Abstract
Epithelial and mesenchymal cells isolated from the amniotic membrane (AM) possess stem cell characteristics, differentiation potential toward lineages of different germ layers, and immunomodulatory properties. While their expansion and differentiation potential have been well studied and characterized, knowledge about their immunomodulatory properties and the mechanisms involved is still incomplete. These mechanisms have been evaluated on various target cells of the innate and the adaptive system and in animal models of different inflammatory diseases. Some results have evidenced that the immunomodulatory effect of AM-derived cells is dependent on cell-cell contact, but many of them have demonstrated that these properties are mediated through the secretion of suppressive molecules. In this review, we present an update on the described immunomodulatory properties of the derived amniotic cells and some of the proposed involved mechanisms. Furthermore, we describe some assays in animal models of different inflammatory diseases which reveal the potential use of these cells to treat such diseases.
Introduction
The amniotic membrane (AM) is an avascular tissue that forms the innermost layer of the fetal membranes. It is composed of five layers: an epithelial cells monolayer, an acellular basement membrane layer, a compact layer, a mesenchymal cells layer, and a spongy layer placed in close proximity to the chorion.Citation1
Using a variety of established protocols, two types of cells have been isolated from the AM and their properties have been studied.Citation2–Citation7 Isolated cells have been identified as human amniotic epithelial cells (HAECs) and human amniotic mesenchymal stromal cells (HAMSCs).Citation2 It has been shown that both types of cells possess stem cell characteristics, differentiation potential toward lineages of different germ layers,Citation5–Citation12 and immunomodulatory properties.Citation13–Citation19
While the expansion and differentiation potential of the AM-derived cells has been well studied and characterized for different groups, the available knowledge about their immunomodulatory behavior is relatively scarce and disperse. However, in the last few years, increasing experimental findings have pointed toward the immunomodulatory properties of these cells, which it is hoped could dramatically expand their therapeutic potential clinical applications.
In this paper, we present an update on the described immunomodulatory properties of the derived amniotic cells, and an overview of the current theories regarding the potential use of these cells to treat inflammatory diseases.
Definition of amniotic membrane-derived cells
Freshly isolated HAECs are medium-sized cells, circular in shape, with a central or eccentric nucleus, one or two nucleoli, and abundant cytoplasm, usually vacuolated.Citation3–Citation6 They express cell surface markers associated with embryonic stem cells such as SSEA-3 and SSEA-4 (stage-specific embryonic antigen 3 and 4), and TRA 1–60 and TRA 1–81 (tumor rejection antigen 1–60 and 1–81). They also express molecules, such as E-Cadherin, CD9, CD29, CD104, CD49e, CD49f, CD49d, and CD44, among other molecules involved in cell-cell interactions and cell adhesionCitation5,Citation6,Citation13,Citation15,Citation20 (). HAECs express transcription factors specific for pluripotential stem cells: Oct-4, Sox-2, Nanog, and Rex-1.Citation2,Citation5,Citation6,Citation8–Citation11 In culture, these cells proliferate, showing numerous mitotic events, and form a confluent single layer with typical cobblestone epithelial morphology.Citation2,Citation3,Citation5,Citation6,Citation8–Citation11 Cultured HAECs undergo epithelial to mesenchymal transition through the autocrine production of transforming growth factor beta (TGF-β).Citation21 Under appropriate culture conditions, these cells can be induced to differentiate in cells of the three germinal layers (ectoderm, mesoderm, and endoderm).Citation2,Citation5,Citation6,Citation8–Citation11
Table 1 Phenotypic characteristics of HAECs
HAMSCs are defined as a population of cells that proliferate in vitro as plastic-adherent, spindle-shaped cells capable of producing fibroblast colony-forming units and displaying a specific pattern of cell surface antigens comparable to that of bone marrow mesenchymal stem cells (BM-MSCs) and other adult sources. They do not express the hematopoietic markers CD45, CD34, or CD14, but they do express variable levels of CD90, CD73, CD105, CD29, CD44, CD49d, CD49e, CD56, and CD166, and they are recognized by the monoclonal antibody against stromal precursor cells-1Citation2,Citation3,Citation5–Citation7,Citation10–Citation12,Citation20 (). These cells are also capable of differentiating toward one or more lineages, including osteogenic, adipogenic, chondrogenic, and vascular/endothelial.Citation2,Citation5–Citation7,Citation10–Citation13 Furthermore, recent reports suggest that, like the amniotic epithelial fraction, HAMSCs have multilineage differentiation potential.Citation22
Table 2 Phenotypic characteristics of HAMSCs
The immunologic profile of HAECs and HAMSCs reveals that they express low levels of major histocompatibility complex (MHC) class I surface antigens and reduced levels of the major components of the antigen processing machinery. They do not express MHC class II antigens,Citation2,Citation13,Citation15 the costimulatory molecules CD80 (B7-1), CD86 (B7-2), CD40, or CD40 ligand, in the presence or absence of interferon gamma (IFN-γ), one of the most potent known inflammatory cytokines.Citation2,Citation15,Citation16 They neither express the programmed cell death receptor 1 (PD1) (an inhibitory receptor that is normally expressed on activated T and B cells), nor its two ligands: programmed death ligands 1 and 2 (PD-L1 and PD-L2).Citation15,Citation16,Citation23 These two are typically upregulated by stimulation with IFN-γ.Citation15,Citation16 AM-derived cells are also negative for the immunoglobulin-like transcript receptors 2, 3, and 4 (ILTR-2, ILTR-3, and ILTR-4).Citation15 There is some controversy about the expression of TRAIL, tumor necrosis factor alpha (TNF-α), and Fas-ligand (Fas-L), all members of the TNF family involved in the induction of apoptosisCitation14,Citation15 ().
