Abstract
In spite of generally accepted dogma that the total number of follicles and oocytes is established in human ovaries during the fetal period of life rather than forming de novo in adult ovaries, some new evidence in the field challenges this understanding. Several studies have shown that different populations of stem cells, such as germinal stem cells and small round stem cells with diameters of 2 to 4 μm, that resembled very small embryonic-like stem cells and expressed several genes related to primordial germ cells, pluripotency, and germinal lineage are present in adult human ovaries and originate in ovarian surface epithelium. These small stem cells were pushed into the germinal direction of development and formed primitive oocyte-like cells in vitro. Moreover, oocyte-like cells were also formed in vitro from embryonic stem cells and induced pluripotent stem cells. This indicates that postnatal oogenesis is not excluded. It is further supported by the occurrence of mesenchymal stem cells that can restore the function of sterilized ovaries and lead to the formation of new follicles and oocytes in animal models. Both oogenesis in vitro and transplantation of stem cell-derived “oocytes” into the ovarian niche to direct their natural maturation represent a big challenge for reproductive biomedicine in the treatment of female infertility in the future and needs to be explored and interpreted with caution, but it is still very important for clinical practice in the field of reproductive medicine.
Keywords:
Introduction
It is still generally accepted that the end number of follicles and oocytes is established in human ovaries during the fetal period of life and does not increase following birth. Nevertheless, since the early days of this field of research there have been several theoretical suggestions that, from an evolutionary point of view, de novo oogenesis is to be expected, as well as with regard to spermatogenesis, which takes place in human testicles during the adult period of life.Citation1–Citation6 Despite this concept, no experimental evidence existed to support it. Now, however, an increasing number of studies show that different populations of stem cells are present in adult human ovaries and the ovaries of other mammalian species. These stem cell populations may represent potential for de novo oogenesis and the capability of adult human ovaries to regenerate.
The aim of this manuscript is to critically review the present literature on the subject in order to estimate the arguments pro and contra postnatal oogenesis in humans.
Introduction to oogenesis in humans
An oocyte is a female germ cell involved in reproduction. It is one of the largest cells in the body and develops in the ovarian follicle, a specialized unit of the ovary, during the process of oogenesis/folliculogenesis in the cortex.Citation7 The process of oogenesis starts in the fetal ovaries with the development of oogonia from primordial germ cells (PGCs). Each oogonium in the fetal ovaries divides and enters the initial stage of meiosis (meiosis I) to become the diploid primary oocyte. The primary oocyte does not complete meiosis I but stops at the first meiotic prophase stage, dictyate. At this stage of development the oocyte nucleus is called the germinal vesicle (GV). GV oocytes are localized within the primordial follicles. By the end of the fetal period, all primary oocytes are formed and have halted their development at the dictyate stage. Although meiosis in primary oocytes is arrested, their chromosomes continue to synthesize amounts of mRNA and rRNA, which are later used to generate a mass of essential proteins needed for further oocyte maturation and the development of any fertilized oocytes and embryos.Citation7 The primary oocytes are maintained for years, until puberty. Upon puberty only a few – 15 to 20 – primary oocytes/follicles are recruited during each menstrual cycle, and only one oocyte in the dominant follicle matures and is ovulated. During this maturation, the primary oocyte finishes meiosis I and divides into two daughter cells: a haploid secondary oocyte and an extruded nonfunctional polar body. Meiosis does not completely finish but is arrested again at the metaphase II stage and terminates after successful fertilization by sperm when the second polar body is extruded. The primary oocytes grow and mature during the development and maturation of primordial, primary, and secondary follicles, whereas the secondary oocytes develop along with the tertiary and pre-ovulating Graafian follicle.Citation7 Developing oocytes are strongly supported by surrounding follicular – granulosa – cells. The process of oogenesis/folliculogenesis is highly regulated by hormones and other substances.
It is generally accepted that human ovaries contain a fixed number of non-growing follicles established before birth that decreases with female age and is depleted in menopause.Citation8–Citation17 Wallace and Kelsey established an age-related model of the population of non-growing follicles in human ovaries from conception to menopause based on eight separate quantitative histological studies in which non-growing follicle populations at known female ages from 7 weeks post-conception to menopause (51 years old) were estimated.Citation18 They found that in the ovaries of 95% of women by the age of 30 years only 12% of their maximal pre-birth non-growing follicle population is present and by the age of 40 years only 3% still remains. In countable terms, at birth approximately 1,000,000 or more follicles were regularly present in the ovaries, at age 30 generally between 10,000 and 100,000 follicles, at age 40 between 1,000 and 10,000 follicles, and at menopause the number of follicles had declined to less than 1,000 follicles. Interestingly, it was found that the rate of non-growing follicle recruitment in the ovaries of most women increased from birth until approximately the age of 14 years and then intensely decreased towards menopause. This model is supposed to be the first model of the human ovarian reserve from conception to menopause and enables one to estimate the number of non-growing follicles present in the ovaries at any given female age. In the future, it will be important to elucidate how infertility, cancer treatment (radiotherapy and chemotherapy), and environmental and other factors affect the model.
