Abstract
Inadequate blood supply to tissues caused by obstruction of arterioles and/or capillaries results in ischemic injuries – these injuries can range from mild (eg, leg ischemia) to severe conditions (eg, myocardial infarction, stroke). Surgical and/or endovascular procedures provide cutting-edge treatment for patients with vascular disorders; however, a high percentage of patients are currently not treatable, owing to high operative risk or unfavorable vascular involvement. Therapeutic angiogenesis has recently emerged as a promising new therapy, promoting the formation of new blood vessels by the introduction of bone marrow–derived stem and progenitor cells. These cells participate in the development of new blood vessels, the enlargement of existing blood vessels, and sprouting new capillaries from existing blood vessels, providing evidence of the therapeutic utility of these cells in ischemic tissues. In this review, the authors describe peripheral arterial disease, an ischemic condition affecting the lower extremities, summarizing different aspects of vascular regeneration and discussing which and how stem cells restore the blood flow. The authors also present an overview of encouraging results from early-phase clinical trials using stem cells to treat peripheral arterial disease. The authors believe that additional research initiatives should be undertaken to better identify the nature of stem cells and that an intensive cooperation between laboratory and clinical investigators is needed to optimize the design of cell therapy trials and to maximize their scientific rigor. Only this will allow the results of these investigations to develop best clinical practices. Additionally, although a number of stem cell therapies exist, many treatments are performed outside international and national regulations and many clinical trials have been not registered on databases such as ClinicalTrials.gov or EudraCT. Therefore, more rigorous clinical trials are required to confirm the first hopeful results and to address the challenging issues.
What is peripheral arterial disease?
Peripheral arterial disease (PAD) is a common circulatory problem in which narrowed arteries reduce blood flow to the limbs, especially the legs. The most common causes of PAD are atherosclerosis obliterans (ASO) and thromboangiitis obliterans (TAO).Citation1
Two major classification systems are currently used to evaluate the spectrum of symptoms: (1) the Fontaine classification, not used in everyday clinical practice but useful for research purposes, and (2) the Rutherford classification, more commonly cited in recent publications in the field of vascular medicine (). The American College of Cardiology/American Heart Association 2005 guidelines noted the usefulness of the Rutherford classification for standardized communication between clinicians.Citation1 Disease staging and classification systems are important for clinical management of these patients. Based on the severity of symptoms, usually two distinct clinical presentations are distinguished in PAD patients: (1) intermittent claudication, characterized by intermittent pain in leg muscles when the person walks, and (2) critical limb ischemia (CLI), a more severe form of PAD, characterized by pain at rest, nonhealing wounds, and gangrene. After 1 year, 30% of patients with CLI will lose their leg and 25% will die.Citation2
Table 1 Two classifications of peripheral arterial disease (PAD): Fontaine and Rutherford
The incidence of CLI in Western societies is approximately 220 new cases per million people per year, and, with an aging population, the population at risk is expected to increase because of persistent rates of tobacco abuse and an increase in diabetes.Citation2 Fifty percent of diabetics (7% of the world population in 2010) suffer from PAD, which may lead to amputation due to CLI.Citation3 Moreover, smoking, hypertension, dyslipidemia, a sedentary lifestyle, and a genetic predisposition all contribute to the development of PAD.Citation4,Citation5
Current treatments for PAD
Revascularization, either surgical or endovascular, is the gold standard treatment for patients with severe PAD. However, despite advances in surgical and endovascular techniques,Citation6 more than 30% of patients do not qualify as candidates for revascularization because of excessive operative risk or adverse vascular involvement. Furthermore, the presence of extensive atherosclerotic plaques in the tibial and/or peroneal arteries renders revascularization unsuccessful. These patients are left to medical therapy, which may only slow disease progression, and the only remaining alternative for relief of rest pain or gangrene is amputation of the affected leg.
An estimated 120–500 amputations are performed per million people per year, and one-quarter of these patients require long-term institutional care or professional assistance at home.Citation2 Medical therapy is limited to antithrombotic therapy,Citation7 the prostaglandin analogue iloprost,Citation8 or recently to cilostazol. Cilostazol has been found to be effective for the treatment of intermittent claudication. This compound has several beneficial effects on platelet aggregation, serum lipids, and endothelial cells (ECs), but how these might relate to improvements in walking is not entirely understood.Citation9 Thus, there is a critical need to develop novel strategies to promote neovascularization in patients with CLI who are not candidates for conventional treatments.
