±0.28 fold) compared to the adult control, which became reduced at the prenatal Day 17. Its expression starts to reduce gradually during the postnatal period. However, the expression in the postnatal period remained higher than that in the adult control (). This finding coincides with previous studies that reported the same pattern of β-catenin expression during embryonic development.Citation34,Citation35 The higher expression in early stages of liver development is because of the important role of β-catenin in liver bud growth through the expansion of hepatoblasts.Citation34 Temporal signaling of Wnt/β-catenin is also important in the hepatoblast differentiation and hepatocyte maturation.Citation36 A gradual decrease in the β-catenin expression was observed in the postnatal period. Even though the expression was decreased, it remained significantly high compared to that in the adult control. The high expression of β-catenin is responsible for the surge of cell proliferation in the postnatal stage. Apte et alCitation37 reported a surge in β-catenin activity during postnatal days 0–20 in mouse models. They also indicated that β-catenin-knockout mouse shows reduced liver size at postnatal Day 30.Citation37
A general pattern that can be seen for most of the adhesion proteins, with few exceptions, is that they are expressed in high concentration in early liver development as well as in the postnatal stage. Because both prenatal and postnatal stages correspond to immense cell proliferation and tissue development, these proteins bind cells together to maintain a true tissue shape and structure. Once proper liver size is achieved in the normal adult liver, these proteins’ active mRNAs are expressed in lower concentrations. Moreover, increased concentrations of these proteins in adult livers are an indication of several melanomas and thus used as hepatocarcinogenic markers.Citation38,Citation39
Conclusion
All the observations presented in this study provide basic knowledge of the pattern of changes in adhesive proteins during different developmental stages. This pattern can be imitated under in vitro conditions to achieve better tissue interactions. However, more in-depth studies are needed to comprehend their exact role in hepatic development.
Disclosure
The authors report no conflicts of interest in this work.
References
- DuanB-WLuS-CWangM-LLiver transplantation in acute-on-chronic liver failure patients with high model for end-stage liver disease (MELD) scores: a single center experience of 100 consecutive casesJ Surg Res2013183293694323558257
- HuCLiLIn vitro culture of isolated primary hepatocytes and stem cell-derived hepatocyte-like cells for liver regenerationProtein Cell20156856257426088193
- Sancho-BruPNajimiMCarusoMStem and progenitor cells for liver repopulation: can we standardize the process from bench to bedside?Gut200958459460319091822
- AmimotoNMizumotoHNakazawaKIjimaHFunatsuKKajiwaraTHepatic differentiation of mouse embryonic stem cells and induced pluripotent stem cells during organoid formation in hollow fibersTissue Eng Part A20111715–162071207821457096
- WangYCuiCBYamauchiMLineage restriction of human hepatic stem cells to mature fates is made efficient by tissue-specific biomatrix scaffoldsHepatology201153129330521254177
- SubramanianKOwensDJRajuRSpheroid culture for enhanced differentiation of human embryonic stem cells to hepatocyte-like cellsStem Cells Dev201323212413124020366
- PiryaeiAValojerdiMRShahsavaniMBaharvandHDifferentiation of bone marrow-derived mesenchymal stem cells into hepatocyte-like cells on nanofibers and their transplantation into a carbon tetrachloride-induced liver fibrosis modelStem Cell Rev Rep201171103118
- PapeleuPElautGRogiersVVanhaeckeTCell cultures as in vitro tools for biotransformation studiesPandalaiSGRecent Research Developments in Drug Metabolism and DispositionIndiaTransworld Research Network12002199234
- WangKShindohHInoueTHoriiIAdvantages of in vitro cytotoxicity testing by using primary rat hepatocytes in comparison with established cell linesJ Toxicol Sci200227322923712238146
- ElautGHenkensTPapeleuPMolecular mechanisms underlying the dedifferentiation process of isolated hepatocytes and their culturesCurr Drug Metab20067662966016918317
