Abstract
Multiple myeloma and non-Hodgkin’s lymphoma remain the most common indications for high-dose chemotherapy and autologous peripheral blood stem cell rescue. While a CD34+ cell dose of 1 × 106/kg is considered the minimum required for engraftment, higher CD34+ doses correlate with improved outcome. Numerous studies, however, support targeting a minimum CD34+ cell dose of 2.0 × 106/kg, and an “optimal” dose of 4 to 6 × 106/kg for a single transplant. Unfortunately, up to 40% of patients fail to mobilize an optimal CD34+ cell dose using myeloid growth factors alone. Plerixafor is a novel reversible inhibitor of CXCR4 that significantly increases the mobilization and collection of higher numbers of hematopoietic progenitor cells. Two randomized multi-center clinical trials in patients with non-Hodgkin’s lymphoma and multiple myeloma have demonstrated that the addition of plerixafor to granulocyte-colony stimulating factor increases the mobilization and yield of CD34+ cells in fewer apheresis days, which results in durable engraftment. This review summarizes the pharmacology and evidence for the clinical efficacy of plerixafor in mobilizing hematopoietic stem and progenitor cells, and discusses potential ways to utilize plerixafor in a cost-effective manner in patients with these diseases.
Introduction
High-dose chemotherapy with autologous stem cell transplantation (ASCT) remains an important treatment modality for patients with non-Hodgkin’s lymphoma (NHL) and multiple myeloma (MM). For patients with aggressive NHL, mostly diffuse large B cell lymphoma, only 40% can be expected to remain disease-free after completing primary chemotherapy.Citation1 However, few patients with relapsed disease can be cured with conventional dose chemotherapy. For the majority of patients who relapse, ASCT remains the best curative option, particularly for patients with chemotherapy-sensitive disease, 40% to 50% of whom remain disease free.Citation2–Citation4 For patients with MM, while not curative, ASCT is associated with the highest complete remission rate, and improved progression-free and overall survival compared with convention chemotherapy.Citation5–Citation7 Furthermore, at least a subset of MM patients, who achieve less than a very good partial response, may benefit from tandem ASCT.Citation8,Citation9 While the role of ASCT in the context of treatment with novel antimyeloma drugs (such as lenalidomide and bortezomib), is debated and requires reinvestigation, it is expected that high-dose therapy with ASCT will remain an important part of front-line and relapsed MM for some years. Indeed, the best reported results for MM patients are with tandem cycles of high-dose chemotherapy with ASCT plus novel antimyeloma agents.Citation10,Citation11 Today, MM and NHL remain the most common indications for high-dose chemotherapy with ASCT.Citation12
Autologous hematopoietic stem and progenitor cells (HSPC) are infused following high-dose chemotherapy to mitigate prolonged or permanent myelosuppression, and can be harvested from the bone marrow or collected from peripheral blood by apheresis. The number of stem cells circulating in the peripheral blood, as defined by the number of CD34+ cells, however, accounts for <0.06% of white blood cells.Citation13,Citation14 Therefore, CD34+ cells residing in the bone marrow have to be mobilized into the circulation prior to apheresis. Over the past 2 decades, mobilized autologous peripheral blood stem cells (PBSC) have replaced bone marrow as the source of hematopoietic stem cells following high-dose chemotherapy, offering a number of advantages over bone marrow harvesting. Infusion of PBSC is associated with significantly shorter durations of neutropenia and thrombocytopenia, reduction in platelet transfusions, faster times to engraftment, and fewer days of hospitalization.Citation15–Citation17 Apheresis of PBSC is less invasive than bone marrow harvesting, and results in a significantly higher yield of CD34+ cells. The yield of CD34+ cells and the number of aphereses required for successful collection, however, is largely determined by the efficiency of stem cell mobilization. In addition, a number of studies have shown a significant correlation between CD34+ cell dose and rapidity of engraftment following high-dose chemotherapy.Citation18
The myeloid growth factors granulocyte macrophage-colony stimulating factor (GM-CSF) and more commonly granulocyte-colony stimulating factor (G-CSF) have been used either alone or in combination with chemotherapy in different mobilization strategies.Citation16 Both are approved for mobilizing PBSC. However, because a significant number of patients fail to mobilize sufficient PBSC with growth factors, particularly those requiring tandem cycles of high-dose chemotherapy,Citation8,Citation9 there has been increasing interest in methods to improve the yield of mobilized CD34+ cells. The CXCR4 antagonist plerixafor is the first noncytokine small molecule recently approved for mobilization of PBSC in combination with G-CSF in patients with NHL and MM. In the following sections, we summarize the current role of plerixafor for increasing mobilization of PBSC and discuss potential directions for its future use.
Cell dose requirement for autologous PBSC transplantation
Defining an optimal CD34+ target stem cell dose is important for identifying patients who mobilize poorly with current mobilizing strategies. The number of aphereses used to achieve the target cell dose also complicates the issue. In addition, it is possible that cells other than CD34+ cells in mobilized PBSC products may affect outcome beyond engraftment. Therefore, the optimal cell dose requirement for autologous transplantation remains uncertain.