In 1997, Asahara et alCitation10 made a big step forward when they identified a class of bone marrow–derived circulating endothelial progenitor cells (EPCs) that contribute to angiogenesis and/or vasculogenesis in ischemic tissues.Citation11 Since then, several studies have reported the capability of stem and progenitor cells to promote neovascularization, reducing ischemic damages.Citation12,Citation13 Encouraging results of several clinical studies have rapidly demonstrated the beneficial effects of autologous stem cell transplantation in patients affected by CLI. Clinical improvements were observed in objective and subjective measurements of perfusion (ie, transcutaneous oxygen tension [TcPO2] and laser Doppler flowmetry [LDF]), pain reduction, increased pain-free total walking distance, and decreased rate of amputation.Citation3,Citation14–Citation69
What is vascular regeneration?
Vascular regeneration involves the restoration of normal vascular function and structure and the growth of new blood vessels. This includes a plethora of processes, such as the distribution of blood flow via the formation of collateral networks; the response of newly generated vessels to hemodynamic, humoral, and local tissue factors; the modulation of the immune response and the trafficking of circulating cells; and the permeation of nutrients and macromolecules through the microvasculature, which can in turn have trophic effects on blood fluidity and hemostasis.Citation9 Vascular regeneration is also important in a variety of processes: during embryonic organogenesis and organ growth in born individuals, in the course of restoration of blood supply to ischemic tissues, and in the establishment of blood supply to tumours.Citation70
Neovascularization involves the growth of new structures from preexisting vessels by migration, proliferation, and differentiation of progenitor cells and the interplay between growth factors and cytokines. The process of neovascularization comprises three distinct phenomena: (1) vasculogenesis, (2) angiogenesis, and (3) arteriogenesis.Citation70
The essential mechanism responsible for new blood vessel formation in adults is based on neoangiogenesis. During angiogenesis, ECs present in vessel walls are activated in response to various stimuli and begin to relase various growth factors, the angiopoietins (Ang1 and Ang2) and Vascular Endothelial Growth Factor (VEGF), which play a crucial role in this process. While Ang1 and Ang2 participate in the “stabilization” of the newly formed vessels, VEGF exerts its pro-angiogenic function by binding to one of its receptors, specifically the VEGF receptor 2 or kinase (VEGFR2 or KDR) insert domain receptor, expressed exclusively by ECs and their precursors. This binding triggers a cascade of events that leads to the formation of new blood vessels and which comprises the migration of ECs into the surrounding tissue in response to angiogenic chemokines; proliferation and differentiation of EPCs; and recruitment of support cells such as pericytes for small capillaries and smooth muscle cells for larger vessels.
The main factor inducing angiogenesis in adults is the availability of oxygen, through the activation of hypoxia-inducible factors.Citation71,Citation72
Stem cells with angiogenic potential
Stem cells are defined as cells with the capacity to self-renew and to generate differentiated cells and are divided into two types: embryonic and adult stem cells.Citation73 Adult stem cells are partially lineage-committed cells and have the capacity to give rise to specialized cells. For this feature, adult stem cells are so-called multipotent cells – as opposed to pluripotent cells (ie, embryonic stem cells), which can give rise to all the cell types in the body. Adult stem cells include three different groups: (1) the bone marrow stem cells, (2) the circulating pool of stem/progenitor cells (also derived from the bone marrow), and (3) the tissue-resident stem cells.Citation74
Bone marrow stem cells include different types of progenitor cells, such as multipotent adult progenitor cells, mesenchymal stem cells (MSCs), and hematopoietic stem cells. The circulating pool of stem and progenitor cells contains a variety of cells, but the most relevant for vascular regeneration are the EPCs. Finally, the tissue-resident stem cells are present in almost all tissues in a quiescent state and can respond efficiently to different stimuli.Citation74
Both EPCs and MSCs show promise for potential utility in therapeutic neovascularization. MSCs are reported to promote angiogenesis because of their capacity to stimulate EC migration and tube formation; furthermore, MSCs support neoangiogenesis, releasing soluble factors that contribute to stimulate angiogenesis.Citation75
What are the features of these cells?