- SkardalASmithLBharadwajSAtalaASokerSZhangYTissue specific synthetic ECM hydrogels for 3-D in vitro maintenance of hepatocyte functionBiomaterials201233184565457522475531
- KimYRajagopalanP3D hepatic cultures simultaneously maintain primary hepatocyte and liver sinusoidal endothelial cell phenotypesPLoS One2010511e1545621103392
- SellaroTLRanadeAFaulkDMMaintenance of human hepatocyte function in vitro by liver-derived extracellular matrix gelsTissue Eng Part A200916310751082
- NitouMIshikawaKShiojiriNImmunohistochemical analysis of development of desmin-positive hepatic stellate cells in mouse liverJ Anat2000197pt 463564611197537
- BilelloJCableEMyersRIsomHRole of paracellular junction complexes in baculovirus-mediated gene transfer to nondividing rat hepatocytesGene Ther200310973374912704412
- TachimoriAYamadaNSakateYUpregulation of ICAM-1 gene expression inhibits tumour growth and liver metastasis in colorectal carcinomaEur J Cancer200541121802181016051479
- LemaigreFZaretKSLiver development update: new embryo models, cell lineage control, and morphogenesisCurr Opin Genet Dev200414558259015380251
- YoungPBoussadiaOHalfterHE-cadherin controls adherens junctions in the epidermis and the renewal of hair folliclesEMBO J200322215723573314592971
- ShiojiriNSugiyamaYImmunolocalization of extracellular matrix components and integrins during mouse liver developmentHepatology200440234635515368439
- StraubBKRickeltSZimbelmannRE–N-cadherin heterodimers define novel adherens junctions connecting endoderm-derived cellsJ Cell Biol201119587388722105347
- GentanabeTHAOzawaME-cadherin but not N-cadherin expression is correlated with the intracellular distribution of catenins in human hepatocellular carcinomasOncol Rep19985110911149683818
- NiemanMTPrudoffRSJohnsonKRWheelockMJN-cadherin promotes motility in human breast cancer cells regardless of their E-cadherin expressionJ Cell Biol1999147363164410545506
- DustinMLRothleinRBhanAKDinarelloCASpringerTInduction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1)J Immunol19861372452543086451
- SugiyamaYKoikeTShiojiriNDevelopmental changes of cell adhesion molecule expression in the fetal mouse liverAnat Rec201029316981710
- AttarMABailieMBChristensenPJBrockeTGWilcoxenSEPaineRInduction of ICAM-1 expression on alveolar epithelial cells during lung development in rats and humansExp Lung Res199925324525910352954
- TadaTWidayatiDFukutaKMorphological study of the transition of haematopoietic sites in the developing mouse during the perinatal periodAnat Histol Embryol200635423524016836587
- KubotaHYaoHLReidLMIdentification and characterization of vitamin A storing cells in fetal liver: implications for functional importance of hepatic stellate cells in liver development and hematopoiesisStem Cells2007252339234917585172
- BalzarMWinterMDe BoerCLitvinovSThe biology of the 17–1A antigen (Ep-CAM)J Mol Med19997769971210606205
- KimJWYeQForguesMCancer-associated molecular signature in the tissue samples of patients with cirrhosisHepatology200439251852714768006
- ElsharkawyAOakleyFMannDThe role and regulation of hepatic stellate cell apoptosis in reversal of liver fibrosisApoptosis200510592793916151628
- GumbinerBMRegulation of cadherin-mediated adhesion in morphogenesisNat Rev Mol Cell Biol20056862263416025097
- ClarkeEJAllanVIntermediate filaments: vimentin moves inCurr Biol20021217R596R59812225682
- FrankeWWGrundCKuhnCJacksonBWIllmenseeKFormation of cytoskeletal elements during mouse embryogenesisDifferentiation19822343596759279
- MicsenyiATanXSneddonTLuoJ-HMichalopoulosGKMongaSPβ-Catenin is temporally regulated during normal liver developmentGastroenterology20041261134114615057752
- LadeAGMongaSPBeta-catenin signaling in hepatic development and progenitors: which way does the WNT blow?Dev Dyn2011240348650021337461
- TanXYuanYZengGβ-Catenin deletion in hepatoblasts disrupts hepatic morphogenesis and survival during mouse developmentHepatology2008471667167918393386
- ApteUZengGThompsonMDβ-Catenin is critical for early postnatal liver growthAm J Physiol Gastrointest Liver Physiol20072926G1578G158517332475
- Nejak-BowenKMongaSPWnt/β-catenin signaling in hepatic organogenesisOrganogenesis20084929919279720
- TsubotaAMatsumotoKMogushiKIQGAP1 and vimentin are key regulator genes in naturally occurring hepatotumorigenesis induced by oxidative stressCarcinogenesis201031350451120015863