Table 3 Immunologic profile of HAECs and HAMSCs
One of the unique characteristics of HAECs and HAMSCs is that they constitutively express the tissue-restricted, nonclassical human leukocyte antigen G (HLA-G).Citation5,Citation8,Citation24,Citation25 Under physiological conditions, constitutive HLA-G expression is found in immune-privileged organs (eg, testis, ovary, and fetal cells) and is associated with tolerogenic properties via the interaction with inhibitory receptors. HLA-G has been shown to have important immunomodulatory functions.Citation24,Citation25 It appears to be recognized mainly by ILTR, which are expressed by T and B lymphocytes, as well as by natural killer and dendritic cells, and abrogate activating signals received by these cells. Levels of HLA-G on cultured AM-derived cells are upregulated by exposure to IFN-γ.Citation15 In conclusion, these characteristics indicate that AM-derived cells are immune-privileged cells, able to survive in immunocompatibility-mismatched allogeneic transplant recipients.
In vitro effects of AM-derived stem cells on different cells of the innate and adaptive immunity
The maternofetal immune tolerance observed during pregnancy has inspired most of the studies done to investigate the immune properties of the human amniotic cells. These studies have demonstrated that human amniotic cells exhibit pleiotropic immune regulatory activities both in vivo and in vitro, which are mediated by complex mechanisms that inhibit the function of different immune cell subpopulations of the innate and adaptive immunity.
In vivo, several preclinical studies have reported that cells derived from the AM can engraft different tissues after xenogeneic and allogeneic transplantation into immunocompetent animals without eliciting an immune response, indicating that these cells are not immunogenic.Citation18,Citation26–Citation32 Additionally, AM has been applied in patients with ophthalmologic disorders,Citation33,Citation34 burn injuries,Citation35 and chronic ulcers to improve wound healing.Citation36–Citation39 In none of these cases acute rejection has been observed in the absence of immunosuppressive treatment.
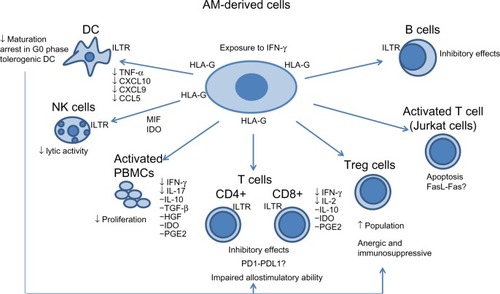
In vitro, the mechanisms involved in immunosuppressive and immunomodulatory properties of the AM-derived cells have been evaluated on various target cells of the innate and the adaptive system (). Some assays have been performed without separating the two types of cells (epithelial and mesenchymal).Citation16,Citation17,Citation19,Citation40–Citation43 Others have been performed on each population of cells.Citation13–Citation15 Wolbank et alCitation13 demonstrated that, although epithelial and mesenchymal fractions show distinct morphology and marker expression, they have similar potency to modulate immunoreactions in vitro.
Figure 1 Proposed mechanisms of the in vitro interaction of AM-derived stem cells with different cells of the immune system.
Innate immunity
Dendritic cells (DCs) have a fundamental role in antigen presentation to naïve T cells following DC maturation, which can be induced by proinflammatory cytokines and/or pathogen associated molecules. During maturation, immature DCs (iDCs) acquire the expression of costimulatory molecules and upregulate expression of MHC class I and class II molecules together with other cell-surface markers (such as CD11c and CD83).Citation44
The immunomodulatory effects of AM-derived cells exerted on antigen-presenting cells have been demonstrated by their ability to block maturation of monocytes into DCsCitation41,Citation42 (). Monocytes exposed to DC differentiation and maturation conditions in the presence of AM-derived cells showed impaired development and failed to express CD1a, to upregulate the costimulatory molecules CD80, CD86, and CD83, and to increase surface expression of HLA-DR.Citation41 The addition of lipopolysaccharide to monocytes cultured toward iDC differentiation in the presence of AM-derived cells did not result in differentiation, even after removal of such cells.Citation41
The mechanisms responsible for this inhibition remain to be elucidated, although several possible explanations have been proposed. It has been suggested that AM-derived cells inhibit DC differentiation by preventing cell cycle entry and inhibiting protein synthesis in stimulated monocytes. Monocytes induced in the presence of AM-derived cells showed almost complete arrest in the G0 phase of the cell cycle.Citation41 HLA-G expressed on HAECs and HAMSCs has also been implicated to explain the tolerogenic DC, through the killing inhibitory receptor ILT pathway present in monocytes, macrophages, and DCs. It has been demonstrated that the tolerogenic DC induced through HLA-G leads to the induction of anergic and immunosuppressive regulatory T cells (Treg)Citation45,Citation46 ().
Using a transwell system, Magatti et al observed that AM-derived cells were able to inhibit monocytes differentiation in the absence of cell-cell contact, suggesting the involvement of soluble factors.Citation41,Citation42 They found that AM-derived cells produce high levels of the Th2-related cytokines CCL2, CXCL8, and interleukin 6 (IL-6), the latter known to be implicated in the inhibition of CD34+ cells and monocyte differentiation to DCs. Additionally, they found that AM-derived cells block the production of inflammatory cytokines TNF-α, CXCL10, CXCL9, and CCL5 in DC differentiation cultures.Citation41 All of these were considered as evidence of the AM-derived cells’ immunosuppressive mechanisms, mediated by anti-inflammatory processes.