MSCs are a subset of cells that express on their surface specific molecules such as CD73, CD90, and CD105; MSCs also express CD54/CD102, CD166, and CD49 (alpha integrin), which regulate cell-to-cell interactions, and they do not express any hematopoietic and/or EPC surface markers.Citation76 MSCs can be found in many fetal and adult tissues and are generally isolated from bone marrow, adipose tissue, umbilical cord blood, and compact bone. Furthermore, MSCs are able to migrate to and home to injured sites, where they act by differentiating into specific cells and by secreting trophic factors, which activate paracrine signaling.Citation77 Moreover, these cells interact with the immune system, particularly modulating the immune response, apparently by inhibiting tumor necrosis factor-alpha (TNFa) and interferon-gamma (IFN-γ) and by increasing interleukin 10 (IL-10).Citation78 This unique immunomodulatory property makes these cells suitable for both autologous and heterologous transplants, since they avoid and/or actively suppress eventual rejection of transplants.Citation79
MSCs display a great therapeutic potential because of their capability to differentiate into muscle, neural precursors, cardiomyocytes, and perivascular cells. Perivascular cells (herein referred to as pericytes) are critical cells in vascular biology. Pericytes typically express alpha smooth muscle actin (α-SMA), platelet-derived growth factor receptor beta (PDGF-β), and nerve/glial antigen-2 (NG-2) proteoglycan. They are branched cells embedded within the basement membrane of capillaries and postcapillary venules, stabilizing the vessel wall.Citation80 Pericytes are considered cells that control EC proliferation and migration, and thereby also the growth of new capillaries. In turn, ECs stimulate expansion and activation of the pericyte precursor cell population. The balance between ECs and pericytes is highly controlled by a series of signaling pathway mechanisms operating in an autocrine and/or a paracrine manner. In pathological conditions in which angiogenic activity is impaired, pericytes and ECs could be partly responsible for abnormalities in blood vessels.
EPCs are adult hemangioblast-derived cells characterized by the expression of CD34, VEGF receptor 2, and CD133. These markers are expressed by precursor cells, but not by differentiated ones.Citation81 In fact, as the hemangioblasts differentiate to become ECs, they downregulate the hematopoietic stem cell marker CD133 expression.Citation81 EPCs can be isolated from human peripheral or umbilical cord blood and can also be found in bone marrow niches. EPCs have shown in vitro all the functional properties of ECs; moreover, EPCs have direct angiogenic action, supporting angiogenesis through their ability to secrete paracrine mediators. In this respect, several studies have shown that these cells release interleukins, growth factors, and chemokines that altogether regulate CD14-positive cells, accelerate vascular network formation, and enhance healing processes.Citation82
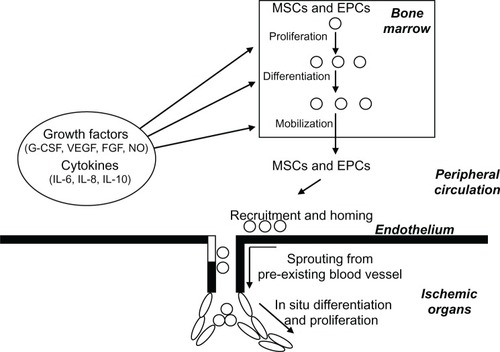
Adult stem cells with angiogenic potential such as EPCs and MSCs will stimulate the production of new blood vessels, as shown in .
Figure 1 Schematic representation of neoangiogenesis promoted by circulating and bone marrow–resident stem cells.
Abbreviations: FGF, fibroblast growth factor; G-CSF, granulocyte colony-stimulating factor; IL, interleukin; NO, nitric oxide; VEGF, vascular endothelial growth factor.
Cell therapy in PAD: clinical results
Promotion of collateral vessel formation and angiogenesis in PAD patients is an important therapeutic strategy to minimize tissue injury associated with severe ischemia. The Therapeutic Angiogenesis using Cell Transplantation trial was the first report on the use of bone marrow–derived mononuclear cells in the treatment of PAD.Citation14 Starting from this, the search of the literature yielded a total of 57 early-phase clinical trials for a total of 1997 enrolled patients. The safety and feasibility of autologous cell transplantation has been reported in 1667 treated patients ( and ). Among these, a total of 303 diabetic patients with CLI and foot ulcers underwent cell therapy. The degree of ischemia varied throughout the groups, ranging from Rutherford category 4/Fontaine stage III through to severe CLI classified as Rutherford category 6/Fontaine stage IV.
Table 2 Clinical trials with cell therapy in peripheral arterial disease (PAD)
Table 3 Clinical trials with intralesional administration of stem cells in foot ulcers
Only a minority of trials (n = 13) included appropriate controls (). In these studies, the follow-up of the untreated or placebo group did not differ from that observed in several large population studies.Citation2,Citation83–Citation87 In addition, the Edinburgh Artery Study defined the prevalence of asymptomatic and symptomatic PAD and related comorbidities in the general population.Citation87 Therefore, since the progression of disease is well defined in CLI patients, the lack of an untreated or a placebo group – even if scientifically compelling – cannot diminish the significance of the studies.
