Abstract
The use of in vitro oligodendrocyte differentiation for transplantation of stem cells to treat demyelinating diseases is an important consideration. In this study, we investigated the effects of serum on glia and oligodendrocyte differentiation from human mesenchymal stem cells (KP-hMSCs). We found that serum deprivation resulted in a reversible downregulation of glial- and oligodendrocyte-specific markers. Serum stimulated expression of oligodendrocyte markers, such as galactocerebroside, as well as Notch1 and JAK1 transcripts. Inhibition of Notch1 activation by the Notch inhibitor, MG132, led to enhanced expression of a serum-stimulated oligodendrocyte marker. This marker was undetectable in serum-deprived KP-hMSCs treated with MG132, suggesting that inhibition of Notch1 function is additive to serum-stimulated oligodendrocyte differentiation. Furthermore, a dominant-negative mutant RBP-J protein also inhibited Notch1 function and led to upregulation of oligodendrocyte-specific markers. Our results demonstrate that serum-stimulated oligodendrocyte differentiation is enhanced by the inhibition of Notch1-associated functions.
Introduction
In the vertebrate central nervous system, axons are sheathed with myelin, the dendritic process of an oligodendrocyte. Demyelinating diseases, such as multiple sclerosis, result from damage to myelin.Citation1 Demyelinated axons may undergo remyelination, but only some of them regain normal structure and function.Citation2 For most disorders, remyelination eventually fails, resulting in progressive demyelination, axonal damage, and persistent neurologic deficits. Immunosuppressive and immunomodulating treatments can relieve symptoms, but have only mild efficacy in the prevention of long-term disability.Citation3
Stem cells are defined as precursor cells with the potential for self-renewal and the ability to generate progeny cells of different lineages. They are considered a source of myelinogenic cells due to their ability to provide an apparently unlimited cell supply for transplantation and to give rise to homogenous populations of myelinogenic phenotypes.Citation4 Bone marrow stromal cells, or human mesenchymal stem cells (KP-hMSCs), have the ability to differentiate into a variety of mesenchymal cells such as osteocytes, adipocytes, and chondrocytes.Citation5 As multipotent progenitor cells, they are also an attractive source of cells for use in therapeutic applications for neural regeneration, and regulation of their differentiation to specific neural cell types is a field of primary interest.Citation6,Citation7 In order to apply KP-hMSCs in demyelinating diseases to enhance remyelination in the central nervous system efficiently, KP-hMSCs should be directed to undergo oligodendrocyte differentiation before transplantation.
Galactocerebroside (Gal-C) and its sulfated derivative, sulfatide, are widely abundant in the vertebrate myelin sheath.Citation8 Deficiency of Gal-C activity causes human disorders, such as inherited globoid cell leukodystrophy, which leads to oligodendrocyte death and subsequent demyelination.Citation9 In vitro culture of cerebellar progenitor cells in serum-free medium promotes oligodendrocyte differentiation.Citation10 The inhibitory effect of serum on oligodendrocyte differentiation has also been observed in the newborn rodent brain and spinal cord.Citation11 In contrast, Schwann cells proliferated but neither ensheathed nor myelinated axons in serum-free medium, while myelination and Gal-C expression were promoted when the cultures were switched to complete medium (serum plus ascorbic acid).Citation12 The controversial effects of serum on Gal-C expression and oligodendrocyte differentiation imply that additional factors may be required to enhance serum-stimulated differentiation.
The role of Notch signaling in oligodendrocyte differentiation has been extensively investigated in several stem cell and progenitor cell models. For example, oligodendrocyte differentiation in the rat optic nerve can be inhibited by the activation of the Notch pathway.Citation13 In contrast, inhibition of gamma-secretase of the Notch signaling pathway promotes oligodendrocyte differentiation in mice with multiple sclerosis, significantly reducing axonal damage through remyelination.Citation14 However, several lines of evidence suggest that F3/contactin-activated Notch signaling enhances oligodendrocyte maturation in rat oligodendrocyte precursor cellsCitation15 and human KP-hMSCs.Citation16 Therefore, the effects of Notch signaling in oligodendrocyte differentiation remain to be elucidated.