The block in the monocyte induced differentiation/maturation toward DC in the presence of AM-derived cells, also resulted in impaired allostimulatory ability on allogeneic T cells.Citation16,Citation41,Citation42 This effect, which persisted even after removal of AM-derived cells and complete reinduction of the monocytes toward DC differentiation, suggested an irreversible functional change of AM-derived cells on differentiating monocytes.Citation41 These findings, similar to those observed by other investigators using BM-MSCs, have been considered as proof that AM-derived cells are tolerogenic, at least in part, through a direct impact on DCs, leading to impaired T cell functions.Citation41,Citation46,Citation47
Natural killer (NK) cells are important effector cells of innate immunity, and they have a key role in antiviral and antitumor immune responses owing to their cytolytic activity and production of pro-inflammatory cytokines.Citation48 The function of NK cells is tightly regulated by cell surface receptors that transduce either inhibitory or activating signals. NK cell-mediated lysis of target cells requires the expression of ligand(s) by the target cells that are recognized by the activating NK receptor, together with low-level to absent expression of MHC class I molecule by the target cell, which is recognized by the MHC class I specific inhibitory receptor of NK cells. The absence of MHC class I antigens on the HAECs provides a degree of immune privilege against cells of the adaptive immune system, but renders these cells potentially vulnerable to attack by NK cells. However, using K562, which is a well-established NK cells target, and MCF-7, a poor NK cells target, it was observed that placenta-derived MSCs were not lysed by NK cells.Citation16 Reverse transcription polymerase chain reaction and enzyme-linked immunosorbent assay analysis revealed that HAECs produce migration inhibitory factor, a potent inhibitor of macrophage migration and, coincidentally, a potent inhibitor of NK cell-mediated lytic activity.Citation14 Additionally, AM-derived cells may inhibit the interaction of HLA-G antigens and the killing inhibitory ILT receptors, which are expressed by NK cells as well as by T and B lymphocytesCitation45–Citation49 (). However, it must be appreciated that activated but not freshly isolated NK cells have been reported to kill MSCs.Citation50
Adaptive immunity
After T-cell receptor (TCR) engagement, T cells proliferate and exert several effector functions, including cytokine release and in the case of CD8+ cells, cytotoxicity.Citation43 The proliferation of T cells stimulated with polyclonal mitogens, allogeneic cells, or specific antigen is inhibited by AM-derived cells.Citation13–Citation17,Citation19,Citation42,Citation51 According to some investigators, HAECs and HAMSCs inhibited proliferation of activated peripheral blood mononuclear cells (PBMCs) by phytohemagglutinin (PHA) or allogeneic cultured cells by cell-to-cell contact and in a dose-dependent manner, in mixed lymphocyte reaction (MLR), as demonstrated by a decrease in proliferation with increasing amounts of AM-derived cells.Citation13,Citation15 They found that in a transwell system, AM-derived cells were unable to suppress the proliferative response of activated PBMCs.Citation13,Citation15 However, other investigators demonstrated that these effects on PBMCs were caused not only by cell-to-cell contact, but also by AM-derived cell culture supernatant or conditioned media from these cultures.Citation14,Citation16,Citation17,Citation19
Using fluid-derived MSCs, Sessarego et al showed that these cells could inhibit the proliferation of T cells when activated by a physiological dual stimulus, through TCR and CD28.Citation51
Roelen et al analyzed the production of cytokines during the primary MLR, secondary MLR, and the mitogenic proliferation response, both in the absence and presence of fetal MSCs.Citation40 They observed that the production of cytokines by the cultures was affected by the addition of fetal MSCs. The cytokines/growth factors that were significantly increased by coculture with fetal MSCs were IL-2, IL-4, IL-7, IL-10, IL-15, IFN-γ (in secondary MLR), and VEGF (vascular endothelial growth factor). Using similar assays, Kang et al observed lower levels of IL-17 and IFN-γ production in the supernatant from cocultures of HAMSCs and PBMCs in the presence of mitogens, compared to the supernatant obtained from cultures of PBMCs alone.Citation19 However, the level of IL-10 and TGF-β production increased significantly in the supernatant obtained from the cocultures of HAMSCs and PBMCs.Citation19 These same authors observed that messenger (m)RNA expression of TGF-β, hepatic growth factor (HGF), indoleamine 2,3-dioxygenase (IDO) and cyclooxygenase 2 (COX-2) were induced more, not only in HAMSCs grown in the presence of PBMCs, but also in HAMSCs separated from PBMCs by transwells, compared to those grown without PBMCs.Citation19 MLR with the addition of neutralizing antibodies to two of the potentially inhibitory cytokines, IL-10 and TGF-β, in a transwell cultures could abrogate the inhibitory effect of placenta-derived multipotent MSCs (fetal in origin), indicating that both are important factors mediating the suppressive capacity of these cells.Citation16,Citation40 Fetal MSCs are more effective in inhibiting T cells than maternal MSCs, probably due to higher IL-10 production by the fetal cells.Citation40
Cultured expanded placenta-derived MSCs have similar inhibitory effects on peripheral blood CD4+ and CD8+ and umbilical cord blood CD4+ and CD8+ lymphocyte proliferation induced by mitogens (PHA) and allogeneic peripheral blood lymphocytes, often in a dose-dependent manner.Citation16,Citation17 These cells in the transwell chamber system could suppress CD4+, CD8+ T cells, and PBMCs, as in the usual coculture system.Citation16,Citation17 In these studies, Li et al assayed the levels of IL-2, IFN-γ, and IL-10 in the supernatant of MLR cultures at different time points.Citation17 They observed that the presence of placental MSCs increased the level of IL-10 (Th2 cytokines) and decreased levels of IL-2 and IFN-γ (Th1 cytokines) (). Following the acceptance of that divergence of naïve T-cells into Th1 or Th2 effectors partly depends on the cytokines that they encounter;Citation52 the authors considered that IL-10 might play a role in MSCs’ regulatory effect.Citation17
There are also some indications that the immunosuppressive effect of placenta-derived MSCs involves Treg cells, which are CD4+ CD25high T cells capable of modulating tolerance in the immune response.Citation16,Citation40,Citation49 Recently, T cells with a regulatory phenotype were detected in the placenta, and there was a greater than threefold increase in the proportion of CD4+ CD25high T cells in lymphocyte proliferation assays stimulated with PHA after 3 days of coculture with placenta-derived MSCs.Citation16 Using the Treg marker, Foxp3, a threefold increase in CD34+/Fox3+ lymphocytes was also found when placenta-derived stem cells were added to MLRCitation16 ().