Table 4 Controlled clinical trials with cell therapy in peripheral arterial disease
Two sources of cells were used in these trials: (1) bone marrow aspiration (n = 46) and (2) apheresis of peripheral blood with or without GSF stimulation (n = 11). The route of cell administration was intramuscular in 39 trials, intra-arterial in nine trials, and combined intra-arterial plus intramuscular in four trials (). Furthermore, two studies compared the therapeutic effects of intramuscular or intra-arterial delivery of bone marrow cells in patients with lower limb ischemia, showing similar beneficial results.Citation36,Citation65
To prevent clot formation, harvested cells were collected in the presence of anticoagulant.Citation3,Citation14–Citation69 Intramuscular administration is usually performed through multiple injections at the level of limb muscles, while intra-arterial infusion is usually performed via classic femoral access. Three studies reported the use of intralesional administration of bone marrow–derived stem cells in 31 diabetic patients with foot ulcers, showing encouraging results ().
In general, bone marrow aspiration was well tolerated, and the most frequent adverse reaction was local pain or mild anemia. However, serious adverse reactions such as angina with ST segment depression were observed in a small number of patients.Citation55
The average follow-up of these clinical studies was 8.4 ± 9.55 months. Considering all studies, the reported outcomes for therapeutic efficacy of cell therapy involved the ankle-brachial index, TcPO2, LDF, pain-free walking distance, ulcer healing, and amputation-free survival. In all studies, symptoms improved after the procedure, as evidenced by clinical evaluation, relief of rest pain, and improvement by at least one level in Rutherford and Fontaine classifications. Furthermore, autologous cell therapy promoted amputation-free survival with an average of 7.8 months and promoted complete wound healing within 3 months in most patients with ulcers prior to bone marrow stem cell transplantation, in comparison with the natural history of PAD patients. Therefore, autologous transplantation of bone marrow–derived cells significantly improved both the objective and the subjective endpoints.
Conclusion
Herein, the authors provide the most comprehensive review of cell therapy trials describing the background and first results of stem and progenitor cell therapy in patients with CLI who are not suitable for revascularization. Both the principle, as far as it is understood, and the methods are described. Compelling evidence suggests that stem cell therapy may become a useful adjunct to the current treatment options. Because of poor prognosis and the increasing number of patients, there is a need for new therapeutic methods.
About 1997 patients without revascularization options were enrolled in these trials and 1667 patients were treated. Cell therapy significantly improved functional outcomes such as ankle-brachial index, TcPO2 or LDF, rest pain, pain-free walking distance, ulcer healing, and limb salvage. Although it is generally agreed that controlled trials yield more reliable results, the authors also included noncontrolled studies, which are the majority of published reports. The authors believe the main reason for this majority is that the authorized studies have chosen to treat end-stage patients, without other therapeutic options. The procedures are generally safe and well tolerated. Reported deaths were expected, given the severe underlying disease, and could not be directly attributed to cell therapy.
Challenges in this new therapeutic option still include open questions regarding cell number, phenotype, processing, route of optimal delivery, and frequency of application. The number of injected cells ranged from 4 × 106 to 109 for bone marrow cells and from 7 × 107 to 3 × 109 for peripheral blood–derived mononuclear cells, with positive effects on blood perfusion, even when low cells were used. Nevertheless, no correlation study between clinical response and cell number has been performed so far, and no proven correlation exists between the phenotype of used cells and efficacy of neoangiogenesis. Answering these two points is critical to understanding which and how many cells are needed to obtain a clinical response.
The question of optimal delivery route remains open. The rationale behind the intramuscular injection is to generate a reservoir of cells near the ischemic area, which can be recruited by active paracrine mechanisms. The intra-arterial injection relies on the fact that the blood flow transports cells up to the ischemia site; however, it is not known how many cells are able to leave the blood stream to reach the ischemic area. Again, no correlation study between the two routes of administration has been performed, although the present trend is for intramuscular administration. It has been reported that the combination of both routes (intramuscular plus intra-arterial)Citation39 has given substantial improvements in clinical outcomes, but this must be confirmed in exhaustive experiments in preclinical models.
In summary, over the past 10 years there has been considerable interest in stem cells, including extensive clinical activity involving stem cells. Unfortunately, the rationale for the clinical application of adult stem cells, particularly in regenerative medicine, has lagged behind initial laboratory observations. At this point, the authors believe that additional research initiatives should be undertaken to better identify the nature of stem cells and that an intensive cooperation between laboratory and clinical investigators is needed to optimize the design of cell therapy trials and to maximize their scientific rigor. Only this will allow the results of these investigations to develop best clinical practices.