While the minimum safest cell dose to provide engraftment appears to be 1.0 to 1.5 × 106 CD34+ cells/kg, delayed engraftment, particularly of platelets, is common, indicating that higher doses should be used.Citation19,Citation20 In 243 patients with NHL, MM, breast cancer and other solid tumors undergoing ASCT, the number of CD34+ cells infused significantly affected the kinetics of neutrophil and platelet engraftment.Citation21 CD34+ cell doses ≥2.5 × 106/kg resulted in more rapid neutrophil engraftment compared with lower doses, although no significant difference in neutrophil recovery was observed between doses of 2.5 to 5.0 × 106/kg and >5.0 × 106/kg.Citation21 The kinetics of platelet recovery, however, appeared more affected by higher doses of CD34+ cells. Patients receiving <2.5 × 106 CD34+ cells/kg had a significant delay in achieving platelet transfusion independence compared with patients receiving 2.5 to 5.0 × 106 CD34+ cells/kg, and patients in this intermediate dose group had slower recovery compared with those receiving >5.0 × 106 CD34+ cells/kg.Citation21 Similar results were also reported in a larger analysis of 692 patients.Citation22 Ninety-five percent of patients who received ≥2.5 × 106 CD34+ cells/kg achieved neutrophil recovery by day 18 post-transplant, although an incremental improvement in neutrophil recovery was observed with increased numbers of CD34+ cells, with “optimal” CD34+ cell doses likely to be greater as evidenced by 95% probabilities of neutrophil recovery at 15 and 13 days post-transplant in patients receiving ≥5.0 or 7.5 × 106 CD34+ cells/kg, respectively.Citation22 Similarly, for platelet recovery to ≥20 × 109/L, a CD34+ cell dose ≥5.0 × 106/kg appeared to be “optimal”, although doses >12 × 106 CD34+ cells/kg resulted in faster recovery.Citation22 Of note in the latter study, patients who required 2 apheresis procedures to collect >2.5 × 106 CD34+ cells/kg had slower platelet engraftment independent of the CD34+ cells dose, suggesting that qualitative differences in CD34+ cells collected may be important.Citation22 While other studies have shown that very high doses of CD34+ cells (>15 × 106/kg) can significantly reduce or eliminate severe thrombocytopenia and platelet transfusion requirements,Citation23,Citation24 it remains uncertain whether this additional benefit is outweighed by the increased resources required to collect such a large number of progenitors. Collectively, these data have been used to support practice patterns targeting a minimal CD34+ cell dose of 2.0 × 106/kg, and an “optimal” dose of 4 to 6 × 106/kg for a single transplant.Citation25,Citation26
There is emerging evidence that the immune cell content of mobilized PBSC products also affects autologous transplant outcomes. Patients achieving higher absolute lymphocyte counts by day 15 or 30 after ASCT have significantly longer survival.Citation27 Furthermore, the early recovery of lymphocytes after transplantation is related to the lymphocyte content of the infused HSPC product, including natural killer cell and CD8+ lymphocytes.Citation28,Citation29 Similarly, higher levels of CD80+ dendritic cells in the graft may be associated with improved survival.Citation30 While these observations require confirmation, they have important implications for mobilization strategies. For example, G-CSF mobilized PBSC contain more lymphocytes compared with G-CSF plus cyclophosphamide mobilized products.Citation27 Sargramostin (recombinant GM-CSF produced in yeast) plus cyclophosphamide mobilizes significantly more CD80+ dendritic cells compared with cyclophosphamide plus G-CSF.Citation30 As discussed below, plerixafor also appears to affect the immune cell content of the mobilized cell product and, therefore, might have a significant impact on long-term outcome of autologous transplantation.
Poor mobilization: risk factors and definitions
Clinical risk factors associated with impaired mobilization of stem cells
Several patient characteristics have been associated with reduced PBSC mobilization (); however these depend on the population studied. Also, for some factors it is not known whether they independently predict reduced mobilization, as not all factors have been included in multivariable analyses. Older age has been associated with poor mobilization in lymphoma and MM patients in some studies,Citation31–Citation35 but not in others.Citation36,Citation37 Among nearly 1000 MM patients, <12 months of prior therapy, a platelet count >200 × 109/L, and lower age were predictive of successful mobilization.Citation38 In other studies, prior use of melphalan,Citation39 interferon,Citation40 and radiation therapy,Citation41 elevated serum lactate dehydrogenase,Citation42 renal impairment, and lower albumin levelCitation43 were associated with reduced mobilization. Prolonged use of lenalidomide is consistently associated with failure to mobilize, particularly with G-CSF alone.Citation44–Citation47 Among patients receiving 3 or more cycles of lenalidomide, 25% failed to mobilize.Citation46 The risk of mobilization failure is also related to the duration of prior treatment with l enalidomide.Citation44 The failure to mobilize sufficient CD34+ cells after lenalidomide, however, may be largely overcome by mobilization using chemotherapy plus G-CSF.Citation46 On the other hand bortezomib does not appear to adversely affect PBSC mobilization.Citation48
Table 1 Factors associated with poor mobilization of stem cells in multiple myeloma and non-Hodgkin’s lymphoma patients
Among NHL patients, the type and extent of prior chemotherapy are important factors affecting CD34+ cell mobilization. Fludarabine is commonly used for treatment of indolent NHL and has been shown to severely affect PBSC mobilization.Citation49,Citation50 In addition, platinum- and etoposide-based regimens commonly used for salvage therapy increase the risk of mobilization failure.Citation51,Citation52 Age ≥60 years, platelet count <150 × 109/L, and marrow cellularity <30% negatively affect PBSC mobilization.Citation35 More recently, elevated serum ferritin levels have also been found to impair mobilization in both lymphoma and myeloma patients.Citation37
While many of the above risk factors have been associated with poor mobilization, their utility in making clinical decisions is somewhat limited as their ability to predict patients who will need additional strategies remains imprecise. Similarly, molecular biomarkers such as lower plasma levels of flt-3,Citation53 higher plasma stromal cell derived factor-1α (SDF-1α) levels, and higher CXCR4 expression on circulating CD34+ cellsCitation54 have been associated with poor mobilization, although prospective studies are needed to better define their role in identifying patients who might be difficult to mobilize.