The state of T-cell activation and differentiation also appear to be critical to the immunomodulatory effects that AM-derived cells may elicit. Banas et al observed that in contrast to the significant inhibition of primary immune responses in the presence of AM-derived cells, preactivated T cells driven by IL-2 were not affected by coculture with AM-derived cells.Citation15 They concluded that when naïve or memory T cells are stimulated they are prone to inhibitory effects of AM-derived cells, whereas activated T cells may be less affected.Citation15 However, Li et al reported that HAECs factors induced apoptosis of activated T cells (Jurkat cells: human acute lymphoblastic T-cell leukemia, clone E6.1).Citation14 Although the mechanism by which HAECs mediated induction of apoptosis of lymphocytes remains unknown, the authors hypothesized that HAECs mediate caspase-dependent killing through the interactions between Fas-L and Fas-positive cellsCitation14 ().
In conclusion, in vitro, at least three interrelated mechanisms have been identified in the interaction of AM-derived cells with different cells of the immune system, both when added in a contact assay or a transwell setting: 1) AM-derived cells are hypoimmunogenic, and they block the generation and maturation of antigen-presenting cells; 2) they are capable of modulating T cell phenotype, modulating the immune response in vitro, and in particular, they are able to inhibit allogeneic lymphocyte proliferation; and 3) they abolish the production of inflammatory cytokines and immunosuppress the local environment.
Mechanisms of immunosuppression by AM-derived stem cells
Although several studies have documented the immunosuppressive activities of AM-derived stem cells, the underlying mechanisms are only partially known. They have been mainly investigated in MSCs isolated from BM.
Some studies indicate that MSCs are not spontaneously immunosuppressive; that priming by inflammatory cytokines is essential for MSCs-mediated immunosuppression.Citation53,Citation54 During an immune response, the inflammatory cytokines produced by T cells and antigen-presenting cells modulate the function of MSCs, leading to the production of growth factors, altered expression of surface molecules, and release of immunosuppressive factors. Several reports have demonstrated that at sites of tissue damage, MSCs produce growth factors, such as epidermal growth factor, fibroblastic growth factor, platelet-derived growth factor, VEGF, insulin-like growth factor-1, stromal cell derived factor-1, TGF-β, and HGF; release large amounts of chemokines, especially CCL2, CXCL9, CXCL10, and CXCL11; and increase the expression of adhesion molecules, such as intercellular adhesion molecules (ICAM)-1 and vascular cell adhesion molecules (VCAM)-1.Citation53–Citation55
Cell adhesion molecules, such as B7-H1, ICAM, and VCAM, may participate in immunomodulation. The immunomodulation by human umbilical cord mesenchymal stem cells (hUC-MSCs) is largely mediated by cell-cell contact via adhesion molecules, particularly B7-H1.Citation56 Moreover, the adhesion molecules, ICAM-1 and VCAM-1, mediated immunosuppression in mouse BM-MSCs induced by IFN-γ from activated T cells; this activity was abrogated by antibodies against ICAM-1 and VCAM-1.Citation56
Levels of HLA-G on cultured AM-derived cells are upregulated by exposure to IFN-γ.Citation15 The expression of these antigens increased substantially in AM-derived cells (from 4% to 36% after 5 days of incubation) when they were titrated into MLR.Citation15 Some of the inhibitory effects of AM-derived cells could be exerted by the HLA-G antigens through the killing inhibitory receptor ILT-4 pathway which directly interacts with HLA-G as well as with HLA class I molecules.Citation15 HLA-G has been observed to upregulate the expression of ILT-2 and ILT-4 on NK and T cells. Although the mechanism is not fully understood HLA-G has been shown to induce apoptosis of activated CD8+ cells and to inhibit CD4+ cell proliferation.Citation15,Citation45,Citation46,Citation49 Moreover, the production of soluble HLA-G5 by amniotic cells has been shown to suppress T cell proliferation and NK cell and T cell cytotoxicity and to promote the generation of regulatory T cells.Citation15,Citation49
Under some experimental conditions, the inhibition of T cell proliferation by amniotic cells requires engagement of the inhibitory surface protein PD1 by its ligand 1 (PD-L1). PD-L1 has been found to be expressed by the syncytiotrophoblast in early pregnancy and PD-L2 on all trophoblast populations throughout pregnancy.Citation23,Citation57 Both seem to play key roles in T-cell mediated tolerance of the semi-allogeneic fetus, as binding of either ligand of the PD1 receptor (expressed on activated T and B cells) inhibited antigen-stimulated T-cell activation and cytokine production in vitro. It has been proposed that, in an early allogeneic environment in which pro-inflammatory cytokines may be present, amniotic cells may upregulate PD-L1 expression, which in turn may inhibit T-cell activation and proliferation.Citation15 In vitro PD-L1 is upregulated in placenta-derived MSCs after stimulation with IFN-γ.Citation16
It is likely that through the synergistic action of the chemokines and adhesion molecules, immune cells accumulate in close proximity to the MSCs, where the high concentration of secreted factors can suppress immune cells effector functions.Citation53,Citation54
In response to immune cells, HAMSCs, as MSCs obtained from other sources, release some soluble factors, resulting in the suppression of proliferation and inhibition of the release of pro-inflammatory cytokines by immune cells; these molecules include IL-10, TGF-β1, HGF, IDO, and prostaglandin E-2 (PGE2)Citation16,Citation17,Citation19,Citation44,Citation53 ().