Additionally, although a number of stem cell therapies exist, many treatments are performed outside international and national regulations and many clinical trials have been not registered on databases such as ClinicalTrials.gov or EudraCT. Therefore, more rigorous clinical trials are required to confirm the first hopeful results and to address the challenging issues.
Acknowledgments
The authors are grateful to Fondazione Luigi Califano, Fondazione Banco di Napoli, and Istituto Superiore di Sanità. The authors thank Prof Anna Maria Molinari and Prof Ferdinando Auricchio for helpful discussions.
Disclosure
The authors report no conflicts of interest in this work.
References
- HirschATHaskalZJHertzerNRAmerican Association for Vascular Surgery/Society for Vascular SurgerySociety for Cardiovascular Angiography and InterventionsSociety for Vascular Medicine and BiologySociety of Interventional RadiologyACC/AHA Task Force on Practice GuidelinesACC/AHA Guidelines for the Management of Patients with Peripheral Arterial Disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Associations for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease); summary of recommendationsJ Vasc Interv Radiol20061791383139716990459
- NorgrenLHiattWRDormandyJANehlerMRHarrisKAFowkesFGTASC (Trans Atlantic Inter-Society Consensus) II Working GroupInter-society consensus for the management of peripheral arterial disease (TASC II)J Vasc Surg200745Suppl SS5S6717223489
- ProcházkaVGumulecJJalůvkaFCell therapy, a new standard in management of chronic critical limb ischemia and foot ulcerCell Transplant201019111413142420529449
- ThorgeirssonTEGellerFSulemPA variant associated with nicotine dependence, lung cancer and peripheral arterial diseaseNature2008452718763864218385739
- WilsonAMSadrzadeh-RafieAHMyersJLow lifetime recreational activity is a risk factor for peripheral arterial diseaseJ Vasc Surg2011542427432432. e1432. e421664093
- ConradMFCrawfordRSHackneyLAEndovascular management of patients with critical limb ischemiaJ Vasc Surg20115341020102521211929
- SahaSPWhayneTFJrMukherjeeDCurrent evidence for antithrombotic therapy after peripheral vascular interventionsCurr Vasc Pharmacol Epub1202012
- LessianiGVazzanaNCuccurulloCInflammation, oxidative stress and platelet activation in aspirin-treated critical limb ischaemia: beneficial effects of iloprostThromb Haemost2011105232132821103664
- VolzKSMiljanEKhooACookeJPDevelopment of pluripotent stem cells for vascular therapyVascul Pharmacol2012565–628829622387745
- AsaharaTMuroharaTSullivanAIsolation of putative progenitor endothelial cells for angiogenesisScience199727553029649679020076
- ShiQRafiiSWuMHEvidence for circulating bone marrow-derived endothelial cellsBlood19989223623679657732
- AsaharaTMasudaHTakahashiTBone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularizationCirc Res199985322122810436164
- CrosbyJRKaminskiWESchattemanGEndothelial cells of hematopoietic origin make a significant contribution to adult blood vessel formationCirc Res200087972873011055974
- Tateishi-YuyamaEMatsubaraHMuroharaTTherapeutic Angiogenesis using Cell Transplantation Study InvestigatorsTherapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trialLancet2002360933142743512241713
- EsatoKHamanoKLiTSNeovascularization induced by autologous bone marrow cell implantation in peripheral arterial diseaseCell Transplant200211874775212588106
- SaigawaTKatoKOzawaTClinical application of bone marrow implantation in patients with arteriosclerosis obliterans, and the association between efficacy and the number of implanted bone marrow cellsCirc J200468121189119315564705
- HigashiYKimuraMHaraKAutologous bone-marrow mononuclear cell implantation improves endothelium-dependent vasodilation in patients with limb ischemiaCirculation2004109101215121815007007
- MiyamotoMYasutakeMTakanoHTherapeutic angiogenesis by autologous bone marrow cell implantation for refractory chronic peripheral arterial disease using assessment of neovascularization by 99 mTc-tetrofosmin (TF) perfusion scintigraphyCell Transplant200413442943715468685
- HuangPPLiSZHanMZAutologous transplantation of peripheral blood stem cells as an effective therapeutic approach for severe arteriosclerosis obliterans of lower extremitiesThromb Haemost200491360660914983238
