Abstract
Avascular necrosis (AVN) of the femoral head is a progressive disease that predominantly affects younger patients. Although the exact pathophysiology of AVN has yet to be elucidated, the disease is characterized by a vascular insult to the blood supply of the femoral head, which can lead to collapse of the femoral head and subsequent degenerative changes. If AVN is diagnosed in the early stages of the disease, it may be possible to attempt surgical procedures which preserve the hip joint, including decompression of the femoral head augmented with concentrated bone marrow. The use of autologous stem cells has shown promise in halting the progression of AVN of the femoral head, and subsequently preventing young patients from undergoing total hip arthroplasty. The purpose of this study was to review the current use of stem cells for the treatment of AVN of the femoral head.
Introduction
Avascular necrosis (AVN, also know as osteonecrosis) of the femoral head occurs when the cells of the trabecular bone spontaneously die, leading to fracture.Citation1 Depending on the amount of femoral head involved, collapse of the articular surface will occur as the disease advances.Citation1,Citation2 In the USA, this occurs in 10,000–20,000 adults every year, typically between the ages of 20 and 60 years.Citation1,Citation3,Citation4 Once collapse of the femoral head occurs in these patients, severe pain ensues, and the disease course rarely regresses.Citation5–Citation8 The primary therapeutic strategy by which these patients experience pain relief is total hip arthroplasty (THA), but at a younger age than in patients undergoing THA for degenerative arthritis.Citation9–Citation11
Pathophysiology of AVN
The pathophysiology of AVN remains unclear despite many attempts to provide a theoretical model.Citation12,Citation13 However, there are several recognized conditions and environmental insults that can predispose patients to AVN (). Although these factors may increase a patient’s risk for developing AVN, others propose that the disease results from a clotting disorder or genetic abnormality that leads to vascular compromise.Citation14 Another working hypothesis asserts that the cell death is due to increased intramedullary pressure in the femoral head, leading to decreased blood flow and cell death via a mechanism similar to compartment syndrome following a traumatic injury.Citation15
Table 1 Risk factors for avascular necrosis of the femoral head
Often an underlying cause cannot be determined. However, these idiopathic cases may actually be attributable to clotting abnormalities or collagen mutations.Citation14,Citation16 Jones et al found that approximately 82% of patients in their study had at least one coagulation factor abnormality.Citation14 Similarly, Liu et al noted that a COL2A1 gene mutation in certain families predisposed to development of AVN.Citation16
Diagnosis
Avascular necrosis tends to affect patients aged 20–40 years, with the average age at presentation being 38 years.Citation3,Citation17,Citation18 Symptoms can vary widely, depending on the stage at presentation. In the earlier stages of AVN, patients may note an insidious onset of pain, without a clear cause or inciting event, and will often have a normal range of motion; however, this can be limited by pain, especially with internal rotation of the hip. With progression of the disease, this insidious groin discomfort may be followed by a sudden onset of severe pain. An event of this nature often signals collapse and fracture of the femoral head, leading to end-stage degenerative changes. Development of late-stage disease is typically marked by increasing mechanical symptoms, which include a decreased and painful range of motion, a Trendelenburg sign, crepitus, and catching of the femoral head.
AVN is usually diagnosed on radiologic imaging. Early-stage disease normally requires high-resolution, three-dimensional imaging such as magnetic resonance imaging (MRI) or computed tomography; however, late-stage disease can be readily apparent on plain radiographs. The features of AVN on imaging are important because accurate staging of the disease is essential in order to be able to treat each patient appropriately. Several classification schemes can be used to determine the clinical stage;Citation19,Citation20 however, we use the Steinberg classification () because we feel it to be the most complete.Citation21 If AVN is identified during the early stages prior to collapse of the subchondral bone (stage 0–2), the joint is amenable to salvage by a variety of hip preservation techniques. However, if the disease has progressed to stage 3 or greater, THA is frequently the only durable option for pain relief and restoration of function.
Table 2 Steinberg classificationCitation21 for avascular necrosis of the fem oral head
Stem cell therapies
Spontaneous regression of AVN is rare, with the vast majority of untreated patients progressing to THA.Citation5,Citation7,Citation11 It is thought that, in patients with AVN, there is an insufficient supply of progenitor cells located in the femoral head and proximal femur to remodel the area of necrosis.Citation22 Although options to halt the progression of AVN are available (core decompression, osteotomy, medical treatments), the results have been disappointing, with up to 40% of patients progressing to THA.Citation15 Since progenitor cells may be lacking in the lesioned area, newer treatment modalities have been developed to introduce stem cells to the areas of necrosis in an attempt to prevent fracture and collapse by restoring the architecture of the femoral head.