Table 4 Primed AM-derived stem cells
IL-10 is a cytokine that functions as a broad spectrum anti-inflammatory cytokine by inhibiting production of IL-1, TNF-α, and other pro-inflammatory factors.Citation53 IL-10 has also been implicated in the inhibitory effect on T cell proliferation exerted by fetal MSCs at the fetomaternal interface.Citation57
TGF-β is a potent anti-inflammatory cytokine that enhances the immunomodulatory properties of placenta-derived MSCs.Citation16 The possible participation of TGF-β in the immunomodulatory effect exerted by HAMSCs on PBMCs has been suggested by several investigators who observed a strong increase in the expression of TGF-β mRNA in HAMSCs after 3 days of IFN-γ treatment Citation16 and coculturing with leukocytes,Citation19 and an increased level of TGF-β in the culture supernatant obtained from HAMSCs and PBMCs cocultured for 3 days.Citation19 The immunosuppressive effect of placenta-derived MSCs has been abrogated with anti-TGF-β antibodies.Citation16,Citation19,Citation49,Citation57
IDO is another well-known immune-suppression factor constitutively expressed by placenta-derived stem cells.Citation16 It is a key regulator of placental immunotolerance during pregnancy that inhibits various immune cell populations, including T cell and NK cells. IDO catalyzes the rate-limiting step in the degradation of tryptophan, an essential amino acid, along the kynurenine pathway.Citation16,Citation19,Citation53 The resulting reduction in local tryptophan concentration and the production of tryptophan metabolites that are immunomodulatory are thought to contribute to the immunosuppressive effects of IDO-expressing cells. IDO mRNA and kynurenine production increased when HAMSCs and PBMCs were cocultured, suggesting that IDO was induced by coculturing and participated in immune modulation by HAMSCs.Citation19 In BM-MSCs, derived IDO was reported to be required to inhibit the proliferation of IFN-γ-producing Th1 cells and, together with PGE2, to block NK-cell activity.Citation58–Citation60
PGE2 is another inflammatory stimulus-induced, immunosuppressive molecule produced by MSCs.Citation53 It regulates the maturation and antigen presentation of DCs and inhibits T cell proliferation and cytokine production.Citation19,Citation53 It is synthesized from arachidonic acid by COX-1 and COX-2 enzymes, which are constitutively expressed by MSCs, indicating that PGE2 is also constitutively expressed.Citation19 HAECs and HAMSCs produce PGE2 in culture, although the basal PGE2 output appears to be significantly greater in HAMSCs than in the epithelial cells.Citation3 The PGE2 production increased in HAMSCs when they were cocultured with PBMCs.Citation19 It has been reported that PGE2 is the most powerful immunomodulatory factor in hUC-MSCs because inhibition of PGE2 synthesis almost completely mitigates the immunosuppressive effect, whereas neutralization of TGF-β and IDO has little effect.Citation60
In summary, it is possible that cell-cell contact and several immunosuppressive mediators produced by AM-derived cells, upon triggering by inflammatory factors, are involved in their immunomodulation properties. The importance of any specific mediator could vary depending upon the local microenvironment leading in a redundant system involving more than one mechanism. However, further studies are needed to clarify the specific role of such factors.
Potential use of AM-derived stem cells to treat inflammatory diseases
The data discussed here on the immunomodulatory properties of AM-derived stem cells are in accordance with those described for MSCs obtained from other sources such as bone marrow,Citation44,Citation49,Citation53,Citation58–Citation60 adipose tissue,Citation13,Citation61,Citation62 and cord blood.Citation49,Citation63–Citation65 However, mainly MSCs isolated from bone marrow have been extensively studied in animal disease models, such as graft versus host disease, experimental autoimmune encephalomyelitis (EAE), collagen-induced arthritis, inflammatory bowel disease, diabetes type 1, and systemic lupus erythematosus. The fact that AM-derived cells are plentiful, easily obtained from a normally discarded amnion tissue, and are not associated with any substantial ethical issues supports the use of HAECs and HAMSCs as a potential therapy to modulate pathogenic immune responses.
Here we present some studies on animal models of diseases with an inflammatory component where the AM-derived stem cells have been used as a therapy to modulate pathogenic immune responses.
Neurological disorders
HAECs have been used for the treatment of spinal cord injury in animal models. In this condition, the inflammation-mediated secondary injury plays an important role in many of the observed deleterious effects.Citation66 After transplantation of HAECs into the damaged areas of a contusion model of spinal cord injury in nonimmunosuppressed monkeys, cells survived up to 120 days in the transplanted environment, supported the growth of host axons, prevented the formation of glial scar, prevented death of axotomized neurons, and induced new collateral sprouting with no evidence of inflammation or rejection. Furthermore, improved performance in locomotion tests was observed in treated animals compared to control animals.Citation27 HAECs transplanted into the injured spinal cord of rats survived during 8 weeks and integrated into the host spinal cord without immune rejection. Compared with the control group, HAECs promoted regeneration and spouting of the axons, improved the hind limb motor function of the rats, and inhibited the atrophy of axotomized cells.Citation28 In the same way, recently it has been demonstrated that HAECs transplanted into the spinal cord of T13 spinal cord hemisected rats suppressed mechanical allodynia and reduced the expression of the microglial marker, F4/80 proteins, known to be involved in spinal cord injury and inflammation.Citation67