Induced differentiation of human KP-hMSCs into oligodendrocytes in vitro has been previously described.Citation6,Citation17 In this study, we further demonstrate the importance of serum in oligodendrocyte differentiation using human KP-hMSCs as a model. We found that inhibition of Notch1 activity enhanced oligodendrocyte differentiation in the presence of serum. In vitro differentiated stem cells may have a therapeutic role in demyelinating disorders.
Materials and methods
Cell cultures and reagents
The human mesenchymal stem cell (MSC) line has been described previously and was originally derived from the bone marrow of a 61-year-old female donor. This line has been transfected with a plasmid containing the type 16 human papilloma virus proteins, E6/E7.Citation18 KP-hMSCs were grown in DMEM-LG (Gibco, Grand Island, NY) supplemented with 10% fetal bovine serum (selected lots), 100 U/mL penicillin, 10 μg/mL streptomycin, and 0.25 μg/mL amphotericin B at 37°C with 5% CO. The medium was changed twice a week, and cells were subcultured 1:4 to 1:5 weekly. Based on the results of flow cytometry, these cells express CD29 (β1 integrin), CD44 (hyaluronan receptor), CD90 (Thy-1), CD105 (endoglin), SH2, and SH3 (human MSC markers).Citation18 These cells maintain the potential to differentiate into mesenchymal and nonmesenchymal tissue even after more than 100 population doublings. The reagents used for cell treatment included JAK1 inhibitor (Merck KGaA, Darmstadt, Germany), MG132, 4′,6-diamidino-2-phenylindole (DAPI; Calbiochem, San Diego, CA), and lactacystin (Sigma, St Louis, MO).
Real-time polymerase chain reaction analysis
Total RNA was extracted from the cell pellet with Trizol (Life Technologies, Bethesda, MD) according to the manufacturer’s specifications. cDNA was synthesized from total RNA using M-MLV reverse transcriptase, and polymerase chain reaction (PCR) was performed using Taq DNA polymerase recombinant (Invitrogen, Carlsbad CA). The reaction products were electrophoresed on a 1.2% agarose gel and visualized using ethidium bromide with the housekeeping gene, β-actin, as a control. The primer sequences for real-time (RT)-PCR of different genes were listed in .
Table 1 The primer sets used for amplification of studied genes by real-time polymerase chain reaction
Western blot analysis
The cells were washed with phosphate-buffered saline (PBS) and lysed with 0.2 mL lysis buffer (20 mM Tris-HCl pH 7.5, 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM b-glycerophosphate, 1 mM Na3VO4, 1 mg/mL leupeptin) for 10 minutes on ice. Protein levels were determined using the BCA assay (Pierce, Rockford IL). After heating for five minutes at 95°C in sample buffer, equal aliquots of the cell lysates were run on 10% or 5% sodium dodecyl sulfate-polyacrylamide gel. Proteins were transferred to polyvinylidene fluoride membranes, blocked probed with antibodies, and detected with the enhanced chemiluminescence system (PerkinElmer Instruments. Waltham, MA). The antibodies, α-nestin (monoclonal, MAB353, 1:1000 dilution), α-neu-N (monoclonal, MAB377, 1:1000 dilution), α-GFAP (monoclonal, MAB360, 1:1000 dilution), α-Gal-C (MAB342, 1:500 dilution), α-MBP (monoclonal, MAB382, 1:1000 dilution), α-rabbit IgG (HRP-conjugated AP132P, 1:5000 dilution), and α-mouse IgG (HRP-conjugated AP124P, 1:5000 dilution) were all purchased from Chemicon (Millipore, Billerica, MA).
Immunofluorescence analysis
Immunofluorescence analysis was used to examine the neural characteristics of the human MSC culture. Cells were washed briefly in PBS, fixed with 4% paraformaldehyde (in PBS) for 10 minutes, permeabilized with 0.1% Triton X-100 in PBS for 10 minutes, and treated with 5% fetal calf serum in PBS for 30 minutes; all at room temperature. They were then incubated with primary antibody overnight at 4°C, washed three times in PBS, then incubated for one hour with fluorescein-conjugated secondary antibody. DAPI was used for nuclear staining. The samples were mounted with mounting medium after the staining procedures have been completed.