Definition of poor mobilization of stem cells
The proportion of patients eligible for ASCT who fail to mobilize an adequate number of CD34+ cells using myeloid growth factors has been variably reported between 5% and 40%,Citation35,Citation55–Citation58 reflecting at least in part a lack of consensus on the definition of “poor mobilizers”. Poor mobilization has been variably defined based on CD34+ cell yield in apheresis products and/or on circulating CD34+ cells following cytokine stimulation. Confounding the definition, a graft anticipated to provide adequate recovery of marrow function at one center may be considered unacceptable in another.Citation59 For example, failure to reach target CD34+ cell yields between 1 and 3 × 106/kg have defined products unsuitable at individual centers.Citation60–Citation63 In addition, the number of aphereses and the blood volume processed also affect the CD34+ cell yield. While some centers perform several apheresis procedures if needed to collect the target number of CD34+ cells,Citation62 others do not;Citation60 and the blood volume processed for each collection has varied from 7 to 35 L.Citation64,Citation65 In one suggested classification based on CD34+ cell yield patients collecting >5 × 106 CD34+ cells/kg after multiple aphereses were classified as easily mobilizable; those collecting 1 to 5 × 106 CD34+ cells/kg as difficult to mobilize; and those collecting <1 × 106 CD34+ cells/kg as nonmobilizable.Citation66
While definitions of poor mobilizers based on CD34+ cell yield may be useful for developing endpoints for clinical trials, cell yield is estimated retrospectively after leukapheresis and does not permit early intervention strategies with the advent of new mobilizing agents such as plerixafor (see below). More recently, a retrospective analysis of 840 NHL and MM patients undergoing PBSC mobilization using cytokines with and without chemotherapy has provided a more precise, and potentially more useful, definition of poor mobilization based on circulating blood CD34+ cell counts after maximal G-CSF stimulation,Citation67 which correlate well with total CD34+ cells collected after 1 to 3 apheresis procedures.Citation13,Citation14 Patients with blood CD34+ cell counts <20/μL, comprising 15.3% of those studied were considered poor mobilizers. Patients with CD34+ cell levels between 11 and 19/μL were defined as “borderline” poor mobilizers (4.5%), those with CD34+ cell levels between 6 and 10/μL defined as “relative” poor mobilizers (5.8%), and those with CD34+ cell levels ≤5/μL were defined as “absolute” poor mobilizers (5.0%).Citation67 Importantly, all good and “borderline” poor mobilizers achieved the collection goal of 2.0 × 106 CD34+ cells/kg after apheresis, although a greater number of aphereses were required. On the other hand, only 77% of “relative” and 40% of “ absolute” poor mobilizers achieved the collection goal, albeit with multiple aphereses.Citation67 The definition of poor mobilizers in this way enables early identification of patients who are likely to mobilize poorly and prediction of those who may benefit from intervention using new mobilization strategies.
Plerixafor
Pharmacology and pharmacokinetics: metabolism, distribution, and excretion
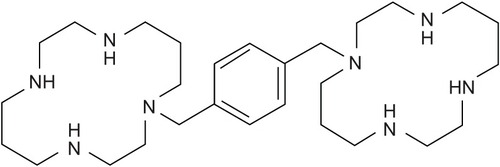
Plerixafor (AMD-3100)(1′-[1,4-phenylenebis (methylene)]-bis-1,4,8,11-tetra azacyclotetradecane) (C28H54N8; MW 502.79 g/mol) is a bicyclam () that reversibly blocks binding of SDF-1α to its cognate receptor CXCR4,Citation68,Citation69 an interaction critical to hematopoietic cell trafficking.Citation70,Citation71 Plerixafor was originally developed for the treatment of human immunodeficiency virus (HIV) infection as it was found to inhibit HIV-1 and HIV-2 viral replication. Plerixafor inhibits virus-cell entry by blocking CXCR4, which interacts with envelope glycoprotein gp120 of T lymphotropic HIV strains, leading to fusion of viral and cell membranes.Citation72 In initial phase I trials with plerixafor conducted as a prelude to investigation in HIV patients, unexpected significant leukocytosis with associated mobilization of hematopoietic progenitor cells was observed.Citation73,Citation74 While the poor oral absorption of plerixafor, related to its high positive charge at physiological pH, has limited its further development as an anti-HIV agent, a number of monocyclam derivatives with better solubility that block CXCR4 are currently under evaluation.Citation75
Stem cells express CXCR4 and bind to stromal cell SDF-1α in the bone marrow matrix, which with other adhesion molecules anchor stem cells within the niche.Citation76–Citation78 Mobilization of stem cells from bone marrow to peripheral blood is observed following SDF-1α peptide analogs,Citation79,Citation80 plerixafor,Citation81,Citation82 and the SDF-1 analog Met-SDF-1β,Citation80 clearly indicating that altering SDF-1α/CXCR4 signaling, most likely by CXCR4 receptor downmodulation, enhances trafficking out of the marrow to the periphery.Citation79,Citation80 Plerixafor induces HSPC mobilization in mice,Citation81 dogs,Citation83 monkeys,Citation84 and humans,Citation81,Citation85–Citation87 and synergizes with G-CSF.Citation81,Citation82,Citation88–Citation90 Relevant to its potential use, however, neoplastic hematopoietic cells also express CXCR4 and interact with stromal cells expressing SDF-1α, and may thus be co-mobilizedCitation91 which may be particularly important for patients with acute leukemia. On December 15, 2008, the Food and Drug Administration (FDA) approved plerixafor (Mozobil®; Genzyme Corporation, Cambridge, MA) for mobilizing PBSC in combination with G-CSF for collection and subsequent ASCT in patients with NHL and MM.
Preparation
Mozobil is available in single-use vials containing 1.2 mL of a 20 mg/mL solution containing 24 mg of plerixafor and 5.9 mg of sodium chloride in sterile water for subcutaneous (SC) injection. Plerixafor is intended for daily administration after patients have received G-CSF once daily for 4 days. Plerixafor should be administered approximately 11 hours prior to initiation of apheresis for up to 4 consecutive days.
Pharmacokinetics
Plerixafor is rapidly absorbed following SC injection. In both normal volunteers and patients with NHL and MM, peak plasma concentrations are reached within 30 to 60 minutes independent of dose.Citation74 The maximum plasma concentrations of plerixafor follow linear dose-dependent kinetics in the dose range of 40 to 240 μg/kg, reaching average maximum concentrations of 121 to 854 ng/mL.Citation90 Similarly, dose-dependent kinetics for the area under the curve (AUC) are also observed with AUC from zero to 10 hours (AUC0→10) ranging from averages of 397 to 3183 ng/h/mL following 40 to 240 μg/kg doses. In a population pharmacokinetic analysis in volunteers and patients, a two-compartment disposition model with first order absorption and elimination was found to best describe the plerixafor concentration-time profile. The distribution half-life (t1/2α) was estimated to be 0.3 hours with a terminal population half-life (t1/2β) of 5.3 hours in subjects with normal renal function. The apparent volume of distribution of plerixafor in healthy human volunteers is 0.28 to 0.33 L/kg after a single SC dose in the dose ranges 40 to 240 μg/kg, and is similar in patients with MM and NHL,Citation92 indicating that it is largely confined to the extravascular fluid space and not metabolized.