Stroke is another neurological disorder in which inflammation has been implicated as a major contributor to the secondary cell death cascade following on from the initial stroke episode. Cells from different sources have been proposed as a novel potential treatment to abrogate the inflammatory side effects observed in this disease.Citation68 Transplantation of HAECs and HAMSCs has been shown to exert beneficial effects in a nonimmunosuppressed rodent stroke model. Transplantation of AM-derived cells directly into the ischemic penumbra at day 1 or 2 poststroke ameliorated behavioral dysfunction, attenuated both motor and neurological deficits, and reduced infarct size associated with occlusion of the middle cerebral artery compared to the vehicle-infused stroke group.Citation29,Citation30 Following the last behavioral test at day 14 poststroke, histology via Nissl staining revealed an increased number of healthy-looking cells in the ischemic penumbra compared to the vehicle-infused stroke group.Citation11,Citation29
In rats with intracerebral hemorrhage, the effects on brain edema and neurological functional recovery after transplantation of HAECs into the lateral ventricle have also been evaluated. The behavior of the animals and brain edema were evaluated after 28 days, and brain sections were made for morphological and immunohistochemical analyses with fluorescence microscopy. Transplanted HAECs were observed along the lateral wall and survived for at least 4 weeks. Around the injury site, activation of microglia was reduced. The water content of intracerebral hemorrhage rats decreased in the treatment group. The behavior test scores were improved in the treatment group compared with those in the control groups.Citation69
Multiple sclerosis
Multiple sclerosis is a T cell-mediated autoimmune inflammatory disease of the central nervous system (CNS). HAECs have been examined for the therapeutic effect in an EAE (experimental autoimmune encephalomyelitis) mouse model of multiple sclerosis.Citation32,Citation70 McDonald et al reported that intraperitoneal injection of HAECs suppressed symptoms and decreased CNS inflammation, demyelination, and axonal degeneration in the spinal cord and brain of a mouse model of multiple sclerosis.Citation32 They also found that HAECs reduced proliferation of T cells and decreased their secretion of proinflammatory cytokines.Citation32 More recently Liu et al reported that intravenously administered HAECs reduced CD3+ T cell and F4/80(+) monocyte/macrophage infiltration and demyelination within the CNS of an EAE mouse.Citation70 HAECs immunosuppression was mediated by PGE2 and TGF-β, as it was demonstrated by the neutralization of TGF-β or PGE2 in splenocyte proliferation assays. Splenocytes from HAEC-treated mice showed a Th2 cytokine shift with significantly elevated IL-5 production.Citation70
Liver disease
Transplantation of HAECs in a liver disease model of fibrosis in mice induced by administration of carbon tetrachloride (CCl4) led to hepatic engraftment observed at 4 weeks after transplantation.Citation31 CCl4-treated immunocompetent mice receiving single or double HAEC doses showed a significant, but similar, decrease in liver fibrosis area associated with decreased activation of collagen-producing hepatic stellate cells, and a decrease in hepatic protein levels of the profibrogenic cytokine, TGF-β1. CCl4 administration caused hepatic T cell infiltration and an increased number of hepatic macrophages compared to normal mice; both types of cells decreased significantly following HAECs transplantation. Treated mice had significantly lower hepatic protein levels of the chemokine monocyte chemoattractant protein-1 (MCP-1), than mice given CCl4 alone, and showed increased expression of genes associated with M2 macrophages, including YM-1, IL-10, and CD206, all associated with fibrosis resolution. The transplantation of HAECs resulted in a reduction of hepatocyte apoptosis and a decrease in inflammation and fibrosis.Citation31
Lung injury
In an inflammatory lung injury model in mice, where damage was induced by administration of bleomycin, a potent stimulator of lung fibrosis, several assays have evidenced that HAECs modulated the host inflammatory response, reduced lung fibrosis, and prevented loss of lung function.Citation71–Citation73 The effect was concomitant with a reduction in proinflammatory cytokines (TNF-α, IFN-γ, MCP-1, and IL-6 in the mouse lung) and resulted in a decrease in inflammatory cells infiltration and an increase in the anti-inflammatory cytokine IL-10.Citation72,Citation73 Lung tissue repair was promoted via paracrine-acting molecules, as demonstrated through the administration in similar animal models of conditioned medium (CM) generated from human amniotic mesenchymal tissue cells (AMTC). At day 14, lung fibrosis scores in AMTC-CM treated mice were significantly lower compared with mice of the bleomycin group in terms of fibrosis distribution, fibroblast proliferation, collagen deposition, and alveolar obliteration. No differences were observed between mice in the bleomycin group and mice treated with control medium. AMTC-CM treatment significantly reduced the fibrosis progression between the two observation time-points, emphasizing the importance of the MSC secretome.Citation74
Conclusion
Results in vitro and from preclinical animal models suggest that human AM-derived cells have the capacity to strongly suppress immune responses, potentially induce peripheral tolerance, and reverse ongoing inflammatory damage. In the preclinical studies mentioned above, the investigators observed that the immunomodulatory effects of AM-derived cells blunt the inflammatory response and allow tissue remodeling after injury, resulting in reduced numbers of fibroblasts and less scarring in the different organs evaluated, such as the spinal cord, liver, and lung. In these studies, the effects were unrelated to the transdifferentiation of AM-derived cells in other cell types. Regardless of the type of injury, the therapeutic effect of AM-derived cells seems to depend on the release of trophic and anti-inflammatory molecules, as reported for BM-derived MSCs.Citation44 However, further research using AM-derived cells in animal models of inflammatory diseases is required to identify the detailed mechanisms responsible for their immunomodulatory effects and to validate the efficacy of these cells in immune-mediated illnesses, with the aim of translating these results into clinical studies in humans.
We thank Dr Antonio Uccelli for critical revision of this manuscript. This study has been supported by the EC10-019 grant from the Department of Pharmacy and Health Products of the Ministry of Health, Social Services, and Equality and the RD12/0019/0001 grant from the Department of Red Cell Therapy, Carlos III Institute of Health, Spain.
Disclosure
The authors declare no conflicts of interest in this work.