In 2002, Hernigou and Beaujean first described a technique for injecting mesenchymal stem cells combined with standard core decompression to introduce biologics into an area of necrosis.Citation23 In this study of 189 hips (116 patients), mesenchymal stem cells (in the form of concentrated iliac crest bone marrow) were injected through a core decompression tract into the area of necrosis.Citation23 Patients with early (precollapse) disease had excellent results at 5 years of clinical follow-up, with only nine of 145 hips requiring THA.Citation23 However, amongst patients who had Stage III or greater disease at the time of therapeutic intervention, over half (25 of 44) hips required THA during the same time period.Citation23 The authors of that study were also able to show that the amount of mesenchymal stem cells injected as well as the underlying etiology of AVN were related to progression of disease. Patients with lower stem cell concentrations (measured by colony-forming units in vitro) or a history of organ transplantation, alcohol use, or corticosteroid exposure were at increased risk of disease progression.Citation23
The above group of patients was included in a recent retrospective review of concentrated bone marrow as a treatment for AVN.Citation22 After an average follow-up of 13 years, it was found that 94 of 534 (17%) hips progressed to THA.Citation22 Patients in this group had a significantly increased Harris Hip Score and a decrease in the size of the necrotic lesion on MRI. Sixty-nine patients (18%) in this group also had complete resolution of their necrotic lesion on MRI.Citation22 The rate of progression to THA in this large study is similar to that previously reported.Citation24 Similarly, they found that a majority of patients experience significant pain relief following injection of concentrated bone marrow into the necrotic femoral head.Citation24
Since the original work by Hernigou and Beaujean,Citation23 four studies have prospectively analyzed the use of core decompression alone and core decompression with autologous bone marrow (CDBM, ). The first such study was performed in 2004 and compared patients with precollapse AVN (stage 1–2 AVN).Citation25 In this investigation, eight hips were treated with core decompression and ten hips received CDBM. At 2-year follow-up, the CDBM group had a significant decrease in pain (P=0.021), with significant improvement in Western Ontario and McMaster/Lequesne osteoarthritis indices.Citation25 Likewise, the size of the AVN lesion at the femoral head decreased significantly in the CDBM group from 15.6% preoperative to 10.1% at final follow-up. In terms of disease progression, five of eight hips in the core decompression group collapsed whereas only one of ten in the CDBM group collapsed.Citation25 The results of this study have been carried out to 5 years of clinical follow-up, with the addition of three patients in each group.Citation26 At the 5-year time point, eight of eleven hips in the core decompression group have fractured and collapsed, while only three of 13 hips in the CDBM have collapsed.Citation26 Similarly, in another small study of 14 hips that underwent core decompression or CDBM, use of CDBM was found to be associated with significant improvement in the patient’s Harris Hip Score and area of femoral head necrosis (P<0.05), with no cases of collapse documented in the CDBM group.Citation27
Table 3 Use of stem cells as treatment for avascular necrosis of the femoral head
Recent larger prospective trials have been completed, and their results are promising and comparable with those of previous smaller studies.Citation28 In one investigation of 25 hips treated with core decompression and 26 hips treated with CDBM, there was a significant improvement in Harris Hip Scores between the CDBM core decompression groups.Citation28 To refine further the identification of which patients may benefit the most from stem cell therapy, the authors noted that patients in the CDBM group with poor preoperative Harris Hip Scores, X-ray changes, and edema or effusion on MRI had better results compared with similar patients in the core decompression group.Citation28 In the largest study, 100 patients underwent core decompression (51 hips) or CDBM (53 hips). In the core decompression group, ten patients progressed and eventually required THA or a vascularized fibular graft, while only two hips in the CDBM group required a vascularized fibular graft.Citation13 The findings of this study are consistent with those of smaller investigations demonstrating that patients treated with CDBM achieved significantly higher Harris Hip Scores at final follow-up.