Plerixafor is mainly eliminated through renal excretion without hepatic metabolism. Approximately 70% of the dose is excreted unchanged in the urine during the first 24 hours. A phase I pharmacokinetic study in otherwise healthy subjects with varying degrees of renal impairment showed an inverse correlation between plerixafor clearance and renal function as determined by the creatinine clearance (CrCl).Citation93 Compared to controls (CrCl > 90 mL/min) the mean AUC from time 0 to 24 hours of plerixafor was 7%, 32%, and 39% higher in subjects with mild (CrCl 51–80 mL/min), moderate (CrCl 31–50 mL/min), and severe (CrCl < 31 mL/min, not requiring dialysis) renal insufficiency, respectively, following a single dose of 240 μg/kg.Citation93 Since some MM patients requiring ASCT have renal impairment, these data indicate the need for dose reduction in patients with moderate to severe renal insufficiency. A plerixafor dose reduction to 160 μg/kg in patients with CrCl ≤ 50 mL/min is expected to result in exposure similar to a dose of 240 μ/kg in patients with normal or mildly impaired renal function.Citation93
Clinical efficacy of plerixafor
The vast majority of studies have investigated the efficacy of plerixafor in enhancing PBSC mobilization after 4 days of G-CSF. An initial phase II trial randomized 25 patients with MM and NHL to receive 10 μg/kg/day G-CSF, starting 4 days before apheresis, with or without 240 μg/kg plerixafor 6 hours before each apheresis on subsequent days.Citation89 After a 13- to 17-day washout, patients underwent a second mobilization attempt using the opposite regimen. Peripheral blood CD34+ cells increased a median of 2.9-fold (range, 1.1–13) within 6 hours after plerixafor injection, which translated into higher CD34+ cells collected and fewer aphereses. Nine of 25 patients failed to collect ≥2 × 106 CD34+ cells/kg after G-CSF alone, while no patient receiving plerixafor plus G-CSF failed to collect this minimum number regardless of the sequence of the mobilization regimen. Only 8/25 patients mobilized with G-CSF alone collected ≥5 × 106 CD34+ cells/kg, compared with 20/25 patients following G-CSF plus plerixafor.Citation89 Compassionate use protocols were approved in the United States and Europe allowing plerixafor to be used in combination with G-CSF in NHL, Hodgkin’s disease, and MM patients who failed to mobilize sufficient CD34+ cells with G-CSF or who were at high risk of failure.Citation94–Citation100 summarizes results for plerixafor used in patients who are poor mobilizers.
Table 2 Protocols evaluating plerixafor for mobilization of stem cells in poor mobilizers
Two randomized double blind placebo-controlled, phase III clinical trials in patients requiring ASCT for MM (n = 302)Citation101 and NHL (n = 298)Citation102 have been reported (summarized in ), leading to FDA approval of plerixafor for mobilization of PBSC in combination with G-CSF in patients with these diseases. In both trials, patients were randomized to receive G-CSF for 4 days prior to starting apheresis on the fifth day, or G-CSF 10 μg/kg/day for 4 days with plerixafor 240 μg/kg added the night before each apheresis started on the fifth day. A maximum of 4 apheresis procedures was allowed, with 3 blood volumes processed per procedure, to collect the target number of CD34+ cells. In both studies, patients could not have failed previous mobilization and were not at high risk for mobilization failure by virtue of having previously received 2 or more cycles of alkylator-based therapy or radiation to >50% of the pelvis for MM patients,Citation101 and lymphomatous involvement of >20% of the bone marrow involvement or pelvic radiation for NHL patients,Citation102 respectively. Both trials were multicentered, providing confidence that the clinical results may be generalized to populations similar to those included.
Table 3 Summary of phase III trials evaluating plerixafor in MM and NHL
In the phase-III trial involving MM patients,Citation101 the primary endpoint was the proportion of patients who collected 6 × 106/kg or more CD34+ cells in 2 apheresis days or less. Secondary endpoints included the proportion of patients who collected ≥6 × 106 CD34+ cells/kg within four aphereses, the number of apheresis days required to reach ≥6 × 106 CD34+ cells/kg, and engraftment kinetics. G-CSF plus phoma; MM, multiple myeloma. plerixafor resulted in more patients yielding ≥6 × 106 CD34+ cells/kg within 2 aphereses compared with G-CSF plus placebo (71.6% vs 34.4%; P < 0.001). A median of 1 apheresis day was required to collect ≥6 × 106 CD34+ cells/kg with G-CSF plus plerixafor compared with 4 days with G-CSF and placebo (P < 0.001). For the secondary mobilization endpoint, more patients in the plerixafor group collected ≥6 × 106 CD34+ cells/kg in four or fewer apheresis days compared to the placebo group (75.7% vs 51.3%; P < 0.001). Patients in the plerixafor group also collected a significantly higher total number of CD34+ cells. All patients receiving plerixafor collected ≥2 × 106 CD34+ cells/kg, the minimum to proceed with transplantation, while 4.6% of those mobilized with G-CSF and placebo failed and required rescue mobilization with plerixafor. More MM patients in the plerixafor group received planned tandem transplantations (21.6%) compared with those in the placebo group (15.6%).
In the phase III trial of NHL patients,Citation102 the primary endpoint of the study was the proportion of patients who collected ≥5 × 106 CD34+ cells/kg in 4 apheresis days. Among patients receiving G-CSF plus plerixafor, 59.3% achieved this target compared with 19.6% in the placebo group (P < 0.001). A greater proportion of patients in the plerixafor group also collected at least 2 × 106 CD34+ cells/kg in four apheresis days (86.7% vs 47.3%; P < 0.001). The time for collecting the minimum number of CD34+ cells was also achieved in significantly fewer apheresis days. Of 10 patients in the plerixafor group who failed to mobilize, 4 were successfully remobilized with plerixafor and G-CSF in an open label rescue phase of the trial, while 33 of 52 (64%) from the placebo group failing to mobilize achieved ≥2 × 106 CD34+ cells/kg following remobilization with plerixafor and G-CSF.