References
- BenirschkeKKaufmanPAnatomy and Pathology of the placental membranesPathology of the Human Placenta4th edNew YorkSpringer-Verlag2000281334
- ParoliniOAlvianoFBagnaraGPConcise review: isolation and characterization of cells from human term placenta: outcome of the first international Workshop on Placenta Derived Stem CellsStem Cells200826230031117975221
- WhittleWLGibbWChallisJRThe characterization of human amnion epithelial and mesenchymal cells: the cellular expression, activity and glucocorticoid regulation of prostaglandin outputPlacenta200021439440110833375
- MikiTMarongiuFDorkoKEllisECStromSCIsolation of Amniotic Epithelial Stem CellsCurrent Protocols in Stem Cell Biology12010 Chapter 1:Unit1E.3
- IlancheranSMoodleyYManuelpillaiUHuman fetal membranes: a source of stem cells for tissue regeneration and repair?Placenta200930121018995896
- InsaustiCLBlanquerMBledaPThe amniotic membrane as a source of stem cellsHistol Histopathol2010251919819924645
- AlvianoFFossatiVMarchionniCTerm Amniotic membrane is a high throughput source for multipotent Mesenchymal Stem Cells with the ability to differentiate into endothelial cells in vitroBMC Dev Biol200771117313666
- MikiTStromSCAmnion-derived pluripotent/multipotent stem cellsStem Cell Rev20062213314217237552
- MikiTLehmannTCaiHStolzDStromSCStem cell characteristics of amniotic epithelial cellsStem Cells200523101549155916081662
- IlancheranSMichalskaAPehGWallaceEMPeraMManuelpillaiUStem cells derived from human fetal membranes display multilineage differentiation potentialBiol Reprod200777357758817494917
- ParoliniOAlvianoFBergwerfIToward cell therapy using placenta-derived cells: disease mechanisms, cell biology, preclinical studies, and regulatory aspects at the round tableStem Cells Dev201019214315419947828
- SonciniMVertuaEGibelliLIsolation and characterization of mesenchymal cells from human fetal membranesJ Tissue Eng Regen Med20071429630518038420
- WolbankSPeterbauerAFahrnerMDose-dependent immunomodulatory effect of human stem cells from amniotic membrane: a comparison with human mesenchymal stem cells from adipose tissueTissue Eng20071361173118317518752
- LiHNiederkornJYNeelamSImmunosuppressive factors secreted by human amniotic epithelial cellsInvest Ophthalmol Vis Sci200546390090715728546
- BanasRATrumpowerCBentlejewskiCMarshallVSingGZeeviAImmunogenicity and immunomodulatory effects of amnion-derived multipotent progenitor cellsHum Immunol200869632132818571002
- ChangCJYenMLChenYCPlacenta-derived multipotent cells exhibit immunosuppressive properties that are enhanced in the presence of interferon-gammaStem Cells200624112466247717071860
- LiCZhangWJiangXMaoNHuman-placenta-derived mesenchymal stem cells inhibit proliferation and function of allogeneic immune cellsCell Tissue Res2007330343744617899199
- BailoMSonciniMVertuaEEngraftment potential of human amnion and chorion cells derived from term placentaTransplantation200478101439144815599307
- KangJWKooHCHwangSYImmunomodulatory effects of human amniotic membrane-derived mesenchymal stem cellsJ Vet Sci2012131233122437532
- RoubelakisMGTrohatouOAnagnouNPAmniotic fluid and amniotic membrane stem cells: marker discoveryStem Cells Int2012201210783622701492
- AlcarazAMrowiecAInsaustiCLAutocrine TGF-β induces epithelial to mesenchymal transition in human amniotic epithelial cellsCell Transplant20132281351136723031712
- KimJKangHMKimHEx vivo characteristics of human amniotic membrane-derived stem cellsCloning Stem Cells20079458159418154518
- OkazakiTHonjoTPD-1 and PD-1 ligands: from discovery to clinical applicationInt Immunol200719781382417606980
- LefebvreSAdrianFMoreauPModulation of HLA-G expression in human thymic and amniotic epithelial cellsHum Immunol200061111095110111137212
- HuntJSPetroffMGMcIntireRHOberCHLA-G and immune tolerance in pregnancyFASEB J200519768169315857883
- MarcusAJCoyneTMBlackIBWoodburyDFate of amnion-derived stem cells transplanted to the fetal rat brain: migration, survival and differentiationJ Cell Mol Med20081241256126418782190
- SankarVMuthusamyRRole of human amniotic epithelial cell transplantation in spinal cord injury repair researchNeuroscience20031181111712676132
- WuZYHuiGZLuYWuXGuoLHTransplantation of human amniotic epithelial cells improves hindlimb function in rats with spinal cord injuryChin Med J (Engl)2006119242101210717199962
- LiuTWuJHuangQHuman amniotic epithelial cells ameliorate behavioral dysfunction and reduce infarct size in the rat middle cerebral artery occlusion modelShock200829560361118414234
- YuSJSonciniMKanekoYHessDCParoliniOBorlonganCVAmnion: a potent graft source for cell therapy in strokeCell Transplant200918211111819499700
- ManuelpillaiUTchongueJLourenszDTransplantation of human amnion epithelial cells reduces hepatic fibrosis in immunocompetent CCl4-treated miceCell Transplant20101991157116820447339
- McDonaldCSiatskasCBernardCCAThe emergence of amnion epithelial stem cells for the treatment of Multiple SclerosisInflamm Regen2011313256271
- DuaHSGomesJAKingAJMaharajanVSThe amniotic membrane in ophthalmologySurv Ophthalmol2004491517714711440
- GomesJARomanASantosMSDuaHSAmniotic membrane use in ophthalmologyCurr Opin Ophthalmol200516423324016000896
- KestingMRWolffKDHohlweg-MajertBSteinstraesserLThe role of allogenic amniotic membrane in burn treatmentJ Burn Care Res200829690791618849850
- WardDJBennettJPBurgosHFabreJThe healing of chronic venous leg ulcers with prepared human amnionBr J Plast Surg19894244634672670029
- ColochoGGrahamWPGreeneAEMathesonDWLynchDHuman amniotic membrane as a physiologic wound dressingArch Surg197410933703734604542