Postnatal oogenesis in nonhuman mammals
As already described, oogonia are characterized by diploidy and mitotic proliferation. In addition, human and mouse oogonia express several factors which are characteristic of pluripotent stem cells, such as octamer-binding transcription factor 4 (OCT4).Citation19 In humans, almost all oogonia enter meiosis between weeks 9 and 22 of prenatal development or undergo mitotic arrest and are eliminated from the ovaries.Citation19 Consequently, neonatal human ovaries generally lack oogonia. Byskov et al reported that there was no evidence for the presence of oogonia in human ovaries after their final clearance during the first 2 years of life.Citation20 They analyzed the sections of fetal ovaries 5 to 10 weeks, 18 weeks, or 32 weeks post-conception and from 2- to 32-year-old ovaries using immunohistochemistry. In fetal ovaries, almost all oogonia expressed pluripotency-related markers, such as stage-specific embryonic antigen-4 (SSEA-4), homeobox gene transcription factor (NANOG), OCT4, and tyrosine kinase receptor for stem cell factor (SCF) (C-KIT), whereas only a small proportion of cells were positively stained for melanoma antigen-4 (MAGE-A4). At birth, only a few oogonia expressing these markers were identified while they completely disappeared before the age of 2 years. Later, only diplotene oocytes that positively stained for C-KIT were found in the ovarian sections. Interestingly, from 18 weeks post-conception to 2 years, the medulla contained conglomerates of healthy and degenerating oogonia and small follicles, waste baskets, and oogonia enclosed in growing follicles.Citation20 Medulla of older ovaries contained groups of primordial, healthy follicles. It is difficult to explain this in consideration of current knowledge because the follicles are only expected to be present in the ovarian cortex, and one may speculate that in the medulla there are some progenitor stem cells enabling their development which perhaps originate from peripheral blood or elsewhere. On the other hand, proliferating oogonia were found in adult ovaries of “lower” primates – prosimians (Strepsirrhines). Ovarian development in the marmoset monkey has not been widely investigated, but the data show that the neonatal marmoset ovary contains numerous oogonia expressing several pluripotency factors such as OCT4A, SALL4, and LIN28A (LIN28).Citation19 Altogether, these data demonstrate the primitiveness of the neonatal marmoset ovary in comparison with the human one while also confirming that oogonia, in fact, persisted in adult ovaries through the evolutionary development of primates. Similarly, some scientific evidence exists that shows oogonia can persist into adulthood in some other prosimian species, such as lemurs.Citation21
Latest evidence for and against postnatal human oogenesis
Germinal stem cells from adult ovaries
Some researchers very early on struggled against the growing dogma about the finite number of follicles and oocytes at birth.Citation22 In spite of the fact that there is no evidence of persistence of oogonia in adult human ovaries, a new idea about germinal (oogonial) stem cells in adult ovaries was developed.Citation23–Citation25 This idea was encouraged by some findings on germinal stem cells in both mouse and human postnatal and adult ovaries. Zou et al have successfully established a neonatal mouse germinal stem cell line expressing a normal karyotype and high telomerase activity.Citation26 These cells were purified by immunomagnetic isolation and cultured for more than 15 months. Moreover, germinal stem cells were also purified from adult mouse ovaries and cultured for a shorter time – but still more than 6 months. These stem cells were infected with green fluorescent protein (GFP) virus and transplanted into the ovaries of infertile mice. The transplanted cells underwent oogenesis and the mice produced offspring that had the GFP transgene.Citation26
The finding in a mouse model was followed by a similar finding in humans. White et al reported the purification of mitotically active germinal stem cells from the ovarian cortex of reproductive-age women.Citation27 These cells were purified from the ovarian tissue by fluorescence-activated cell sorting based on DDX4 (VASA) expression. They were rare but mitotically active, and their gene expression profile was quite comparable to primitive germ cells. The authors found that these cells were able to persist in vitro for months and to spontaneously develop into oocyte-like cells with diameters of 35–50 μm, which were also found to be haploid. When these cells were marked by GFP and xenotransplanted into the human ovarian cortex biopsies or immunodeficient mice, the formation of GFP-positive “oocytes” was observed. It may be criticized that this work was performed in reproductive-age women where potential contamination with the naturally present immature oocytes is not excluded. In addition, the germinal stem cells were isolated from frozen–thawed ovarian tissue that may be damaged, which also increased the possibility of contamination with naturally present immature oocytes. There has also been some criticism of the specificity of the DDX4 (VASA) marker for purification of germinal stem cells from adult ovaries because it can also select immature oocytes from existing early follicles and because experimental evidence of no mitotically active female germinal progenitors in postnatal mouse ovaries was provided, using comparable methodology.Citation28 Recently, it was reported that lifelong in vivo cell-lineage tracing of oocytes and follicles in mice did not confirm de novo oogenesis from putative germinal stem cells in adult mice ovaries.Citation29 It was shown that the initial pool of oocytes was the only source of germ cells throughout the life span of the studied mice and that no de novo oogenesis occurred under the physiological condition. In addition, there was no evidence for existence of germinal stem cells and neogenesis in adult monkey ovaries.Citation30 These findings largely show that more time and further research are needed to clarify the presence and role of germinal stem cells in adult human ovaries.
Ovarian surface epithelium as a source of stem cells