- KawamuraAHorieTTsudaIPrevention of limb amputation in patients with limbs ulcers by autologous peripheral blood mononuclear cell implantationTher Apher Dial200591596315828908
- LenkKAdamsVLurzPTherapeutical potential of blood-derived progenitor cells in patients with peripheral arterial occlusive disease and critical limb ischaemiaEur Heart J200526181903190915855189
- HuangPLiSHanMXiaoZYangRHanZCAutologous transplantation of granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cells improves critical limb ischemia in diabetesDiabetes Care20052892155216016123483
- IshidaAOhyaYSakudaHAutologous peripheral blood mononuclear cell implantation for patients with peripheral arterial disease improves limb ischemiaCirc J200569101260126516195628
- DurduSAkarARAratMSancakTErenNTOzyurdaUAutologous bone-marrow mononuclear cell implantation for patients with Rutherford grade II–III thromboangiitis obliteransJ Vasc Surg200644473273916926085
- KoshikawaMShimodairaSYoshiokaTTherapeutic angiogenesis by bone marrow implantation for critical hand ischemia in patients with peripheral arterial disease: a pilot studyCurr Med Res Opin200622479379816684440
- AraiMMisaoYNagaiHGranulocyte colony-stimulating factor: a noninvasive regeneration therapy for treating atherosclerotic peripheral artery diseaseCirc J20067091093109816936417
- MiyamotoKNishigamiKNagayaNUnblinded pilot study of autologous transplantation of bone marrow mononuclear cells in patients with thromboangiitis obliteransCirculation2006114242679268417145986
- KawamuraAHorieTTsudaIClinical study of therapeutic angiogenesis by autologous peripheral blood stem cell (PBSC) transplantation in 92 patients with critically ischemic limbsJ Artif Organs20069422623317171401
- BartschTBrehmMZeusTKöglerGWernetPStrauerBETransplantation of autologous mononuclear bone marrow stem cells in patients with peripheral arterial disease (the TAM-PAD study)Clin Res Cardiol2007961289189917694378
- HuangPPYangXFLiSZWenJCZhangYHanZCRandomised comparison of G-CSF-mobilized peripheral blood mononuclear cells versus bone marrow-mononuclear cells for the treatment of patients with lower limb arteriosclerosis obliteransThromb Haemost20079861335134218064333
- KajiguchiMKondoTIzawaHSafety and efficacy of autologous progenitor cell transplantation for therapeutic angiogenesis in patients with critical limb ischemiaCirc J200771219620117251666
- HernándezPCortinaLArtazaHAutologous bone-marrow mononuclear cell implantation in patients with severe lower limb ischaemia: a comparison of using blood cell separator and Ficoll density gradient centrifugationAtherosclerosis20071942e52e5616982058
- SaitoSNishikawaKObataHGotoFAutologous bone marrow transplantation and hyperbaric oxygen therapy for patients with thromboangiitis obliteransAngiology200758442943417652224
- MatobaSTatsumiTMuroharaTLong-term clinical outcome after intramuscular implantation of bone marrow mononuclear cells (Therapeutic Angiogenesis by Cell Transplantation [TACT] trial) in patients with chronic limb ischemiaAm Heart J200815651010101819061721
- NapoliCFarzatiBSicaVBeneficial effects of autologous bone marrow cell infusion and antioxidants/L-arginine in patients with chronic critical limb ischemiaEur J Cardiovasc Prev Rehabil200815670971819050436
- GuYQZhangJGuoLRTransplantation of autologous bone marrow mononuclear cells for patients with lower limb ischemiaChin Med J (Engl)20081211196396718706241
- ChocholaMPytlíkRKobylkaPAutologous intra-arterial infusion of bone marrow mononuclear cells in patients with critical leg ischemiaInt Angiol200827428129018677289
- WesterTJørgensenJJStrandenETreatment with autologous bone marrow mononuclear cells in patients with critical lower limb ischaemia: a pilot studyScand J Surg2008971566218450207
- Van TongerenRBHammingJFFibbeWEIntramuscular or combined intramuscular/intra-arterial administration of bone marrow mononuclear cells: a clinical trial in patients with advanced limb ischemiaJ Cardiovasc Surg (Torino)20084915158
- De VrieseASBillietJVan DroogenbroeckJGhekiereJDe LetterJAAutologous transplantation of bone marrow mononuclear cells for limb ischemia in a Caucasian population with atherosclerosis obliteransJ Intern Med2008263439540318221334
- CobellisGSilvestroniALilloSLong-term effects of repeated autologous transplantation of bone marrow cells in patients affected by peripheral arterial diseaseBone Marrow Transplant2008421066767218695661