In both randomized trials, engraftment kinetics and durability were reported to be similar for both the plerixafor and placebo groups in patients who underwent transplantation.Citation101,Citation102 In a post hoc analysis, there was a significant trend between CD34+ cell dose and the proportion of patients maintaining a platelet count of ≥150 × 109/L on and beyond day 100 for NHL patients, but only at day 100 after transplantation for MM patients.Citation103 While the clinical significance of this finding remains uncertain, it may reflect better marrow reserves in patients who receive a larger dose of CD34+ cells, which, in turn, may result in improved tolerance of subsequent treatments in patients who relapse, particularly those with MM where relapse is almost universal. As noted above, both NHL and MM patients who received plerixafor yielded higher CD34+ cell collections,Citation101,Citation102 and this may have important implications for subsequent management.
Side effects and adverse reactions
Plerixafor is generally safe and well tolerated. In the two randomized trials in patients with MM and NHL,Citation101,Citation102 the most common adverse events that were considered related to plerixafor were injection site erythema (20%–29%), fatigue (8%), and gastrointestinal symptoms, including nausea (16%–17%), vomiting (5%), diarrhea (18%–38%), abdominal pain (6%), and flatulence (5%). Mild to moderate systemic reactions including urticaria, periorbital swelling, dyspnea, and hypoxia were observed in <1% of patients approximately 30 minutes after plerixafor administration and responded to treatments or resolved spontaneously. Symptoms were generally mild with good patient compliance and treatment only rarely led to discontinuation of drug. In the MM trial, only 1 patient receiving plerixafor discontinued treatment after 3 doses because of diarrhea and fatigue, and 2 patients in the placebo group discontinued treatment because of an enlarged spleen in 1 patient and nausea, vomiting, and abdominal pain in the other.Citation101 In the NHL study, no patient discontinued treatment because of plerixafor-related side effects.Citation102 No interactions of plerixafor with other drugs are known.
A clinical perspective on the use of plerixafor for mobilization of autologous stem cells
The safety and efficacy of plerixafor in mobilizing autologous PBSC is clinically proven, and from a scientific perspective, the results support the routine use of plerixafor in combination with G-CSF for mobilizing PBSC in all patients with NHL and MM undergoing ASCT. A significant limitation to routine use of plerixafor, however, remains the cost, particularly as one-third or more of unselected patients will collect an adequate number of CD34+ cells within two apheresis days using G-CSF alone. A US nationwide inpatient sample study recently reported the average cost of an autologous PBSC transplant performed between 2000 and 2001 for NHL and MM patients, including collection and cryopreservation of stem cells, was approximately US$51,000, with significantly higher costs if complications occurred.Citation104 The wholesale price of a vial (20 mg/1.2 mL) of plerixafor is approximately US$7,500.Citation105 Therefore, for an average adult, a 2-day course of plerixafor would cost US$15,000. Furthermore, plerixafor plus G-CSF mobilization has also been reported to lead to an apheresis product with a lower ratio of CD34+ cells to total nucleated cells, resulting in an increased requirement for storage bags and, in turn, cost of PBSC storage.Citation106 A cost-effectiveness analysis demonstrating that the high cost of plerixafor can be offset by a decreased number of aphereses required to collect a target CD34+ cell dose is likely required before routine use of plerixafor can be recommended for all patients. While one study has shown that the cost of plerixafor plus G-CSF mobilization is similar to that of cyclophosphamide and G-CSF mobilization with less morbidity,Citation107 an analysis comparing with G-CSF alone is not currently available.
An alternative, and possibly more cost effective strategy, may be to reserve the use of plerixafor to patients who are “poor mobilizers”. As reviewed above and summarized in , 63% to 76% of patients who fail to collect a sufficient CD34+ cell dose will collect successfully following a remobilization attempt with G-CSF plus plerixafor. However, such a second mobilization attempt would be expected to significantly add to total cost. While clinical risk factors are significantly associated with mobilization failure, their predictive value is not sufficiently strong. A more practical approach may be to begin mobilization with G-CSF alone in the standard manner, assess peripheral blood CD34+ cell counts on the fourth day of mobilization, and, if the CD34+ cell count is less than 10 to 20/μL, add plerixafor on the evening of the fourth day onward, beginning apheresis on the fifth day as initially planned. The validity of this patient-targeted, decision-making algorithm has recently been shown to be potentially cost saving.Citation108,Citation109
Future directions
To date, most research investigating plerixafor for mobilization has largely focused on increasing the number of CD34+ cells mobilized and collected by apheresis compared to G-CSF alone. However, there is increasing data showing that the plerixafor-mobilized PBSC product is also qualitatively different. Plerixafor in combination with G-CSF appears to mobilize more primitive HSPC with higher repopulation potential than G-CSF alone.Citation110 Furthermore, HSPC mobilized with plerixafor plus G-CSF have different microRNA and gene expression profiles compared to those mobilized with G-CSF alone.Citation111 The clinical significance of these qualitative differences remains unknown.
In addition to HSPC content, the immunological cell composition of apheresis products mobilized with plerixafor requires further investigation. As reviewed above, the lymphocyte and dendritic cell content of PBSC products may significantly affect relapse after ASCT.Citation28–Citation30 As PBSC products mobilized following plerixafor have been shown to contain more lymphocytesCitation112 and dendritic cells,Citation31,Citation113 the ability to modify long-term outcome requires further study. In particular, to further increase dendritic cell content, investigation of the combination of plerixafor and GM-CSF might also be an additional avenue of investigation.
Although plerixafor is approved for use only in NHL and MM patients, a significant proportion of patients with Hodgkin’s disease also mobilize poorly and could be candidates for plerixafor. Such patients were included in the previous series but not in the registration trials of plerixafor. Finally, patients with resistant and relapsed germ cell tumors have a good outcome with tandem ASCT.Citation114 Since these patients are usually exposed to platinum drugs in primary therapy, many are difficult to mobilize following G-CSF alone. I nvestigation of plerixafor in this population is indicated.