It is possible that the confusion surrounding germinal stem cells could be explained by the fact that the wrong cells were sought in adult human/mammalian ovaries. The experimental evidence showed that in adult human ovaries there might be some other populations of stem cells that may serve as oocyte progenitor cells. Ovarian surface epithelium was early on proposed as a potential source of stem cells, or even primordial follicles, in adult ovaries and was therefore entitled “germinal” epithelium.Citation31 Moreover, it has also been found to be an important source of ovarian neoplasms, and it is estimated that approximately 70% of ovarian cancers are of epithelial origin, specifically originating from the ovarian surface epithelium.Citation32 In 2005, Bukovsky et al indeed confirmed in vitro development of oocyte-like cells in cultures of ovarian surface epithelium.Citation33 They scraped the ovarian surface epithelium of ovarian cortex biopsies in postmenopausal women, set up cell cultures, and observed the development of oocyte-like cells in vitro. These cells expressed some of the oocyte-specific markers, as revealed by immunocytochemistry, but were not analyzed according to their genetic status. The immunocytochemistry data showed that these cells may exhibit oocyte-specific processes, such as GV breakdown, expulsion of the polar body, and surface expression of zona pellucida proteins. The authors found that development of oocyte-like cells was triggered by the presence of phenol red in the culture medium, which could express weak estrogenic activity.Citation34 Although the confirmation of oocyte-like cells was weak, this work opened up a new way to study and think about the ovarian surface epithelium. Nevertheless, the putative stem cells from ovarian surface epithelium that developed into oocyte-like cells were not identified, with the cytokeratin-positive mesenchymal progenitor cells being theoretically proposed instead. It has also been proposed that oocyte precursors may develop from ovarian surface epithelium crypts, and granulosa cells from epithelial cords containing stem cells; this principle was suggested to be a base for de novo oogenesis and follicular renewal in postnatal mammalian ovaries.Citation35
Small putative stem cells in the ovarian surface epithelium of adult ovaries
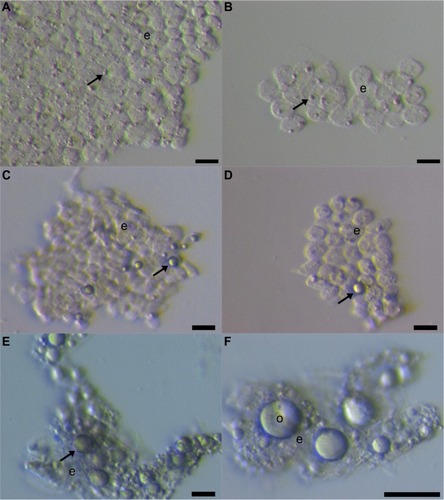
Our group further researched the ovarian surface epithelium cell cultures in terms of potential oogenesis in vitro.Citation36 We scraped the ovarian cortex biopsies in postmenopausal women and of young women with premature ovarian failure (POF), characterized by nonfunctional ovaries and no naturally present follicles and oocytes. Additionally, we developed a laparoscopic procedure to brush the ovarian surface epithelium in women with POF. Similarly to Bukovsky,Citation33 we found development of oocyte-like cells in cultures of ovarian surface epithelium and confirmed the expression of some oocyte-specific genes, showing that there is really “something” relevant present.Citation36 The oocyte-like cells were able to develop into parthenogenetic embryo-like structures.Citation37 Moreover, we discovered the first presence of small round cells of unknown origin with diameters of 2 to 4 μm among epithelial cells of ovarian surface epithelium brushings (), in cultures of ovarian surface epithelium, and also in ovarian sections, as revealed by immunocytochemistry, which we then suggested to be putative stem cells.Citation36,Citation37 At that time, the work of BukovskyCitation33 and our work was criticized by Notarianni,Citation38 who theoretically provided several aspects (including too simple a culture medium in comparison to human embryonic stem cells [hESCs]) to show that we may be wrong in our interpretation, but did not provide any experimental data to confirm this. Alternatively, Parte et al confirmed our results in human, monkey, sheep, and rabbit testing.Citation39 They termed these small putative stem cells from adult ovarian surface epithelium as very small embryonic-like cells (VSELs) according to a very similar population of stem cells expressing pluripotency that was discovered by the Ratajczak research group in human and murine adult bone marrow, peripheral blood, and umbilical cord blood.Citation40–Citation43 The VSELs have been proposed to originate from embryonal epiblast and to persist as pluripotent stem cells in adult human tissues and organs.Citation44–Citation46 Although the population of small putative stem cells from ovarian surface epithelium and VSELs from bone marrow seem to be quite similar, there has been no direct experimental evidence until now that they represent the same population of cells. It needs to be noted that very similar populations of small ovarian stem cells have also been discovered in monkey, sheep, rabbit, and pig adult ovaries, thus indicating that they might proliferate among different mammalian species.Citation39,Citation47
Figure 1 Small putative stem cells among epithelial cells after laparoscopic brushing of the ovarian surface epithelium in the same patient with premature ovarian failure and no naturally present follicles and oocytes.
Abbreviations: e, epithelial cells; o, oocyte-like cells.
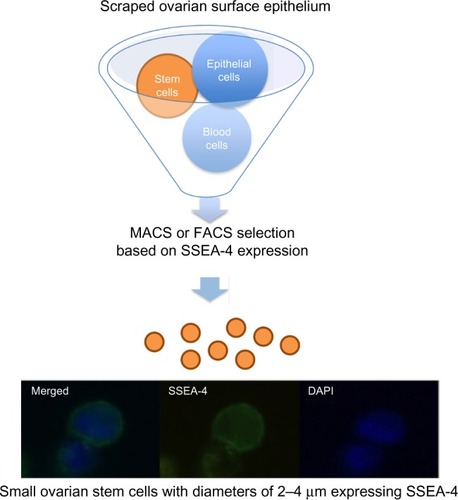
Our further work showed that it is possible to purify small ovarian stem cells from ovarian surface epithelium scrapings by two different methods, based on the expression of SSEA-4: magnetic-activated and fluorescence-activated cell sorting ().Citation48 The small SSEA-4-positive cells represented a relatively small proportion (up to 10%) of all scraped cells. The purification of small putative stem cells from human adult ovaries enabled the genetic analysis and gene expression profiling of these cells using microarrays. The results showed that they expressed several genes related to pluripotency and germinal lineage, especially PGCs.Citation48,Citation49 The most prominent genes related to pluripotency were SALL4, CDH1, LIN28B, SOX11, LEFTY1, PP1R9A, MYBL2, and HESRG. They also strongly expressed some genes related to PGCs such as PRDM1 (BLIMP1), PRDM14, and DPPA3 (STELLA).Citation49 All genes related to pluripotency were downregulated in small ovarian stem cells in comparison with hESCs while the PGC-related gene PRDM1 was upregulated. Interestingly, small ovarian stem cells also expressed some germinal markers, such as DDX4 (VASA), which were upregulated in comparison with hESCs.Citation49 All these data showed that small ovarian stem cells expressed a specific pattern of pluripotency and are definitely related to germinal lineage. Moreover, it was found that a very similar population of SSEA-4-positive small cells can be observed and purified from human testicular cell cultures and hESC cultures.Citation49 Similarly, it has already been reported that small putative stem cells are present in human testicular tissue.Citation50 On the other hand, these small putative stem cells were then discovered in hESC cultures for the first time. We suggest them to be the “real” hESCs that may persist in adult human tissues and organs, including gonads, in a dormant state from the embryonic period of life; however, it is too early to make a real conclusion.
Figure 2 Selection of small putative stem cells from cells scraped from the ovarian surface epithelium based on the expression of SSEA-4 surface antigen.
Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; FACS, fluorescence-activated cell sorting; MACS, magnetic-activated cell sorting.