DNA delivery methods
Wild-type and dominant negative (DN) mutant RBP-J plasmidsCitation19 were a gift from the Riken BioResource Center DNA Bank with permission from Dr. T Honjo (Riken, Kyoto, Japan). For the transfection of cells with plasmids, Nucleofector® technology (AMAXA Biosystems, Cologne, Germany) was used as described previously.Citation20 Each nucleofection sample contained 2 μg DNA, 4 × 105 cells, and 100 μL Human MSC Nucleofector Solution. The transfection was carried out under program C-17 of the Nucleofector device, as recommended by the manufacturer. The efficiency of transfection, as evaluated by the expression of green fluorescence protein in cells transfected with pmaxGFP vector®, was 50%–70%.
Statistical analysis
Three independent experiments were conducted to measure statistical differences between the control and experimental groups. Statistical differences were determined using the Student’s t-test, and significance was set at P < 0.05.
Results
Serum deprivation induces loss of glial markers in KP-hMSCs and C6 cells
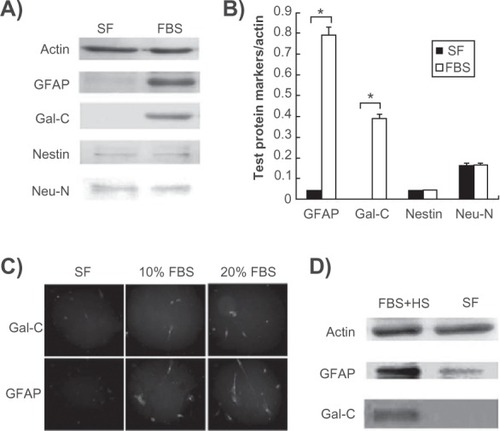
We previously demonstrated that KP-hMSCs are able to express neuronal and glial markers under normal growth conditions, and deprivation of serum in defined neuronal induction medium containing isobutyl 1-methyl xanthine-induced neurite outgrowth and neuronal differentiation, with an increase in expression of neuronal markers, such as Neu-N and Tuj-1.Citation6 Because KP-hMSCs can differentiate into either glial or neural cells, we investigated whether serum deprivation influences the expression of glial markers by seeding 1 × 106 cells in either serum-free or serum-containing medium for 24 hours. Western blot analysis showed that serum deprivation caused an apparent decrease in glial markers, including glial fibrillary acidic protein and Gal-C, but they were significantly increased after serum stimulation (). However, expression of the Neu-N neuronal marker and nestin precursor marker did not change in serum-free medium, suggesting that deprivation of serum alone without an induction stimulus was not sufficient to enhance neuronal differentiation (). These immunoblot results were also quantified by densitometric analysis, by which each marker examined was normalized by actin (). The effects of serum on the expression of Gal-C and glial fibrillary acidic protein were further examined by immunofluorescence staining, which showed that these glial markers were unable to be expressed in cells without serum stimulation (). Also, 10% serum stimulation was sufficient to induce the expression of the glial markers, Gal-C and glial fibrillary acidic protein, while 20% serum treatment slightly enhanced this effect. This phenomenon was further observed in the C6 rat glial cell line in which the expressions of glial fibrillary acidic protein and Gal-C were induced by adding 5% fetal bovine serum and 15% horse serum ().Citation21 These results suggest that serum is essential for the upregulation of glial markers in both KP-hMSCs and C6 glial cells for subsequent cell differentiation.
Figure 1 Serum induces the upregulation of markers for glial differentiation. A) Western blot analysis for comparison of glial and neuronal markers in human mesenchymal stem cells cultured with or without 10% FBS. B) Densitometric measurement of Western blot protein bands (*P < 0.05). C) Immunofluorescence staining of Gal-C and GFAP expression in human mesenchymal stem cells cultured in different concentration of FBS. D) effects of serum deprivation on C6 rat glial cells expressing GFAP and Gal-C proteins.