Beyond its use in mobilization of HSPC for transplantation, the appreciation that CXCR4 chemokine receptors are expressed by neoplastic cells from patients with acute and chronic leukemias, as well as a variety of solid tumors, has raised interest in the potential therapeutic role of plerixafor in a variety of cancers.Citation91,Citation115,Citation116 Within the tumor microenvironment (including outside the bone marrow), the interaction of SDF-1α on stromal cells with CXCR4 on tumor cells has been shown to promote growth and survival signals to a variety of cancer cell types,Citation117,Citation118 facilitate tumor progression by recruiting endothelial progenitor cells for tumor angiogenesis,Citation118 and confer cell adhesion-mediated drug resistance to both solid tumor cells and leukemia.Citation119 By blocking CXCR4-SDF-1α interactions in the microenvironment, a rationale for investigating plerixafor in the treatment of acute myeloid leukemia,Citation120–Citation123 BCR-ABL+ leukemia,Citation124 chronic lymphocytic leukemia,Citation91 mantle cell lymphoma,Citation125 multiple myeloma,Citation126 breast cancer,Citation118 and lung cancer,Citation127 has been reported. Clinical investigation of plerixafor in combination with chemotherapeutic agents will be important to determine the efficacy of the novel approach of CXCR4 blockade in the treatment of in these diseases.
Conclusions
Plerixafor is a novel small molecule inhibitor of CXCR4 and has been shown to significantly increase the mobilization and collection of higher numbers of PBSC in 2 randomized trials, and is now approved in combination with G-CSF for mobilization in NHL and MM patients undergoing ASCT. Although well tolerated and efficacious, use of plerixafor in all such patients undergoing transplantation is limited by high cost. Pre-emptive strategies that target only patients who mobilize poorly with G-CSF may result in more cost-effective utilization of plerixafor. Investigation of plerixafor in patient populations other than those approved for its use, including Hodgkin’s disease and patients with germ cell tumors undergoing transplantation, is important, as many of these patients tend to mobilize poorly because of prior therapy. In addition, investigation of qualitative differences in PBSC products mobilized with plerixafor compared with G-CSF alone will lead to better understanding of the significance of graft composition in the autologous setting and may lead to better long-term outcomes in patients undergoing ASCT.
Disclosure
The authors report no conflict of interest in this work.
References
- FisherRIGaynorERDahlbergSComparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin’s lymphomaN Engl J Med199332814100210067680764
- GuglielmiCGomezFPhilipTTime to relapse has prognostic value in patients with aggressive lymphoma enrolled onto the Parma trialJ Clin Oncol19981610326432699779700
- MartelliMVignettiMZinzaniPLHigh-dose chemotherapy followed by autologous bone marrow transplantation versus dexamethasone, cisplatin, and cytarabine in aggressive non-Hodgkin’s lymphoma with partial response to front-line chemotherapy: a prospective randomized italian multicenter studyJ Clin Oncol19961425345428636768
- PhilipTGuglielmiCHagenbeekAAutologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphomaN Engl J Med199533323154015457477169
- AttalMHarousseauJLStoppaAMA prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du MyelomeN Engl J Med1996335291978649495
- ChildJAMorganGJDaviesFEHigh-dose chemotherapy with hematopoietic stem-cell rescue for multiple myelomaN Engl J Med2003348191875188312736280
- PalumboABringhenSPetrucciMTIntermediate-dose melphalan improves survival of myeloma patients aged 50 to 70: results of a randomized controlled trialBlood2004104103052305715265788
- AttalMHarousseauJLFaconTSingle versus double autologous stem-cell transplantation for multiple myelomaN Engl J Med2003349262495250214695409
- CavoMTosiPZamagniEProspective, randomized study of single compared with double autologous stem-cell transplantation for multiple myeloma: Bologna 96 clinical studyJ Clin Oncol200725172434244117485707
- PalumboAGayFFalcoPBortezomib as induction before autologous transplantation, followed by lenalidomide as consolidation-maintenance in untreated multiple myeloma patientsJ Clin Oncol201028580080720048187
- Van RheeFSzymonifkaJAnaissieETotal Therapy 3 for multiple myeloma: prognostic implications of cumulative dosing and premature discontinuation of VTD maintenance components, bortezomib, thalidomide, and dexamethasone, relevant to all phases of therapyBlood201011681220122720501894
- PasquiniMCHeKPerezWSCIBMTR summary slides part 1CIBMTR Newsletters20061257
- FruehaufSHaasRConradtCPeripheral blood progenitor cell (PBPC) counts during steady-state hematopoiesis allow to estimate the yield of mobilized PBPC after filgrastim (R-metHuG-CSF)-supported cytotoxic chemotherapyBlood1995859261926267537123
- FruehaufSSchmittKVeldwijkMRPeripheral blood progenitor cell (PBPC) counts during steady-state haemopoiesis enable the estimation of the yield of mobilized PBPC after granulocyte colony-stimulating factor supported cytotoxic chemotherapy: an update on 100 patientsBr J Haematol1999105378679410354148
- SchmitzNLinchDCDregerPRandomised trial of filgrastim-mobilised peripheral blood progenitor cell transplantation versus autologous bone-marrow transplantation in lymphoma patientsLancet199634789983533578598700
- ToLBHaylockDNSimmonsPJJuttnerCAThe biology and clinical uses of blood stem cellsBlood1997897223322589116266
- WeaverCHBucknerCDCurtisLHEconomic evaluation of filgrastim, sargramostim, and sequential sargramostim and filgrastim after myelosuppressive chemotherapyBone Marrow Transplant200229215916411850711