Oocyte-like cells from small ovarian stem cells
Our data showed that small putative ovarian stem cells from human adult ovaries – ovarian surface epithelium – can grow and develop into oocyte-like cells in vitro.Citation36,Citation37,Citation48,Citation51 They can also grow into oocyte-like cells in cell cultures of scraped ovarian surface epithelium spontaneously or, especially, after triggering by follicular fluid retrieved in the in vitro fertilization program upon oocyte retrieval after ovarian hormonal stimulation. This could be expected, as follicular fluid is known to be rich in reproductive hormones (estrogens, progesterone, follicle-stimulating hormone [FSH], androgens) and several other substances important for oocyte growth and maturation, such as proteins, amino acids, lipids (including free cholesterol and meiosis-activating sterol), growth factors, and more.Citation52–Citation56 Oocyte-like cells have even been retrieved in women with POF, diagnosed by histology, serum hormonal level, and ultrasound; these women were characterized by having no naturally present follicles and oocytes.Citation36,Citation51,Citation57 The oocyte-like cells grew up to 60–80 μm and did not express zona pellucida. The oocyte-like cells were taken from cell cultures and, as single cells, analyzed by real-time quantitative polymerase chain reaction in comparison with non-fertilized oocytes from the in vitro fertilization program, hESCs, and somatic fibroblasts.Citation51 It was found that oocyte-like cells were heterogeneous at the genetic level, but some of them expressed several genes related to pluripotency and oocytes (including meiosis-related genes) and matched with oocytes and hESCs closer than with adult fibroblasts.Citation51 In spite of that, the oocyte-like cells were far from being competent oocytes (even immature oocytes). They more represented stem cells “pushed” into the germinal direction than “real” oocytes.
Based on experimental experience, we proposed small ovarian stem cells to be germ cell progenitors related to PGCs that might be pre-stage of germinal stem cells, oogonia, and oocytes.
Small ovarian stem cells, blood cells, cells of the immune system, or microvesicles?
The small ovarian stem cells could be mixed with something else, like blood cells (erythrocytes) or cells from the immunological system (lymphocytes). Moreover, there was also some suggestion that small ovarian stem cells might be microvesicles, yet several characteristics about them dispel such suggestions ().Citation57–Citation60 After May-Grünwald-Giemsa staining, commonly used to stain blood smears, the small ovarian stem cells scraped from the ovarian surface epithelium either did not stain or only weakly stained, while all blood cells, including lymphocytes, strongly stained, as evidenced by our previous workCitation57 and . The small stem cells scraped from the ovarian surface epithelium had completely round nuclei which filled almost the entire cell volumes, as revealed by Hoechst 33258 (Sigma Aldrich Co, St Louis, MO, USA) staining (). Erythrocytes were indeed the predominating blood cells in a population of cells scraped from the ovarian surface epithelium, but small ovarian stem cells were differentiated by being significantly smaller (). Blood cells represented about 1% of all scraped cells, a minority among other types of scraped cells, such as epithelial cells, as revealed by May-Grünwald-Giemsa staining. The small, round stem cells could be comparable to lymphocytes due to their round shape and high nuclear/cytoplasm ratio, but they were significantly smaller. Moreover, the lymphocytes were positively stained after May-Grünwald-Giemsa staining, while small round cells were not.Citation57 The small ovarian stem cells always appeared to be completely round, did not change their shape, and were significantly larger than microvesicles (). The abovementioned populations of cells also expressed different biomarkers, therefore they cannot be mixed regarding their molecular status. Small ovarian stem cells composed the only population of cells that expressed at least some markers of pluripotency and germinal lineage and were shown being pushed into the germinal direction, while all other types of cells did not ().Citation36,Citation37,Citation39,Citation48,Citation51,Citation57
Figure 3 Small putative stem cells (arrows) from ovarian surface epithelium in a patient with premature ovarian failure and no naturally present follicles and oocytes.
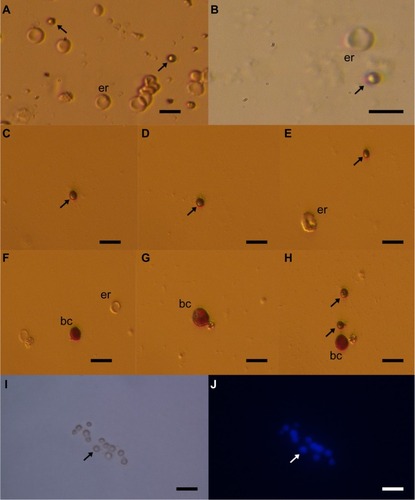
Table 1 Comparison of small ovarian stem cells, blood cells (erythrocytes), cells of the immune system (lymphocytes), and microvesiclesCitation49,Citation57–Citation60
Oocytes from other pluripotent stem cells: ESCs, induced pluripotent stem cells, and others
The ovarian stem cells are the most interesting population of cells for potential autologous de novo oogenesis and regeneration of nonfunctional ovaries in infertile women, although the data show that oocyte-like cells which express some oocyte-specific markers can also be developed from other populations of pluripotent stem cells, such as ESCs and induced pluripotent stem cells.Citation61–Citation67 Moreover, it has also been reported that oocyte-like cells can be developed from human amniotic fluid stem cells, and even from pancreatic stem cells and embryonal dermal skin cells in animal models.Citation68–Citation71 In all these cases, the oocyte-like cells were developed from stem cells that expressed at least a degree of pluripotency, and the resulting oocyte-like cells expressed some germinal markers but were still far from being real and competent oocytes.Citation49,Citation51,Citation62,Citation71 Despite this limited outcome, all these studies are quite promising and indicate that development of gametes – oocytes – from stem cells might be shown as valid one day.
In vitro oogenesis or transplantation of pluripotent stem cells into insufficient ovaries?
In vitro maturation of human oocytes is one of the most difficult tasks in the in vitro fertilization program. On one hand, the procedure of human oocyte in vitro maturation is still a low-success procedure due to suboptimal culture conditions and, on the other hand, it may also possess some epigenetic risk, although, for the most part, not experimentally confirmed in humans.Citation72–Citation75 One of the main reasons for this lies in several known and unknown substances and factors, which are involved in the oocyte maturation process in the follicular/ovarian niche and are not present when the immature oocyte is exposed to in vitro conditions. Epigenetic regulation is being established only near the end of oocyte maturation in natural conditions and may be incomplete or even broken when immature oocytes are exposed to in vitro conditions and suboptimal in vitro maturation procedures. In the in vitro fertilization program, immature GV oocytes can be retrieved from the ovaries of women with polycystic ovary syndrome to be matured in vitro, both to avoid the hormonal stimulation of their ovaries and to prevent potential ovarian hyperstimulation syndrome.Citation76,Citation77 In spite of such effort, the data show that the oocytes matured in vitro result in low clinical success.Citation78 The in vitro matured oocytes result in worse embryo development and poor clinical outcomes in terms of pregnancy and birth. This may be a consequence of deleterious effects of in vitro maturation on the organization of the meiotic spindle and of the chromosome alignment of human oocytes particular to oocyte-like cells developed from stem cells, which are still far from the GV stage of oocyte maturity.Citation79 This means that the procedure of oocyte in vitro maturation, including oocyte-like cells developed from stem cells, needs to be further researched.