Serum deprivation-induced loss of glial markers is reversible
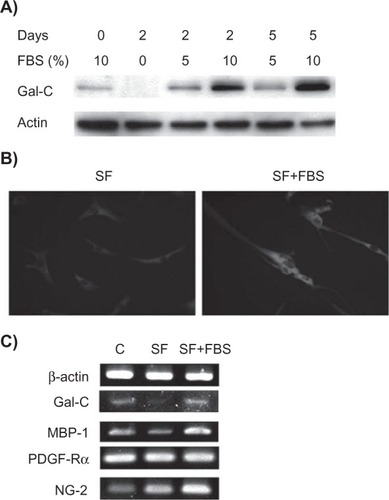
We next tested whether serum deprivation-induced loss of glial markers is reversible. We found that loss of the Gal-C marker in serum-deprived KP-hMSCs was rescued with the addition of fetal bovine serum (5% and 10%) for up to five days (). Immunofluorescence staining confirmed that glial fibrillary acidic protein also recovered under these conditions (). We next compared the expression of oligodendrocyte markers for different stages of differentiation. KP-hMSCs were cultured in serum-deprived medium, then supplemented with 10% fetal bovine serum for 48 hours. The mRNA levels for the markers expressed in these two conditions were compared. The markers (PDGF-Rα) for progenitor and pro- oligodendrocyte cells was not change under either condition, while NG-2 was not repressed under serum deprivation but was upregulated after the addition of serum (). NG-2 has been reported to be expressed in both progenitors and nonmyelinating oliogodendrocytes,Citation22,Citation23 and is not influenced by serum deprivation as reported for OLN-93 oligodendrocytes.Citation24 Also, the addition of serum rescued the decrease in Gal-C and myelin basic protein markers for myelinating oligodendrocytes (). These results suggest that serum deprivation causing loss of glial and oligodendrocyte markers is reversible.
Figure 2 Recovery of glial markers by serum supplementation in serum-deprived human mesenchymal stem cells. A) Western blot analysis detecting the expression of Gal-C in human mesenchymal stem cells that were cultured in serum-free medium for two days and then supplemented with FBS. B) Immunofluorescence staining of glial fibrillary acidic protein expression in human mesenchymal stem cells cultured in either SF for two days or thereafter supplemented with 10% FBS for an additional two days (SF + FBS). C) Semiquantitative real-time polymerase chain reaction detecting mRNA levels of glial and oligodendrocyte markers using the above experimental conditions. C is a control that was continuously cultured in FBS-supplemented medium.
Inhibition of Notch signaling enhances serum-stimulated oligodendrocyte differentiation
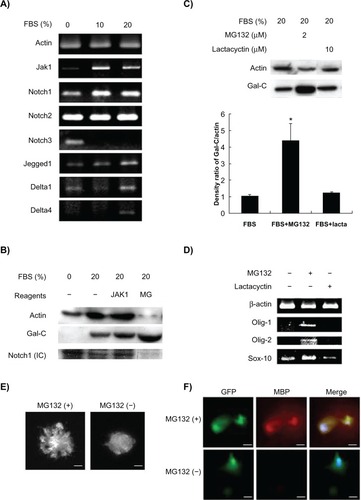
Stimulation of MSC differentiation by serum is accompanied by activation of various signaling pathways. As shown in , semiquantitative RT-PCR showed that mRNA levels of JAK1 and Notch1 were downregulated in response to serum deprivation. In contrast, Notch2 and Notch3 were unchanged and upregulated, respectively. Generally, the Notch signaling pathway seemed upregulated in the presence of serum, as demonstrated by the upregulation of Jagged1 and Delta1/4 in KP-hMSCs treated with 20% fetal bovine serum (). To understand better the role of the JAK and Notch signaling pathways in serum-induced expression of Gal-C markers for oligodendrocyte differentiation, cells in serum-containing medium were treated with JAK1 inhibitor and the Notch1-specific inhibitor, MG132. The Western blot data showed that MG132 repressed cleavage of the Notch1 intracellular domain, and induced an apparent upregulation of Gal-C compared with the vehicle control and JAK1 inhibitor (). To exclude the possibility of MG132 proteasome inhibition activity influencing Gal-C expression, a control experiment was conducted using the proteasome-specific inhibitor, lactacystin. The results showed that Gal-C expression was only induced by MG132 but not by lactacystin, suggesting that inhibition of Notch signaling is important for serum-stimulated Gal-C expression in KP-hMSCs (). We further analyzed the effects of Notch1 inhibition on gene expression of transcription factors required for differentiation of oligodendrocytes in KP-hMSCs. As demonstrated by semiquantitative RT-PCR, inhibition of Notch1 by MG132, but not lactacystin, in the presence of serum led to the induction of Olig-1, Olig-2, and Sox10 gene expression in KP-hMSCs (). The effects of Notch1 inhibition by MG132 on the differentiation of oligodendrocyte were also demonstrated using matrigel-coated culture dishes. KP-hMSCs were transfected with green fluorescence protein for detection of extended processes after cells were treated with MG132 (). Furthermore, an increase in the myelin basic protein-1 oligodendrocyte marker was detected in KP-hMSCs (). The cells have been transfected with green fluorescence protein and stained with DAPI for localization of upregulated myelin basic protein-1 and cell nucleus, respectively. These data suggest that inhibition of Notch1 expression, at least in part, can enhance the serum-stimulated oligodendrocyte differentiation of KP-hMSCs.