- SienaSSchiavoRPedrazzoliPCarlo-StellaCTherapeutic relevance of CD34 cell dose in blood cell transplantation for cancer therapyJ Clin Oncol20001861360137710715309
- DesikanKRTricotGMunshiNCPreceding chemotherapy, tumour load and age influence engraftment in multiple myeloma patients mobilized with granulocyte colony-stimulating factor aloneBr J Haematol2001112124224711167811
- WattsMJSullivanAMLeverettDBack-up bone marrow is frequently ineffective in patients with poor peripheral-blood stem-cell mobilizationJ Clin Oncol1998164155415609552065
- BensingerWAppelbaumFRowleySFactors that influence collection and engraftment of autologous peripheral-blood stem cellsJ Clin Oncol19951310254725557595706
- WeaverCHHazeltonBBirchRAn analysis of engraftment kinetics as a function of the CD34 content of peripheral blood progenitor cell collections in 692 patients after the administration of myeloablative chemotherapyBlood19958610396139697579367
- BenedettiGPatoiaLGigliettiAAlessioMPelicciPGrignaniFVery large amounts of peripheral blood progenitor cells eliminate severe thrombocytopenia after high-dose melphalan in advanced breast cancer patientsBone Marrow Transplant199924997197910556956
- KettererNSallesGRabaMHigh CD34(+) cell counts decrease hematologic toxicity of autologous peripheral blood progenitor cell transplantationBlood1998919314831559558369
- GiraltSStadtmauerEAHarousseauJLInternational myeloma working group (IMWG) consensus statement and guidelines regarding the current status of stem cell collection and high-dose therapy for multiple myeloma and the role of plerixafor (AMD 3100)Leukemia200923101904191219554029
- GertzMAKumarSKLacyMQComparison of high-dose CY and growth factor with growth factor alone for mobilization of stem cells for transplantation in patients with multiple myelomaBone Marrow Transplant200943861962518997825
- HiwaseDKHiwaseSBaileyMBollardGSchwarerAPThe role of stem cell mobilization regimen on lymphocyte collection yield in patients with multiple myelomaCytotherapy200810550751718608354
- AttaEHde AzevedoAMMaiolinoAHigh CD8+ lymphocyte dose in the autograft predicts early absolute lymphocyte count recovery after peripheral hematopoietic stem cell transplantationAm J Hematol2009841212819006229
- PorrataLFMarkovicSNTimely reconstitution of immune competence affects clinical outcome following autologous stem cell transplantationClin Exp Med200442788515672944
- GazittYAkayCThomasC3rdNo polarization of type 1 or type 2 precursor dendritic cells in peripheral blood stem cell collections of non-hodgkin’s lymphoma patients mobilized with cyclophosphamide plus G-CSF, GM-CSF, or GM-CSF followed by G-CSFStem Cells Dev200615226927716646673
- GazittYCallanderNFreytesCOPeripheral blood stem cell mobilization with cyclophosphamide in combination with G-CSF, GM-CSF, or sequential GM-CSF/G-CSF in non-Hodgkin’s lymphoma patients: a randomized prospective studyJ Hematother Stem Cell Res20009573774811091498
- KotasekDShepherdKMSageREFactors affecting blood stem cell collections following high-dose cyclophosphamide mobilization in lymphoma, myeloma and solid tumorsBone Marrow Transplant19929111171347478
- KoumakisGVassilomanolakisMHatzichristouHPredictive factors affecting mobilization and peripheral blood stem cell (PBSC) collection using single apheresis (SA) for rescuing patients after highdose chemotherapy (HD.CHE) in various malignanciesBone Marrow Transplant1996186106510728971374
- FietzTRiegerKDimeoFBlauIWThielEKnaufWUStem cell mobilization in multiple myeloma patients: do we need an age-adjusted regimen for the elderly?J Clin Apher200419420220715597345
- HosingCSalibaRMAhlawatSPoor hematopoietic stem cell mobilizers: a single institution study of incidence and risk factors in patients with recurrent or relapsed lymphomaAm J Hematol200984633533719384931
- CanalesMAFernandez-JimenezMCMartinAIdentification of factors associated with poor peripheral blood progenitor cell mobilization in Hodgkin’s diseaseHaematologica200186549449811410412
- OzkurtZNYeginZASuyaniEFactors affecting stem cell mobilization for autologous hematopoietic stem cell transplantationJ Clin Apher20102528028620623783
- MorrisCLSiegelEBarlogieBMobilization of CD34+ cells in elderly patients (≥ 70 years) with multiple myeloma: influence of age, prior therapy, platelet count and mobilization regimenBr J Haematol2003120341342312580955
- BoccadoroMPalumboABringhenSOral melphalan at diagnosis hampers adequate collection of peripheral blood progenitor cells in multiple myelomaHaematologica200287884685012161361
- PutkonenMRauhalaAPelliniemiTTRemesKSepsis, low platelet nadir at mobilization and previous IFN use predict stem cell mobilization failure in patients with multiple myelomaCytotherapy20079654855417882719
- PrinceHMImrieKSutherlandDRPeripheral blood progenitor cell collections in multiple myeloma: predictors and management of inadequate collectionsBr J Haematol19969311421458611448
- EganKSinghVGidronAMehtaJCorrelation between serum lactate dehydrogenase and stem cell mobilizationBone Marrow Transplant2007401093193417846596
- NakasoneHKandaYUedaTRetrospective comparison of mobilization methods for autologous stem cell transplantation in multiple myelomaAm J Hematol2009841280981419862826
- KumarSDispenzieriALacyMQImpact of lenalidomide therapy on stem cell mobilization and engraftment post-peripheral blood stem cell transplantation in patients with newly diagnosed myelomaLeukemia20072192035204217581613
- MazumderAKaufmanJNiesvizkyRLonialSVesoleDJagannathSEffect of lenalidomide therapy on mobilization of peripheral blood stem cells in previously untreated multiple myeloma patientsLeukemia200822612801281 author reply 1281–128218033320
- PopatUSalibaRThandiRImpairment of filgrastim-induced stem cell mobilization after prior lenalidomide in patients with multiple myelomaBiol Blood Marrow Transplant200915671872319450756