It has been proposed that transplantation of stem cells or stem cell-derived “oocytes” into the ovary or other organs would be of great advantage because it would provide a natural niche for maturation of oocytes and bypass several obstacles related to oocyte in vitro maturation.Citation80 In a mouse model it was confirmed that ESC-derived oocyte maturation ultimately fails in vitro, and to overcome this obstacle the transplantation of ESC-derived “oocytes” into an ovarian niche has been proposed in order to direct their natural and functional maturation. Indeed, the ESC-derived “oocyte” maturation was shown in newly formed ovarian follicles after transplantation into the mouse ovarian niche.Citation80 The ESC-derived “oocytes” were enclosed in ovarian follicles, and some of them reached the primordial or primary stage of development. The primary follicles consisted of an ESC-derived “oocyte”, expressing some germinal markers, with a single layer of endogenous cuboidal granulosa cells, surrounded by a basement membrane.Citation80 Recently, it was reported that artificial mouse/rat PGCs were induced in vitro from pluripotent stem cells, enzymatically dispersed, and transplanted together with PGC-free gonadal cells under the kidney capsule of xenogenic immunodeficient animals.Citation81 The transplanted PGCs formed ovary-like tissue that was histologically similar to the normal ovary and also expressed the oocyte-related markers Vasa and Stella. In addition, the mouse GV-stage oocyte-like cells were collected from the ovary-like tissue in rats. These oocytes were matured in vitro to metaphase II stage and gave rise to offspring using intracytoplasmic sperm injection.Citation81 All these data show that transplantation of stem cells or “oocytes” derived from this process into the natural ovarian niche may represent a more optimal and reliable approach to deriving competent “oocytes” from stem cells in infertile women than in vitro oogenesis.Citation82 Moreover, the assisted conception is based on well-developed and experienced ovarian hormonal stimulation protocols under perfectly controlled conditions and results in the functional in vivo maturation of follicles and oocytes in humans. These protocols might be quite helpful in supporting the in vivo maturation of stem cell-derived “oocytes” in the future.
Therapeutic/clinical potential of postnatal oogenesis in humans
Postnatal oogenesis could be of great importance for clinical practice in the field of reproductive medicine. Some young patients suffer from nonfunctional ovaries (ovarian insufficiency or premature ovarian failure) or from poor ovarian response to hormonal stimulation, performed to retrieve oocytes for in vitro fertilization. POF is a common condition which affects approximately one out of 100 women and is characterized by amenorrhea, hypoestrogenism, and elevated gonadotropin levels in women under the age of 40 years.Citation83 Nonfunctional ovaries may be a natural condition related to abnormalities of chromosome X, such as fragile X permutation (~5%); familial genetic causes including abnormalities of genes such as FOXL2, FSHR, and INHA (~20%); autoimmunity with anti-ovary antibodies (~10%) or other idiopathic factors (~65%); or an artificial consequence of aggressive therapy (chemotherapy or radiotherapy) of different cancers including childhood cancers, Hodgkin’s lymphoma, and breast cancer.Citation84 Autologous stem cells in these patients could be used to develop into “oocytes” to be fertilized in vitro, or they may be transplanted into the insufficient ovaries to regenerate them. This approach would be safer than the cryopreservation and autotransplantation of thawed ovarian tissue in cancer patients that is an established practice worldwide but without the risk of retransplantation of malignant cells into the body.Citation85
Regeneration of ovaries by mesenchymal stem cell transplantation
Development of oocytes from stem cells does not represent the only challenge in reproductive medicine. The limits between different types of stem cells are not clear, and a greater number of reports confirmed that mesenchymal stem cells (MSCs) may also possess a degree of pluripotency, including those from human testicles.Citation86 Consequently, they may be used to regenerate the nonfunctional ovaries. Indeed, several studies in animal models showed that transplantation of animal or human MSCs from different sources may regenerate nonfunctional ovaries, pre-sterilized by cyclophosphamide, an alkylating antineoplastic agent, which is also used in human medicine (chemotherapy), and/or busulfan.Citation87–Citation94 The transplanted cells appear not to develop into oocytes but mostly support the surrounding granulosa cells, which are known to be of mesodermal (mesenchymal) origin, and the general ovarian physiology, thus indirectly supporting the postnatal oogenesis/folliculogenesis.
It was found that intravenous injection of male bone marrow-derived MSCs into rabbits with chemotherapy-induced ovarian damage improved ovarian function.Citation87 The MSCs accomplished this function by direct differentiation into specific cellular phenotypes and by decreasing FSH while increasing estrogen and vascular endothelial growth factor (VEGF) levels to positively influence the regeneration of the ovaries. Cytological and histological examinations confirmed the increased numbers of follicles with normal structure in the MSC recipient group of animals.Citation87 Similarly, in another study, the transplantation of bone marrow-derived MSCs decreased germ cell apoptosis and DNA damage while increasing the number of primordial follicles after chemotherapy regimens in rats.Citation88
It was also discovered that human amniotic fluid contains a population of CD44/CD105-positive human amniotic fluid stem cells (hAFCs) that rapidly proliferate and highly express the proliferative markers, a number of biomarkers of MSCs, and even some biomarkers and properties of pluripotent stem cells and germinal lineage under continuous subculture in vitro.Citation89,Citation90 Moreover, they had the ability to restore ovarian morphology after transplantation into the ovaries of mice pre-sterilized by intraperitoneal injection of cyclophosphamide and busulfan. In these transplanted mice, the ovaries were restored and displayed increased levels of anti-Müllerian hormone (AMH), a functional marker of folliculogenesis, and many follicle-enclosed oocytes at all stages of development, while in control sterilized mice without transplantation they did not.Citation90,Citation91 Furthermore, it was confirmed that transplantation of MSCs from other sources, such as human umbilical cord blood matrix (Wharton’s jelly) or mouse adipose tissue-derived stem cells (ADSC), can effectively restore ovarian functionality and reduce apoptosis of granulosa cells in animals with cyclophosphamide/busulfan-induced premature ovarian failure.Citation92–Citation94 The main worry of these studies is the potentially incomplete sterilization of ovaries in animals; therefore, some additional and more relevant models of premature ovarian failure would need to be applied to these types of studies.