- GrussJSJirschDWHuman amniotic membrane: a versatile wound dressingCan Med Assoc J19781181012371246647542
- InsaustiCLAlcarazAGarcía-VizcaínoEMAmniotic membrane induces epithelialization in massive posttraumatic woundsWound Repair Regen201018436837720636551
- RoelenDLvan der MastBJin’t AnkerPSDifferential immunomodulatory effects of fetal versus maternal multipotent stromal cellsHum Immunol2009701162319010366
- MagattiMDe MunariSVertuaEAmniotic mesenchymal tissue cells inhibit dendritic cell differentiation of peripheral blood and amnion resident monocytesCell Transplant200918889991419523334
- MagattiMDe MunariSVertuaEGibelliLWenglerGSParoliniOHuman amnion mesenchyme harbors cells with allogeneic T-cell suppression and stimulation capabilitiesStem Cells200826118219217901399
- HaoYMaDHHwangDGKimWSZhangFIdentification of antiangiogenic and antiinflammatory proteins in human amniotic membraneCornea200019334835210832697
- UccelliAMorettaLPistoiaVMesenchymal stem cells in health and diseaseNat Rev Immunol20088972673619172693
- RistichVLiangSZhangWWuJHoruzskoATolerization of dendritic cells by HLA-GEur J Immunol20053541133114215770701
- LeMaoultJCaumartinJDaouyaMImmune regulation by pretenders: cell-to-cell transfers of HLA-G make effector T cells act as regulatory cellsBlood200710952040204817077329
- ChiesaSMorbelliSMorandoSMesenchymal stem cells impair in vivo T-cell priming by dendritic cellsProc Natl Acad Sci U S A201110842173841738921960443
- MorettaLMorettaANatural killer cell immune regulation: Coordination of immune function in tissueNatural Killer Cells: Basic Science and Clinical ApplicationsMichaelT LotzeAngusW ThompsonLondonElsevier2010433444
- ChenPMYenMLLiuKJSytwuHKYenBLImmunomodulatory properties of human adult and fetal multipotent mesenchymal stem cellsJ Biomed Sci2011184921762539
- SpaggiariGMCapobiancoAAbdelrazikHBecchettiFMingariMCMorettaLMesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2Blood200811131327133317951526
- SessaregoNParodiAPodestàMMultipotent mesenchymal stromal cells from amniotic fluid: solid perspectives for clinical applicationHaematologica200893333934618268281
- MacatoniaSEHoskenNALittonMDendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cellsJ Immunol199515410507150797730613
- ShiYSuJRobertsAIShouPRabsonABRenGHow mesenchymal stem cells interact with tissue immune responsesTrends Immunol201233313614322227317
- RenGZhangLZhaoXMesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxideCell Stem Cell20082214115018371435
- RenGZhaoXZhangLInflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppressionJ Immunol201018452321232820130212
- TipnisSViswanathanCMajumdarASImmunosuppressive properties of human umbilical cord-derived mesenchymal stem cells: role of B7-H1 and IDOImmunol Cell Biol201088879580620386557
- PetroffMGImmune interactions at the maternal-fetal interfaceJ Reprod Immunol2005681–211316236361
- AggarwalSPittenger M. Human mesenchymal stem cells modulate allogeneic immune cell responsesBlood200510541815182215494428
- SotiropoulouPAPerezSAGritzapisADBaxevanisCNPapamichailMInteractions between human mesenchymal stem cells and natural killer cellsStem Cells20062417885
- SpaggiariGMCapobiancoAAbdelrazikHBecchettiFMingariMCMorettaLMesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2Blood200811131327133317951526
- McIntoshKZvonicSGarrettSThe immunogenicity of human adipose-derived cells: temporal changes in vitroStem Cells20062451246125316410391
- PuissantBBarreauCBourinPImmunomodulatory effect of human adipose tissue-derived adult stem cells: comparison with bone marrow mesenchymal stem cellsBr J Haematol2005129111812915801964
- ChenKWangDDuWTHuman umbilical cord mesenchymal stem cells hUC-MSCs exert immunosuppressive activities through a PGE2-dependent mechanismClin Immunol2010135344845820207200
- EnnisJGötherströmCLe BlancKDaviesJEIn vitro immunologic properties of human umbilical cord perivascular cellsCytotherapy200810217418118368596
- WangMYangYYangDThe immunomodulatory activity of human umbilical cord blood-derived mesenchymal stem cells in vitroImmunology2009126222023218624725
- HausmannONPost-traumatic inflammation following spinal cord injurySpinal Cord200341736937812815368
- RohDHSeoMSChoiHSTransplantation of human umbilical cord blood or amniotic epithelial stem cells alleviates mechanical allodynia after spinal cord injury in ratsCell Transplant20132291577159023294734
- BroughtonBRLimRArumugamTVDrummondGRWallaceEMSobeyCGPost-stroke inflammation and the potential efficacy of novel stem cell therapies: focus on amnion epithelial cellsFront Cell Neurosci201266623335880
- DongWChenHYangXGuoLHuiGTreatment of intracerebral haemorrhage in rats with intraventricular transplantation of human amniotic epithelial cellsCell Biol Int201034657357720184556
- LiuYHVaghjianiVTeeJYAmniotic epithelial cells from the human placenta potently suppress a mouse model of multiple sclerosisPLoS One201274e3575822563398
- CargnoniAGibelliLTosiniATransplantation of allogeneic and xenogeneic placenta-derived cells reduces bleomycin-induced lung fibrosisCell Transplant200918440542219622228
- MoodleyYIlancheranSSamuelCHuman amnion epithelial cell transplantation abrogates lung fibrosis and augments repairAm J Respir Crit Care Med2010182564365120522792
- MurphySLimRDickinsonHHuman amnion epithelial cells prevent bleomycin-induced lung injury and preserve lung functionCell Transplant201120690992321092408
- CargnoniAResselLRossiDConditioned medium from amniotic mesenchymal tissue cells reduces progression of bleomycin-induced lung fibrosisCytotherapy201214215316121954836