Although these initial results have been promising, a recent study showed no difference in outcome with regard to core decompression versus multiple drilling of the AVN lesion and augmenting the procedure with concentrated bone marrow.Citation29 It has been demonstrated that mesenchymal stem cells may not be able to survive in the necrotic lesions.Citation30,Citation31 However, previous studies in patients with AVN of the femoral head have shown that labeled stem cells stay within the necrotic lesion, and are able to expand and survive in the avascular environment for up to 12 weeks following adequate decompression.Citation26,Citation32 In order to explain the results of the study by Lim et al,Citation29 it may be that the decompression obtained was not adequate to induce a complete healing response.
One other factor that has been shown to influence healing of necrotic lesions in the femoral head has been the number of stem cells injected.Citation22,Citation23 Ex vivo amplification of the iliac crest stem cells has been proposed in order to increase the number of stem cells injected.Citation33,Citation34 In both of these studies, the authors found that mesenchymal stem cells isolated from the iliac crest can be successfully expanded in an ex vivo setting and transplanted to the area of osteonecrosis. The authors found that no patient has had a progression in the size or stage of their AVN lesions.Citation33,Citation34
Use of bone marrow for the treatment of AVN has also been attempted in osteonecrosis of the knee.Citation35 In a small study, the investigators debrided the areas of AVN in the femoral condyles, and then used iliac crest bone marrow along with a bone matrix to fill the lesions.Citation35 At 2 years of clinical follow-up, they noted no progression in the size of the necrotic lesions and all patients had an increase in their American Knee Society scores, as well as a decrease in pain.Citation35
A small case series has been published looking at injecting adipose-derived mesenchymal stem cells directly into the hip joints of patients with AVN of the femoral head.Citation36 The patients in this study noted a reduction in pain that coincided with a decrease in the necrotic volume within the femoral head.Citation36 Although this study shows promise by using a plentiful and easily accessible source of mesenchymal stem cells, the results of the study remain under debate.Citation37
Our surgical technique
Patients are selected to undergo minimally invasive hip decompression augmented by concentrated bone marrow aspirate if they meet our inclusion criteria (). The procedure is only performed in patients with stage 0, 1, or 2 AVN of the femoral head according to the Steinberg classification ().Citation21 In order to obtain mesenchymal stem cells, a small (2–3 mm) incision is made over the anterior iliac crest. A small trochar (MarrowStim, Biomet Biologics, Warsaw, IN, USA) is inserted into the iliac crest and bone marrow is aspirated. The bone marrow is then concentrated using a Bio-Cue System (Biomet Biologics), whereby 60 cc of bone marrow is concentrated 10× to produce 6 mL of concentrated bone marrow. Typically, 12 mL of concentrated bone marrow is used for each hip. During this time, 120 mL of blood is obtained to produce 12 mL of platelet-rich plasma, which serves as a cell growth adjuvant.
Figure 1 Magnetic resonance image from a patient with an avascular necrosis lesion (star) at the femoral head (A). Intraoperative radiograph of the patient undergoing minimally invasive core decompression with injection of autologous concentrated bone marrow aspirate (B).
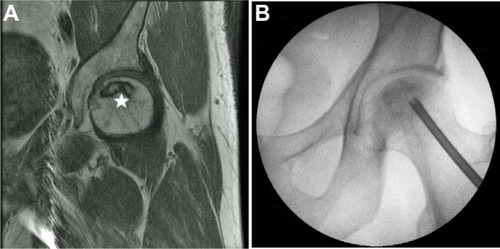
Table 4 Indications for stem cell treatment
Hip decompression and injection of concentrated bone marrow
During the time in which the bone marrow and peripheral blood is being concentrated and processed, we start the hip decompression. An incision is made over the lateral aspect of the femur at the level of the vastus ridge. When the ideal starting point has been obtained, just above the level of the lesser trochanter and distal to the vastus ridge, a 6 mm trochar is advanced with gentle tapping using a mallet under fluoroscopy, to end “in” the necrotic lesion. Correct trochar positioning is then confirmed using fluoroscopy (). The inner core of the trochar is removed, leaving a hollow trochar in place. Once optimal positioning has been achieved, the concentrated bone marrow and platelet-rich plasma are combined and injected through the trochar into the necrotic lesion of the femoral head. When the injection has been completed, the tract is then packed with autologous cancellous bone to prevent back flow of the concentrated bone marrow and platelet-rich plasma products.