Figure 3 Effects of Notch signaling on serum-induced oligodendrocyte differentiation. A) Semiquantitative real-time polymerase chain reaction for detecting mRNA levels of JAK1 and Notch-associated markers in human mesenchymal stem cells cultured in different concentration of FBS. B) Western blotting for detecting the Gal-C and Notch1 cleaved internal domain in human mesenchymal stem cells treated with JAK1 inhibitor and Notch inhibitor MG132 for 24 hours. C) Comparison of effects of MG132 and lactacystin on expression of Gal-C. Upper panel: Western blot analysis. Lower panel: densitometric measurement of protein bands (*P < 0.05 compared with FBS control). D) Comparison of mRNA levels of oligodendrocyte-specific transcription factors (Olig-1, Olig-2, and SOX-10) in human mesenchymal stem cells treated with MG132 or lactacystin. E) Morphologic changes in GFP-labeled human mesenchymal stem cells. F) Comparison of the expression of MBP-1 in GFP-labeled human mesenchymal stem cells before (−) and after (+) MG132 treatment for 24 hours. Human mesenchymal stem cells were maintained in FBS during the treatment. The nuclei were stained by 4′,6-diamidino-2-phenylindole (DAPI). The sizes of scale bars for E and F were 20 and 40 μm, respectively.
Upregulation of oligodendrocyte markers and transcriptional factors by dominant-negative RBP-J Notch-targeting transcription mediator
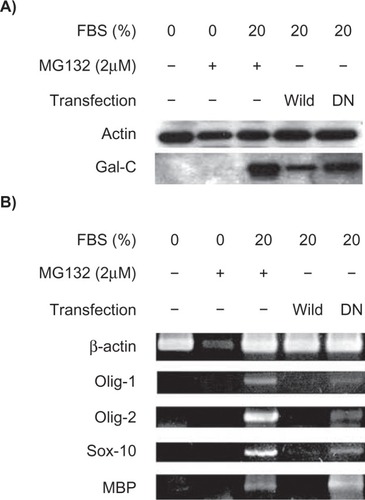
To elucidate further the role of the Notch signaling pathway on MSC oligodendrocyte differentiation, cells were transfected with a DN mutated RBP-J cDNA. The DN mutated RBP-J cDNA contains an insertion of oligonucleotide linkers between 218Arg and 227Arg to interfere with Notch function.Citation19 KP-hMSCs transfected with DN mutated RBP-J or treated with MG132 had increased Gal-C protein levels compared with cells transfected with wild-type RBP-J cDNA (). We also noticed that cells treated with MG132 under serum deprivation could not induce Gal-C expression (), indicating that inhibition of Notch signaling is an additive factor in serum-stimulated oligodendrocyte differentiation. Similarly, cells transfected with mutated RBP-J had increased Olig-1, Olig-2, Sox10, and myelin basic protein mRNA levels in the presence of serum, while cells transfected with wild-type RBP-J suppressed serum-induced expression of Olig-1, Olig-2, Sox10, and myelin basic protein mRNAs (). Taken together, these data suggest that chemical or molecular Notch inhibition can enhance serum-stimulated oligodendrocyte differentiation in vitro.
Figure 4 Inhibition of Notch activity by mutant RBP-J protein enhances the serum-stimulated expression of oligodendrocyte markers. A) Western blot analysis for comparison of Gal-C expression in human mesenchymal stem cells transfected with wild-type or the DN mutant form of RBP-J cDNA. B) Semiquantitative real-time polymerase chain reaction detecting the mRNA levels of other oligodendrocyte markers. MG132 (24 hours of treatment) was unable to induce these markers under serum-free conditions.