Recently, such studies have also been applied in human medicine, but the results have not been published yet. Recently, putative MSCs were also found in human adult ovaries and represent an extremely interesting tool for regenerating the sterile ovaries in humans.Citation95 Nevertheless, the MSCs would need to be applied with the same caution as pluripotent stem cells to prevent potential teratoma formation or other complications in humans.
Conclusion
Studies on this topic are intensely ongoing, and time will tell which approach could be optimal for use in clinical practice to treat ovarian infertility in young women at reproductive age who wish to have a child. Nevertheless, more and deeper research into this area is needed for future and better understanding of stem cells and competent oocytes in humans. The data show that there are different populations of stem cells in adult human ovaries; therefore, the postnatal oogenesis/folliculogenesis cannot be excluded. Perhaps existing stem cells enable the persistence of a sufficient number of follicles in adult ovaries to produce functional oocytes during the reproductive period of life, although the overall number of follicles decreases over a long lifespan. Both oogenesis in vitro and transplantation of stem cell-derived “oocytes” into the ovarian niche to direct their natural maturation represent major challenges in reproductive biomedicine to treat female infertility in the future. Above all, this needs to be researched and interpreted with great caution to avoid the risk of tumor formation (stem cell expression of pluripotency) and false hope in patients.
Disclosure
The author reports no conflict of interest in this work.
References
- WaldeyerWEierstock und Ei. Ein Beitrag zur Anatomie und Entwicklungsgeschichte der Sexualorgane [Ovary and Egg. A Contribution to the Anatomy and Evolution of the Sexual Organs]LeipzigEngelmann1870 German
- KingeryHMOogenesis in the white mouseJ Morphol191730261315
- AllenEOvogenesis during sexual maturityAm J Anat192331439481
- EvansHMSwezyOOvogenesis and the normal follicular cycle in adult MammaliaCal West Med19323616018742022
- BukovskyACopasPVirant-KlunIPotential new strategies for the treatment of ovarian infertility and degenerative diseases with autologous ovarian stem cellsExpert Opin Biol Ther20066434136516548762
- RodriguesPLimbackDMcGinnisLKPlanchaCEAlbertiniDFOogenesis: prospects and challenges for the futureJ Cell Physiol2008216235536518452183
- JohnsonMHEverittBJEssential Reproduction5th edOxfordBlackwell Science2000
- BlockEQuantitative morphological investigations of the follicular system in women; variations at different agesActa Anat (Basel)1952141–210812314932631
- BlockEA quantitative morphological investigation of the follicular system in newborn female infantsActa Anat (Basel)195317320120613050278
- BakerTGA quantitative and cytological study of germ cells in human ovariesProc R Soc Lond B Biol Sci196315841743314070052
- GougeonAChainyGBMorphometric studies of small follicles in ovaries of women of different agesJ Reprod Fertil19878124334423430463
- RichardsonSJSenikasVNelsonJFFollicular depletion during the menopausal transition: evidence for accelerated loss and ultimate exhaustionJ Clin Endocrinol Metab1987656123112373119654
- HansenKRKnowltonNSThyerACCharlestonJSSoulesMRKleinNAA new model of reproductive aging: the decline in ovarian non-growing follicle number from birth to menopauseHum Reprod200823369970818192670
- BendsenEByskovAGAndersenCYWestergaardLGNumber of germ cells and somatic cells in human fetal ovaries during the first weeks after sex differentiationHum Reprod2006211303516155081
- ForaboscoASforzaCEstablishment of ovarian reserve: a quantitative morphometric study of the developing human ovaryFertil Steril200788367568317434504
- FaddyMJGosdenRGGougeonARichardsonSJNelsonJFAccelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopauseHum Reprod1992710134213461291557
- FaddyMJGosdenRGA model conforming the decline in follicle numbers to the age of menopause in womenHum Reprod1996117148414868671489
- WallaceWHKelseyTWHuman ovarian reserve from conception to the menopausePLoS One201051e877220111701
- FereydouniBDrummerCAeckerleNSchlattSBehrRThe neonatal marmoset monkey ovary is very primitive exhibiting many oogoniaReproduction2014148223724724840529
- ByskovAGHøyerPEYding AndersenCKristensenSGJespersenAMøllgårdKNo evidence for the presence of oogonia in the human ovary after their final clearance during the first two years of lifeHum Reprod20112682129213921572085
- GosdenRGOvulation 1: oocyte development throughout lifeGrudzinskasJGYovichJLGametes. The OocyteCambridgeCambridge University Press1995119127
- ZuckermanSThe number of oocytes in the mature ovaryRec Prog Horm Res1951663108
- OatleyJHuntPAOf mice and (wo)men: purified oogonial stem cells from mouse and human ovariesBiol Reprod201286619622402962
- VogelGReproductive biology. Potential egg stem cells reignite debateScience201233560721029103022383817
- GhazalSOogonial stem cells: do they exist and may they have an impact on future fertility treatment?Curr Opin Obstet Gynecol201325322322823587798
- ZouKYuanZYangZProduction of offspring from a germline stem cell line derived from neonatal ovariesNat Cell Biol200911563163619363485
- WhiteYAWoodsDCTakaiYIshiharaOSekiHTillyJLOocyte formation by mitotically active germ cells purified from ovaries of reproductive-age womenNat Med201218341342122366948
- ZhangHZhengWShenYAdhikariDUenoHLiuKExperimental evidence showing that no mitotically active female germline progenitors exist in postnatal mouse ovariesProc Natl Acad Sci U S A201210931125801258522778414
- ZhangHLiuLLiXLife-long in vivo cell-lineage tracing shows that no oogenesis originates from putative germline stem cells in adult miceProc Natl Acad Sci U S A201411150179831798825453063