Postoperative care
This is an outpatient surgical procedure, after which all patients are discharged home and allowed to weight-bear as tolerated with crutches for 2 weeks. We have found that a small number of patients initially have an increase in pain after the injection; however, this pain is typically short-lived, with most patients reporting significant pain relief within a few weeks.
Hip decompression with injection of concentrated bone marrow and platelet-rich plasma has been performed on 73 hips at our institution. Over the follow-up period (average 17 months), 16 hips (21%) have progressed to further stages of osteonecrosis, ultimately requiring total hip replacement.Citation24 Although some patients have progressed to needing THA, others have had complete resolution of their symptoms as well as their lesions on MRI (). Importantly, there have been no significant complications in any patient undergoing this procedure. Significant pain relief, based on the visual analog scale, has been reported in 60 patients (86%), while the remainder reported little or no pain relief.Citation24
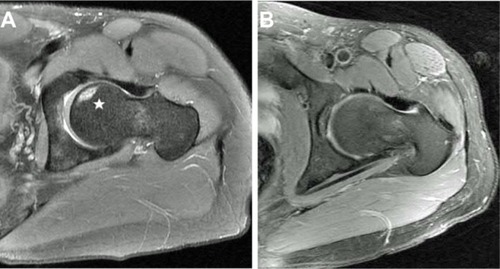
Figure 2 Preoperative magnetic resonance image (A) from a patient with an avascular necrosis lesion at the femoral head (star). The patient underwent minimally invasive core decompression with injection of autologous concentrated bone marrow aspirate and noted a significant reduction and pain. Follow-up magnetic resonance imaging 2 years following decompression and concentrated bone marrow injection showed complete resolution of the avascular necrosis lesion (B).
In conclusion, the use of core decompression combined with mesenchymal stem cells in the form of concentrated bone marrow can provide significant pain relief, improvement in function, and ultimately halt the progression of AVN of the femoral head. Using this minimally invasive regenerative hip preservation therapy, young patients can hopefully avoid the need for THA and be able to return to normal function and activities of daily living.
Disclosure
The authors have received research support from Biomet Biologics (Warsaw, IN, USA).
References
- HerndonJHAufrancOEAvascular necrosis of the femoral head in the adult. A review of its incidence in a variety of conditionsClin Orthop Relat Res19728643624558626
- MwaleFWangHJohnsonAJMontMAAntoniouJAbnormal vascular endothelial growth factor expression in mesenchymal stem cells from both osteonecrotic and osteoarthritic hipsBull NYU Hosp Jt Dis201169Suppl 1S56S6122035487
- LaverniaCJSierraRJGriecoFROsteonecrosis of the femoral headJ Am Acad Orthop Surg1999725026110434079
- GangjiVHauzeurJPTreating osteonecrosis with autologous bone marrow cellsSkeletal Radiol20103920921119760411
- HernigouPBachirDGalacterosFThe natural history of symptomatic osteonecrosis in adults with sickle-cell diseaseJ Bone Joint Surg Am200385-A50050412637438
- HernigouPPoignardANogierAManicomOFate of very small asymptomatic stage-I osteonecrotic lesions of the hipJ Bone Joint Surg Am200486-A2589259315590840
- HernigouPHabibiABachirDGalacterosFThe natural history of asymptomatic osteonecrosis of the femoral head in adults with sickle cell diseaseJ Bone Joint Surg Am2006882565257217142405
- ChengEYThongtranganILaorrASalehKJSpontaneous resolution of osteonecrosis of the femoral headJ Bone Joint Surg Am200486-A2594259915590841
- BozicKJZurakowskiDThornhillTSSurvivorship analysis of hips treated with core decompression for nontraumatic osteonecrosis of the femoral headJ Bone Joint Surg Am19998120020910073583
- IorioRHealyWLAbramowitzAJPfeiferBAClinical outcome and survivorship analysis of core decompression for early osteonecrosis of the femoral headJ Arthroplasty19981334419493536
- ItoHMatsunoTOmizuNAokiYMinamiAMid-term prognosis of non-traumatic osteonecrosis of the femoral headJ Bone Joint Surg Br20038579680112931794
- AldridgeJM3rdUrbaniakJRAvascular necrosis of the femoral head: etiology, pathophysiology, classification, and current treatment guidelinesAm J Orthop (Belle Mead NJ)20043332733215344574