Discussion
To develop therapeutic applications, it is important to investigate the mechanisms involved in determining the fate of stem or progenitor cells. Based on previous knowledge of the signaling pathways regulating neural differentiation of KP-hMSCs,Citation6 we tested the relationship between the effects of serum and Notch signaling activity on regulation of oligodendrocyte differentiation. It has been reported that Schwann cells important for insulation of the peripheral nervous system can only express Gal-C and myelination in the presence of serum in vitro.Citation12 Moreover, a recent report shows that human bone marrow-derived MSC can differentiate into Schwann-like cells in complete medium.Citation25 Our current data support the importance of serum in expression of markers for glial and oligodendrocyte differentiation from human KP-hMSCs in vitro. Because glia and oligodendrocytes are important for myelination of the central nervous system, it suggests that human KP-hMSCs are one of the stem cell sources for regenerating functional glial and Schwann cells for neural transplantation. We conclude that serum is required for glial-like differentiation from human KP-hMSCs, at least in vitro.
Our data indicate that serum can induce the expression of Notch1 in vitro. It appears that Notch1 provides negative feedback inhibition of the effects of serum on oligodendrocyte differentiation because inhibition of Notch1 activity enhances serum-induced differentiation. We also examined the expression of Notch2 and Notch3 in response to serum stimulation, and we found that Notch3 exhibits an opposite expressive pattern to that of Notch1 mRNA. Given that Notch3 is structurally and functionally different from both Notch1 and Notch2, it is possible that Notch3 and Notch1 may behave differently in response to serum stimulation.Citation26 Whether Notch3 is also important for serum-stimulated oligodendrocyte differentiation remains to be investigated. To our knowledge, this is the first report delineating the function of serum stimulation and Notch1 regulating the oligodendrocyte differentiation of KP-hMSCs.
Although Notch signaling is known to inhibit growth and differentiation of neighboring cells laterally, its role in vertebrate neural development is still unclear. This may be due to a poor understanding of the diverse fates of neural cell lineages. However, several spinal cord developmental models illustrate single types of neurons produced from distinct precursor subdomains that align at the dorsoventral axis.Citation27–Citation29 The literature shows that each precursor population produces various cell types. For example, motor neuron progenitor precursors produce oligodendrocytes and motoneurons.Citation30,Citation31 Analysis of transgenically marked lineages shows that Olig2+ motor neuron progenitor cells also give rise to astrocytes and ependymal cells.Citation32 Thus, vertebrate neural precursors can give rise to various cell types, raising the possibility that, as in insects, Notch functions to diversify the fates of differentiating cells that have a common origin.
Wang et al have described the role of the Notch signaling pathway in oligodendrocyte differentiation.Citation13 They showed that developing and mature oligodendrocytes express the Notch1 receptor and that retinal ganglion cell expression of Jagged, which is distributed along the axon, decreases developmentally in a manner that correlates with optic nerve myelination. They also demonstrated that oligodendrocyte precursor cell differentiation in vitro is potentially inhibited by Notch signaling, suggesting that Notch-Jagged interactions play an inhibitory role in regulating the timing of central nervous system myelination. Recently, Genoud et al supported this conclusion in vivo using the Cre/Lox approach to eliminate Notch signaling selectively from oligodendrocyte precursor cells, which resulted in ectopic and premature oligodendrocyte differentiation.Citation33 Moreover, it has been demonstrated that heterozygous deletion of the Notch1 gene leads to increased myelination in mice.Citation34 Our current data are consistent with previous reports that the Notch signaling pathway plays an inhibitory role in oligodendrocyte differentiation. Recently, the Notch signaling pathway has been demonstrated to have an important role in the development of a variety of glial cell types, including Müller glia, radial glia, astrocytes, and Schwann cells, which are the myelinating cells of the peripheral nervous system.Citation35 Furthermore, Notch signaling may also be required for oligodendrocyte precursor cell specification. Notably, neurospheres derived from mouse embryos with a null allele of the Delta-like Notch ligand produce fewer oligodendrocyte precursor cells, and this deficiency can be rescued by reintroducing the Jagged gene.Citation36 Moreover, oligodendrocyte precursor cells are not detectable in the spinal cord of zebra fish embryos with defective Delta, while the conditional expression of constitutively active Notch promotes the generation of excessive oligodendrocyte precursor cells in the spinal cord.Citation37 Thus, it appears that Notch signaling plays an important role in oligodendrocyte precursor cell generation.