- MotukuruVSureshKRVivekanandVRajSGirijaKRTherapeutic angiogenesis in Buerger’s disease (thromboangiitis obliterans) patients with critical limb ischemia by autologous transplantation of bone marrow mononuclear cellsJ Vasc Surg200848Suppl 6S53S60
- AmannBLüdemannCRückertRDesign and rationale of a randomized, double-blind, placebo-controlled phase III study for autologous bone marrow cell transplantation in critical limb ischemia: the BONe Marrow Outcomes Trial in Critical Limb Ischemia (BONMOT-CLI)Vasa200837431932519003741
- AmannBLüdemannCRateiRSchmidt-LuckeJAAutologous bone marrow cell transplantation increases leg perfusion and reduces amputations in patients with advanced critical limb ischemia due to peripheral artery diseaseCell Transplant200918337138019500466
- CapiodJCTournoisCVitryFCharacterization and comparison of bone marrow and peripheral blood mononuclear cells used for cellular therapy in critical leg ischaemia: towards a new cellular productVox Sang200996325626519207166
- FranzRWParksAShahKJHankinsTHartmanJFWrightMLUse of autologous bone marrow mononuclear cell implantation therapy as a limb salvage procedure in patients with severe peripheral arterial diseaseJ Vasc Surg20095061378139019837539
- FranzRWShahKJJohnsonJDShort- to mid-term results using autologous bone-marrow mononuclear cell implantation therapy as a limb salvage procedure in patients with severe peripheral arterial diseaseVasc Endovascular Surg201145539840621669864
- ZafarghandiMRRavariHAghdamiNSafety and efficacy of granulocyte-colony-stimulating factor administration following autologous intramuscular implantation of bone marrow mononuclear cells: a randomized controlled trial in patients with advanced lower limb ischemiaCytotherapy201012678379120078390
- ProcházkaVGumulecJChmelováJAutologous bone marrow stem cell transplantation in patients with end-stage chronical critical limb ischemia and diabetic footVnitr Lek200955317317819378841
- Lara-HernandezRLozano-VilardellPBlanesPTorreguitart-MiradaNGalmésABesalduchJSafety and efficacy of therapeutic angiogenesis as a novel treatment in patients with critical limb ischemiaAnn Vasc Surg201024228729420142004
- IsoYSodaTSatoTImpact of implanted bone marrow progenitor cell composition on limb salvage after cell implantation in patients with critical limb ischemiaAtherosclerosis2010209116717219748620
- SprengersRWMollFLTeraaMVerhaarMCJUVENTAS Study GroupRationale and design of the JUVENTAS trial for repeated intra-arterial infusion of autologous bone marrow-derived mononuclear cells in patients with critical limb ischemiaJ Vasc Surg20105161564156820488328
- MurphyMPLawsonJHRappBMAutologous bone marrow mononuclear cell therapy is safe and promotes amputation-free survival in patients with critical limb ischemiaJ Vasc Surg20115361565157421514773
- WalterDHKrankenbergHBalzerJOIntraarterial administration of bone marrow mononuclear cells in patients with critical limb ischemia: a randomized start, placebo-controlled pilot trial (PROVASA)Circ Cardiovasc Interv201141263721205939
- IafratiMDHallettJWGeilsGEarly results and lessons learned from a multicenter, randomized, double-blind trial of bone marrow aspirate concentrate in critical limb ischemiaJ Vasc Surg20115461650165822019148
- IdeiNSogaJHataTAutologous bone-marrow mononuclear cell implantation reduces long-term major amputation risk in patients with critical limb ischemia: a comparison of atherosclerotic peripheral arterial disease and Buerger diseaseCirc Cardiovasc Interv201141152521205941
- Ruiz-SalmeronRde la Cuesta-DiazAConstantino-BermejoMAngiographic demonstration of neoangiogenesis after intra-arterial infusion of autologous bone marrow mononuclear cells in diabetic patients with critical limb ischemiaCell Transplant201120101629163922289660
- LuDChenBLiangZComparison of bone marrow mesenchymal stem cells with bone marrow-derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: a double-blind, randomized, controlled trialDiabetes Res Clin Pract2011921263621216483
- GabrHHedayetAImamUNasserMLimb salvage using intramuscular injection of unfractionated autologous bone marrow mononuclear cells in critical limb ischemia: a prospective pilot clinical trialExp Clin Transplant20119319720221649569
- BenoitEO’DonnellTFJrIafratiMDThe role of amputation as an outcome measure in cellular therapy for critical limb ischemia: implications for clinical trial designJ Transl Med2011916521951607