- ZhaoDCuiDWangBTreatment of early stage osteonecrosis of the femoral head with autologous implantation of bone marrow-derived and cultured mesenchymal stem cellsBone20125032533022094904
- JonesLCMontMALeTBProcoagulants and osteonecrosisJ Rheumatol20033078379112672200
- HungerfordDSPathogenesis of ischemic necrosis of the femoral headInstr Course Lect1983322522606546072
- LiuYFChenWMLinYFType II collagen gene variants and inherited osteonecrosis of the femoral headN Engl J Med20053522294230115930420
- MontMAHungerfordDSNon-traumatic avascular necrosis of the femoral headJ Bone Joint Surg Am1995774594747890797
- MontMAJonesLCEinhornTAHungerfordDSReddiAHOsteonecrosis of the femoral head. Potential treatment with growth and differentiation factorsClin Orthop Relat Res1998355SupplS314S3359917651
- FicatRPIdiopathic bone necrosis of the femoral head. Early diagnosis and treatmentJ Bone Joint Surg Br198567393155745
- KerboulMThomineJPostelMMerle d’AubignéRThe conservative surgical treatment of idiopathic aseptic necrosis of the femoral headJ Bone Joint Surg Br1974562912964854691
- SteinbergMEHaykenGDSteinbergDRA quantitative system for staging avascular necrosisJ Bone Joint Surg Br19957734417822393
- HernigouPPoignardAZilberSRouardHCell therapy of hip osteonecrosis with autologous bone marrow graftingIndian J Orthop200943404519753178
- HernigouPBeaujeanFTreatment of osteonecrosis with autologous bone marrow graftingClin Orthop Relat Res2002405142312461352
- MartinJRHoudekMTSierraRJUse of concentrated bone marrow aspirate and platelet rich plasma during minimally invasive decompression of the femoral head in the treatment of osteonecrosisCroat Med J20135421922423771751
- GangjiVHauzeurJPMatosCDe MaertelaerVToungouzMLambermontMTreatment of osteonecrosis of the femoral head with implantation of autologous bone-marrow cells. A pilot studyJ Bone Joint Surg Am200486-A1153116015173287
- GangjiVDe MaertelaerVHauzeurJPAutologous bone marrow cell implantation in the treatment of non-traumatic osteonecrosis of the femoral head: five year follow-up of a prospective controlled studyBone2011491005100921821156
- ChangTTangKTaoXTreatment of early avascular necrosis of femoral head by core decompression combined with autologous bone marrow mesenchymal stem cells transplantationZhongguo Xiu Fu Chong Jian Wai Ke Za Zhi201024739743 Chinese20632513
- SenRKTripathySKAggarwalSMarwahaNSharmaRRKhandelwalNEarly results of core decompression and autologous bone marrow mononuclear cells instillation in femoral head osteonecrosis: a randomized control studyJ Arthroplasty20122767968622000577
- LimYWKimYSLeeJWKwonSYStem cell implantation for osteonecrosis of the femoral headExp Mol Med201345e6124232260
- PotierEFerreiraEAndriamanalijaonaRHypoxia affects mesenchymal stromal cell osteogenic differentiation and angiogenic factor expressionBone2007401078108717276151
- PotierEFerreiraEMeunierASedelLLogeart-AvramoglouDPetiteHProlonged hypoxia concomitant with serum deprivation induces massive human mesenchymal stem cell deathTissue Eng2007131325133117518749
- YanZHangDGuoCChenZFate of mesenchymal stem cells transplanted to osteonecrosis of femoral headJ Orthop Res20092744244618925660
- MüllerIVaeglerMHolzwarthCSecretion of angiogenic proteins by human multipotent mesenchymal stromal cells and their clinical potential in the treatment of avascular osteonecrosisLeukemia2008222054206118719618
- RackwitzLEdenLReppenhagenSStem cell- and growth factor-based regenerative therapies for avascular necrosis of the femoral headStem Cell Res Ther20123722356811
- LeeKGoodmanSBCell therapy for secondary osteonecrosis of the femoral condyles using the Cellect DBM System: a preliminary reportJ Arthroplasty200924434818534437
- PakJAutologous adipose tissue-derived stem cells induce persistent bone-like tissue in osteonecrotic femoral headsPain Physician201215758522270740
- KimHJAutologous adipose tissue-derived stem cells induce persistent bone-like tissue in osteonecrotic femoral heads: not bone-like, but fat-like tissuePain Physician201215E74922996870