We used both Notch inhibitor MG132 and mutant DN RBP-J cDNA transfection to demonstrate the effects of Notch1 inhibition on oligodendrocyte differentiation. It is important to use mutant RBP-J for a parallel experiment because MG132 is toxic to cells and may cause nonspecific responses. For example, MG132 has been reported to repress β-actin gene transcription,Citation38 and this gene was used as a loading and internal control in this study. Although we have overexpressed mutant DN RBP-J cDNA in KP-hMSCs to inhibit Notch1, the phenomenon of oligodendrocyte differentiation may be limited by transfection efficiency. The transfection efficiency of KP-hMSCs was around 50%–70%, suggesting that a portion of cells would not be altered after cells were transiently transfected with DN RBP-J cDNA. Also, it is difficult to quantify the extent of Notch1 inhibition and the resulting cell differentiation by this mutant construct. To confirm directly the association between Notch1 inhibition and oligodendrocyte differentiation, viral-mediated siRNA targeting on RBP-J and Notch1 would be important alternatives for this research purpose.
In summary, our results demonstrate that serum deprivation in cultured KP-hMSCs leads to reduced expression of glial and oligodendrocyte markers. These effects are reversible with the addition of serum. Additionally, the serum-mediated stimulation of MSC oligodendrocyte differentiation is enhanced by Notch1 inhibition. Therefore, our data suggest the optimal culture conditions for differentiation of oligodendrocytes in vitro include the addition of serum, and this may increase the efficacy of differentiation via downregulation of Notch activity. These findings may be beneficial to MSC-based in vitro differentiation of oligodendrocytes intended for therapeutic purposes.
Acknowledgments
This research was supported in part by grants from the Taipei Veterans General Hospital (V96E2-009), National Science Council (95-2314-B-075-047-MY3, 96-3111-B-010-003, 96-2627-B-010-009), and National Yang-Ming University, Ministry of Education. This work was assisted in part by the Division of Experimental Surgery of the Department of Surgery, Taipei Veterans General Hospital. We thank Dr T Honjo for plasmids support.
Disclosure
The authors report no conflicts of interest in this work.
References
- BruckWThe pathology of multiple sclerosis is the result of focal inflammatory demyelination with axonal damageJ Neurol2005252Suppl 5v3v916254699
- LubetzkiCWilliamsAStankoffBPromoting repair in multiple sclerosis: Problems and prospectsCurr Opin Neurol20051823724415891406
- LublinFHistory of modern multiple sclerosis therapyJ Neurol2005252Suppl 3iii3iii916170498
- KeirsteadHSStem cells for the treatment of myelin lossTrends Neurosci20052867768316213602
- PittengerMFMackayAMBeckSCMultilineage potential of adult human mesenchymal stem cellsScience199928414314710102814
- ChuMSChangCFYangCCBauYCHoLLHungSCSignalling pathway in the induction of neurite outgrowth in human mesenchymal stem cellsCell Signal20061851953016098715
- KondoTJohnsonSAYoderMCRomandRHashinoESonic hedgehog and retinoic acid synergistically promote sensory fate specification from bone marrow-derived pluripotent stem cellsProc Natl Acad Sci U S A20051024789479415778294
- KirschningERutterGHohenbergHHigh-pressure freezing and freeze-substitution of native rat brain: Suitability for preservation and immunoelectron microscopic localization of myelin glycolipidsJ Neurosci Res1998534654749710266
- ZakaMWengerDAPsychosine-induced apoptosis in a mouse oligodendrocyte progenitor cell line is mediated by caspase activationNeurosci Lett200435820520915039117
- LevineJMReynoldsRFawcettJWThe oligodendrocyte precursor cell in health and diseaseTrends Neurosci200124394711163886
- EcclestonPASilberbergDHThe differentiation of oligodendrocytes in a serum-free hormone-supplemented mediumBrain Res1984318196386106
- OwensGCBungeRPEvidence for an early role for myelin-associated glycoprotein in the process of myelinationGlia198921191282470674