- YuanJZhangDWangLNo evidence for neo-oogenesis may link to ovarian senescence in adult monkeyStem Cells201331112538255023897655
- LudwigKSOn the question of primordial follicles from the germinal epithelium during puberty in the human ovaryExperientia1965218469471 German5870921
- AbellMRThe nature and classification of ovarian neoplasmsCan Med Assoc J19669421110211244286898
- BukovskyASvetlikovaMCaudleMROogenesis in cultures derived from adult human ovariesReprod Biol Endocrinol200531715871747
- OrtmannOSturmRKnuppenREmonsGWeak estrogenic activity of phenol red in the pituitary gonadotroph: re-evaluation of estrogen and antiestrogen effectsJ Steroid Biochem199035117222407899
- BukovskyAGuptaSKVirant-KlunIUpadhyayaNBCopasPVan MeterSEStudy origin of germ cells and formation of new primary follicles in adult human and rat ovariesMethods Mol Biol200845023326518370063
- Virant-KlunIZechNRozmanPPutative stem cells with an embryonic character isolated from the ovarian surface epithelium of women with no naturally present follicles and oocytesDifferentiation200876884385618452550
- Virant-KlunIRozmanPCvjeticaninBParthenogenetic embryo-like structures in the human ovarian surface epithelium cell culture in postmenopausal women with no naturally present follicles and oocytesStem Cells Dev200918113714918605894 Erratum in: Stem Cells Dev20091871109
- NotarianniEReinterpretation of evidence advanced for neo-oogenesis in mammals, in terms of a finite oocyte reserveJ Ovarian Res201141121211009
- ParteSBhartiyaDTelangJDetection, characterization, and spontaneous differentiation in vitro of very small embryonic-like putative stem cells in adult mammalian ovaryStem Cells Dev20112081451146421291304
- KuciaMRecaRCampbellFRA population of very small embryonic-like (VSEL) CXCR4(+)SSEA-1(+)Oct-4+ stem cells identified in adult bone marrowLeukemia200620585786916498386
- KuciaMZuba-SurmaEWysoczynskiMPhysiological and pathological consequences of identification of very small embryonic like (VSEL) stem cells in adult bone marrowJ Physiol Pharmacol200657Suppl 5518
- KuciaMJWysoczynskiMWuWZuba-SurmaEKRatajczakJRatajczakMZEvidence that very small embryonic-like stem cells are mobilized into peripheral bloodStem Cells20082682083209218511604
- KuciaMHalasaMWysoczynskiMMorphological and molecular characterization of novel population of CXCR4+ SSEA-4+ Oct-4+ very small embryonic-like cells purified from human cord blood: preliminary reportLeukemia200721229730317136117
- RatajczakMZMachalinskiBWojakowskiWRatajczakJKuciaMA hypothesis for an embryonic origin of pluripotent Oct-4(+) stem cells in adult bone marrow and other tissuesLeukemia200721586086717344915
- ShinDMLiuRWuWGlobal gene expression analysis of very small embryonic-like stem cells reveals that the Ezh2-dependent bivalent domain mechanism contributes to their pluripotent stateStem Cells Dev201221101639165222023227
- ShinDMZuba-SurmaEKWuWNovel epigenetic mechanisms that control pluripotency and quiescence of adult bone marrow-derived Oct4(+) very small embryonic-like stem cellsLeukemia200923112042205119641521
- BuiHTVan ThuanNKwonDNIdentification and characterization of putative stem cells in the adult pig ovaryDevelopment2014141112235224424866115
- Virant-KlunISkutellaTHrenMIsolation of small SSEA-4-positive putative stem cells from the ovarian surface epithelium of adult human ovaries by two different methodsBiomed Res Int2013201369041523509763
- Virant-KlunIStimpfelMCvjeticaninBVrtacnik-BokalESkutellaTSmall SSEA-4-positive cells from human ovarian cell cultures: related to embryonic stem cells and germinal lineage?J Ovarian Res201362423570331
- BhartiyaDKasiviswananthanSShaikhACellular origin of testis-derived pluripotent stem cells: a case for very small embryonic-like stem cellsStem Cells Dev201221567067421988281
- Virant-KlunISkutellaTKubistaMVoglerASinkovecJMeden-VrtovecHExpression of pluripotency and oocyte-related genes in single putative stem cells from human adult ovarian surface epithelium cultured in vitro in the presence of follicular fluidBiomed Res Int2013201386146023555100
- DuijkersIJWillemsenWNHollandersHMHamiltonCJThomasCMVemerHMFollicular fluid hormone concentrations after ovarian stimulation using gonadotropin preparations with different FSH/LH ratios. II. Comparison of hMG and recombinant FSHInt J Fertil Womens Med19974264314359459089
- VelazquezAReyesAChargoyJRosadoAAmino acid and protein concentrations of human follicular fluidFertil Steril19772819610064370
- BokalEVTacerKFVrbnjakMFollicular sterol composition in gonadotrophin stimulated women with polycystic ovarian syndromeMol Cell Endocrinol20062491–2929816516374
- SmitzJPictonHMPlatteauPPrincipal findings from a multicenter trial investigating the safety of follicular-fluid meiosis-activating sterol for in vitro maturation of human cumulus-enclosed oocytesFertil Steril200787494996417198705
- UlugUTuranETosunSBErdenHFBahceciMComparison of preovulatory follicular concentrations of epidermal growth factor, insulin-like growth factor-I, and inhibins A and B in women undergoing assisted conception treatment with gonadotropin-releasing hormone (GnRH) agonists and GnRH antagonistsFertil Steril200787499599817280662
- Virant-KlunISkutellaTStimpfelMSinkovecJOvarian surface epithelium in patients with severe ovarian infertility: a potential source of cells expressing markers of pluripotent/multipotent stem cellsJ Biomed Biotechnol2011201138192822187524
- VömelTPlattDStrobeltWDiameters of erythrocytes of different ages measured by scanning electron-microscopyMech Ageing Dev19801343573657442327
- KuseRSchusterSSchübbeHDixSHausmannKBlood lymphocyte volumes and diameters in patients with chronic lymphocytic leukemia and normal controlsBlut19855042432483857080
- LeeYEl AndaloussiSWoodMJExosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapyHum Mol Genet201221R1R125R13422872698