- PowellRJComerotaAJBerceliSAInterim analysis results from the RESTORE-CLI, a randomized, double-blind multicenter phase II trial comparing expanded autologous bone marrow-derived tissue repair cells and placebo in patients with critical limb ischemiaJ Vasc Surg20115441032104121684715
- PerinECSilvaGGahremanpourAA randomized, controlled study of autologous therapy with bone marrow-derived aldehyde dehydrogenase bright cells in patients with critical limb ischemiaCatheter Cardiovasc Interv20117871060106721594960
- SmadjaDMDuong-van-HuyenJPDal CortivoLEarly endothelial progenitor cells in bone marrow are a biomarker of cell therapy success in patients with critical limb ischemiaCytotherapy201214223223922040109
- PowellRJMarstonWABerceliSACellular therapy with ixmyelocel-T to treat critical limb ischemia: the randomized, double-blind, placebo-controlled RESTORE-CLI trialMol Ther20122061280128622453769
- KlepanecAMistrikMAltanerCNo difference in intraarterial and intramuscular delivery of autologous bone-marrow cells in patients with advanced critical limb ischemiaCell Transplant Epub422012
- SchiavettaAMaioneCBottiCA phase II trial of autologous transplantation of bone marrow stem cells for critical limb ischemia: results of NAPLES studyStem Cells Trans MedIn press2012
- VojtassákJDanisovicLKubesMAutologous biograft and mesenchymal stem cells in treatment of the diabetic footNeuro Endocrinol Lett200627Suppl 2S134S137
- DashNRDashSNRoutrayPMohapatraSMohapatraPCTargeting nonhealing ulcers of lower extremity in human through autologous bone marrow-derived mesenchymal stem cellsRejuvenation Res200912535936619929258
- SubrammaniyanRAmalorpavanathanJShankarRApplication of autologous bone marrow mononuclear cells in six patients with advanced chronic critical limb ischemia as a result of diabetes: our experienceCytotherapy201113899399921671823
- CarmelietPAngiogenesis in health and diseaseNat Med20039665366012778163
- BenoitEO’DonnellTFJrPatelANSafety and efficacy of autologous cell therapy in critical limb ischemia: a systematic review of the literatureCell Transplant Epub3282012
- KaelinWGJrRatcliffePJOxygen sensing by metazoans: the central role of the HIF hydroxylase pathwayMol Cell200830439340218498744
- GopallJHuangWZhaoYProspects of adult stem cells therapy in peripheral vascular diseasesBJMP201034a345
- DimmelerSBurchfieldJZeiherAMCell-based therapy of myocardial infarctionArterioscler Thromb Vasc Biol200828220821617951319
- CobellisGMaioneCBottiCBeneficial effects of VEGF secreted from stromal cells in supporting endothelial cell functions: therapeutic implications for critical limb ischemiaCell Transplant201019111425143720587143
- VolarevicVArsenijevicNLukicMLStojkovicMConcise review: mesenchymal stem cell treatment of the complications of diabetes mellitusStem Cells201129151021280154
- PittengerMFMackayAMBeckSCMultilineage potential of adult human mesenchymal stem cellsScience1999284541114314710102814
- AggarwalSPittengerMFHuman mesenchymal stem cells modulate allogeneic immune cell responsesBlood200510541815182215494428
- StaggJImmune regulation by mesenchymal stem cells: two sides to the coinTissue Antigens20076911917212702
- RibattiDNicoBCrivellatoEThe role of pericytes in angiogenesisInt J Dev Biol201155326126821710434
- SchattemanGCAwadOHemangioblasts, angioblasts, and adult endothelial cell progenitorsAnat Rec A Discov Mol Cell Evol Biol20042761132114699630
- JarajapuYPGrantMBThe promise of cell-based therapies for diabetic complications: challenges and solutionsCirc Res2010106585486920299675
- DormandyJAMurrayGDThe fate of the claudicant: a prospective study of 1969 claudicantsEur J Vasc Surg1991521311332037083
- CriquiMHLangerRDFronekAMortality over a period of 10 years in patients with peripheral arterial diseaseN Engl J Med199232663813861729621
- HirschATCriquiMHTreat-JacobsonDPeripheral arterial disease detection, awareness, and treatment in primary careJAMA2001286111317132411560536
- DormandyJARutherfordRBTrans Atlantic Inter-Society Consensus Working GroupManagement of peripheral arterial disease (PAD)J Vasc Surg2000311 Pt 2S1S29610666287
- FowkesFGHousleyECawoodEHMacintyreCCRuckleyCVPrescottRJEdinburgh Artery Study: prevalence of asymptomatic and symptomatic peripheral arterial disease in the general populationInt J Epidemiol19912023843921917239