- WangSSdrullaADdiSibioGNotch receptor activation inhibits oligodendrocyte differentiationNeuron19982163759697852
- JurynczykMJurewiczABieleckiBRaineCSSelmajKInhibition of Notch signaling enhances tissue repair in an animal model of multiple sclerosisJ Neuroimmunol200517031016290267
- HuQDAngBTKarsakMF3/contactin acts as a functional ligand for Notch during oligodendrocyte maturationCell200311516317514567914
- LuLChenXZhangCWMorphological and functional characterization of predifferentiation of myelinating glia-like cells from human bone marrow stromal cells through activation of F3/Notch signaling in mouse retinaStem Cells20082658059017975227
- HungSCChengHPanCYTsaiMJKaoLSMaHLIn vitro differentiation of size-sieved stem cells into electrically active neural cellsStem Cells20022052252912456960
- HungSCYangDMChangCFImmortalization without neoplastic transformation of human mesenchymal stem cells by transduction with HPV16 E6/E7 genesInt J Cancer200411031331915095294
- ChungCNHamaguchiYHonjoTKawaichiMSite-directed mutagenesis study on DNA binding regions of the mouse homologue of Suppressor of Hairless, RBP-J kappaNucleic Acids Res199422293829448065905
- ChenTHChenWMHsuKHKuoCDHungSCSodium butyrate activates ERK to regulate differentiation of mesenchymal stem cellsBiochem Biophys Res Commun200735591391817331472
- YangIHCoCCHoCCSpatially controlled co-culture of neurons and glial cellsJ Biomed Mater Res A20057597698416138329
- NishiyamaAChangATrappBDNG2+ glial cells: A novel glial cell population in the adult brainJ Neuropathol Exp Neurol1999581113112410560654
- ZhangSCDefining glial cells during CNS developmentNat Rev Neurosci2001284084311715061
- BuckinxRSmoldersISahebaliSMorphological changes do not reflect biochemical and functional differentiation in OLN-93 oligodendroglial cellsJ Neurosci Methods20091841919595704
- YangLYZhengJKWangCYLiWYDifferentiation of adult human bone marrow mesenchymal stem cells into Schwann-like cells in vitroChin J Traumatol20058778015769304
- BellaviaDChecquoloSCampeseAFFelliMPGulinoAScrepantiINotch3: From subtle structural differences to functional diversityOncogene2008275092509818758477
- BriscoeJEricsonJSpecification of neuronal fates in the ventral neural tubeCurr Opin Neurobiol200111434911179871
- JessellTMNeuronal specification in the spinal cord: Inductive signals and transcriptional codesNat Rev Genet20001202911262869
- ShirasakiRPfaffSLTranscriptional codes and the control of neuronal identityAnnu Rev Neurosci20022525128112052910
- RichardsonWDSmithHKSunTPringleNPHallAWoodruffROligodendrocyte lineage and the motor neuron connectionGlia20002913614210625331
- RowitchDHGlial specification in the vertebrate neural tubeNat Rev Neurosci2004540941915100723
- MasahiraNTakebayashiHOnoKOlig2-positive progenitors in the embryonic spinal cord give rise not only to motoneurons and oligodendrocytes, but also to a subset of astrocytes and ependymal cellsDev Biol200629335836916581057
- GenoudSLappe-SiefkeCGoebbelsSNotch1 control of oligodendrocyte differentiation in the spinal cordJ Cell Biol200215870971812186854
- GivogriMICostaRMSchonmannVSilvaAJCampagnoniATBongarzoneERCentral nervous system myelination in mice with deficient expression of Notch1 receptorJ Neurosci Res20026730932011813235
- GaianoNFishellGThe role of notch in promoting glial and neural stem cell fatesAnnu Rev Neurosci20022547149012052917
- GrandbarbeLBouissacJRandMHrabe de AngelisMArtavanis-TsakonasSMohierEDelta-Notch signaling controls the generation of neurons/glia from neural stem cells in a stepwise processDevelopment20031301391140212588854
- ParkHCAppelBDelta-Notch signaling regulates oligodendrocyte specificationDevelopment20031303747375512835391
- DennisAPLonardDMNawazZO’MalleyBWInhibition of the 26S proteasome blocks progesterone receptor-dependent transcription through failed recruitment of RNA polymerase IIJ Steroid Biochem Mol Biol20059433734615857753