- HübnerKFuhrmannGChristensonLKDerivation of oocytes from mouse embryonic stem cellsScience200330056231251125612730498
- NovakILightfootDAWangHErikssonAMahdyEHöögCMouse embryonic stem cells form follicle-like ovarian structures but do not progress through meiosisStem Cells20062481931193616644921
- WestFDMachacekDWBoydNLPandiyanKRobbinsKRSticeSLEnrichment and differentiation of human germ-like cells mediated by feeder cells and basic fibroblast growth factor signalingStem Cells200826112768277618719225
- WestFDRoche-RiosMIAbrahamSKIT ligand and bone morphogenetic protein signaling enhances human embryonic stem cell to germ-like cell differentiationHum Reprod201025116817819840987
- RichardsMFongCYBongsoAComparative evaluation of different in vitro systems that stimulate germ cell differentiation in human embryonic stem cellsFertil Steril201093398699419064262
- MedranoJVRamathalCNguyenHNSimonCReijo PeraRADivergent RNA-binding proteins, DAZL and VASA, induce meiotic progression in human germ cells derived in vitroStem Cells201230344145122162380
- EguizabalCMontserratNVassenaRComplete meiosis from human induced pluripotent stem cellsStem Cells20112981186119521681858
- ChengXChenSYuXZhengPWangHBMP15 gene is activated during human amniotic fluid stem cell differentiation into oocyte-like cellsDNA Cell Biol20123171198120422356426
- DannerSKajahnJGeismannCKlinkEKruseCDerivation of oocyte-like cells from a clonal pancreatic stem cell lineMol Hum Reprod2007131112017114208
- DycePWWenLLiJIn vitro germline potential of stem cells derived from fetal porcine skinNat Cell Biol20068438439016565707
- DycePWShenWHuynhEAnalysis of oocyte-like cells differentiated from porcine fetal skin-derived stem cellsStem Cells Dev201120580981921054136
- El HajjNHaafTEpigenetic disturbances in in vitro cultured gametes and embryos: implications for human assisted reproductionFertil Steril201399363264123357453
- WangNLeFZhanQTEffects of in vitro maturation on histone acetylation in metaphase II oocytes and early cleavage embryosObstet Gynecol Int2010201098927820613962
- YoshidaHAbeHArimaTQuality evaluation of IVM embryo and imprinting genes of IVM babiesJ Assist Reprod Genet201330222122523378104
- KuhtzJRomeroSDe VosMSmitzJHaafTAnckaertEHuman in vitro oocyte maturation is not associated with increased imprinting error rates at LIT1, SNRPN, PEG3 and GTL2Hum Reprod20142991995200524963167
- TrounsonAWoodCKauscheAIn vitro maturation and the fertilization and developmental competence of oocytes recovered from untreated polycystic ovarian patientsFertil Steril19946223533628034085
- GuzmanLOrtega-HrepichCPolyzosNPA prediction model to select PCOS patients suitable for IVM treatment based on anti-Mullerian hormone and antral follicle countHum Reprod20132851261126623427238
- RoesnerSVon WolffMEberhardtIBeuter-WinklerPTothBStrowitzkiTIn vitro maturation: a five-year experienceActa Obstet Gynecol Scand2012911222721995801
- LiYFengHLCaoYJConfocal microscopic analysis of the spindle and chromosome configurations of human oocytes matured in vitroFertil Steril200685482783216580356
- NicholasCRHastonKMGrewallAKLongacreTAReijo PeraRATransplantation directs oocyte maturation from embryonic stem cells and provides a therapeutic strategy for female infertilityHum Mol Genet200918224376438919696121
- HayamaTYamaguchiTKato-ItohMGeneration of mouse functional oocytes in rat by xeno-ectopic transplantation of primordial germ cellsBiol Reprod20149148925165118
- BukovskyAOvarian stem cell niche and follicular renewal in mammalsAnat Rec (Hoboken)201129481284130621714105
- WoadKJWatkinsWJPrendergastDShellingANThe genetic basis of premature ovarian failureAust N Z J Obstet Gynaecol200646324224416704481
- SwerdlowAJCookeRBatesAEngland and Wales Hodgkin Lymphoma Follow-up GroupRisk of premature menopause after treatment for Hodgkin’s lymphomaJ Natl Cancer Inst20141069
- HoekmanEJSmitVTFlemingTPLouweLAFleurenGJHildersCGSearching for metastases in ovarian tissue before autotransplantation: a tailor-made approachFertil Steril2015103246947725497447
- GonzalezRGriparicLVargasVA putative mesenchymal stem cells population isolated from adult human testesBiochem Biophys Res Commun2009385457057519482010
- Abd-AllahSHShalabySMPashaHFMechanistic action of mesenchymal stem cell injection in the treatment of chemically induced ovarian failure in rabbitsCytotherapy2013151647523260087
- KilicSPinarliFOzogulCTasdemirNNaz SaracGDelibasiTProtection from cyclophosphamide-induced ovarian damage with bone marrow-derived mesenchymal stem cells during pubertyGynecol Endocrinol201430213514024308768
- LiuTHuangYGuoLChengWZouGCD44+/CD105+ human amniotic fluid mesenchymal stem cells survive and proliferate in the ovary long-term in a mouse model of chemotherapy-induced premature ovarian failureInt J Med Sci20129759260223028242
- LaiDWangFChenYWangLWangYChengWHuman amniotic fluid stem cells have a potential to recover ovarian function in mice with chemotherapy-induced sterilityBMC Dev Biol2013133424006896
- WangFWangLYaoXLaiDGuoLHuman amniotic epithelial cells can differentiate into granulosa cells and restore folliculogenesis in a mouse model of chemotherapy-induced premature ovarian failureStem Cell Res Ther20134512424406076
- WangSYuLSunMThe therapeutic potential of umbilical cord mesenchymal stem cells in mice premature ovarian failureBiomed Res Int2013201369049123998127
- SunMWangSLiYAdipose-derived stem cells improved mouse ovary function after chemotherapy-induced ovary failureStem Cell Res Ther2013448023838374
- TakeharaYYabuuchiAEzoeKThe restorative effects of adipose-derived mesenchymal stem cells on damaged ovarian functionLab Invest201393218119323212100
- StimpfelMCerkovnikPNovakovicSMaverAVirant-KlunIPutative mesenchymal stem cells isolated from adult human ovariesJ Assist Reprod Genet201431895997424845159