- ParipatiHStewartAKCabouSCompromised stem cell mobilization following induction therapy with lenalidomide in myelomaLeukemia20082261282128418216870
- OakerveeHPopatRCavenaghJDUse of bortezomib as induction therapy prior to stem cell transplantation in frontline treatment of multiple myeloma: impact on stem cell harvesting and engraftmentLeuk Lymphoma200748101910192117917960
- LaszloDGalieniPRaspadoriDTozziMLauriaFMartinelliGFludarabine combination regimen severely affected peripheral blood stem cell mobilizationActa Haematol2004111422822915153717
- TournilhacOCazinBLepretreSImpact of frontline fudarabine and cyclophosphamide combined treatment on peripheral blood stem cell mobilization in B-cell chronic lymphocytic leukemiaBlood2004103136336512969985
- FordCDGreenWWarenskiSPetersenFBEffect of prior chemotherapy on hematopoietic stem cell mobilizationBone Marrow Transplant200433990190515004541
- OlivieriABrunoriMCapelliDSalvage therapy with an outpatient DHAP schedule followed by PBSC transplantation in 79 lymphoma patients: an intention to mobilize and transplant analysisEur J Haematol2004721101714962257
- GazittYLiuQHigh steady-state plasma levels of flt3-ligand in the peripheral blood is a good predictor for poor mobilization of CD34+ PBSC in patients undergoing high-dose chemotherapy and stem cell rescueJ Hematother Stem Cell Res20009228529310813543
- GazittYLiuQPlasma levels of SDF-1 and expression of SDF-1 receptor on CD34+ cells in mobilized peripheral blood of non-Hodgkin’s lymphoma patientsStem Cells2001191374511209089
- GordanLNSugrueMWLynchJWPoor mobilization of peripheral blood stem cells is a risk factor for worse outcome in lymphoma patients undergoing autologous stem cell transplantationLeuk Lymphoma200344581582012802919
- PavoneVGaudioFConsoleGPoor mobilization is an independent prognostic factor in patients with malignant lymphomas treated by peripheral blood stem cell transplantationBone Marrow Transplant200637871972416518434
- AkhtarSWeshiAERahalMFactors affecting autologous peripheral blood stem cell collection in patients with relapsed or refractory diffuse large cell lymphoma and Hodgkin lymphoma: a single institution result of 168 patientsLeuk Lymphoma200849476977818398746
- PusicIJiangSYLanduaSImpact of mobilization and remobilization strategies on achieving sufficient stem cell yields for autologous transplantationBiol Blood Marrow Transplant20081491045105618721768
- KessingerASharpJGThe whys and hows of hematopoietic progenitor and stem cell mobilizationBone Marrow Transplant200331531932912634722
- WattsMJIngsSJFlynnMDoddsDGoldstoneAHLinchDCRemobilization of patients who fail to achieve minimal progenitor thresholds at the first attempt is clinically worthwhileBr J Haematol2000111128729111091215
- FraipontVSautoisBBaudouxESuccessful mobilization of peripheral blood HPCs with G-CSF alone in patients failing to achieve sufficient numbers of CD34+ cells and/or CFU-GM with chemotherapy and G-CSFTransfusion200040333934710738037
- WeaverCHTauerKZhenBSecond attempts at mobilization of peripheral blood stem cells in patients with initial low CD34+ cell yieldsJ Hematother1998732412499621257
- BasheyACorringhamSGilpinESimultaneous administration of G-CSF and GM-CSF for re-mobilization in patients with inadequate initial progenitor cell collections for autologous transplantationCytotherapy20002319520012042042
- KessingerAArmitageJOLandmarkJDSmithDMWeisenburgerDDAutologous peripheral hematopoietic stem cell transplantation restores hematopoietic function following marrow ablative therapyBlood19887137237272894230
- ComenzoRLMalachowskiMEMillerKBLarge-volume leukapheresis for collection of mononuclear cells for hematopoietic rescue in Hodgkin’s diseaseTransfusion199535142457998067
- StiffPJManagement strategies for the hard-to-mobilize patientBone Marrow Transplant199923Suppl 2S29S3310335874
- WuchterPRanDBrucknerTPoor mobilization of hematopoietic stem cells-definitions, incidence, risk factors, and impact on outcome of autologous transplantationBiol Blood Marrow Transplant201016449049919925876
- GerlachLOSkerljRTBridgerGJSchwartzTWMolecular interactions of cyclam and bicyclam non-peptide antagonists with the CXCR4 chemokine receptorJ Biol Chem200127617141531416011154697
- HatseSPrincenKBridgerGDe ClercqEScholsDChemokine receptor inhibition by AMD3100 is strictly confined to CXCR4FEBS Lett20025271–325526212220670
- LapidotTKolletOThe essential roles of the chemokine SDF-1 and its receptor CXCR4 in human stem cell homing and repopulation of transplanted immune-deficient NOD/SCID and NOD/SCID/B2m(null) miceLeukemia200216101992200312357350
- PeledAPetitIKolletODependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4Science199928354038458489933168
- EsteJATelentiAHIV entry inhibitorsLancet20073709581818817617275
- De ClercqEThe bicyclam AMD3100 storyNat Rev Drug Discov20032758158712815382
- HendrixCWFlexnerCMacFarlandRTPharmacokinetics and safety of AMD-3100, a novel antagonist of the CXCR-4 chemokine receptor, in human volunteersAntimicrob Agents Chemother20004461667167310817726
- PetterssonSPerez-NuenoVIMenaMPNovel monocyclam derivatives as HIV entry inhibitors: Design, synthesis, anti-HIV evaluation, and their interaction with the CXCR4 co-receptorChem Med Chem2010581272128120533501
- NilssonSKJohnstonHMCoverdaleJASpatial localization of transplanted hemopoietic stem cells: inferences for the localization of stem cell nichesBlood20019782293229911290590
- LordBITestaNGHendryJHThe relative spatial distributions of CFUs and CFUc in the normal mouse femurBlood197546165721131427
- LordBIWrightEGSpatial organisation of CFU-S proliferation regulators in the mouse femurLeuk Res198486107310836513579
- PelusLMBianHFukudaSWongDMerzoukASalariHThe CXCR4 agonist peptide, CTCE-0021, rapidly mobilizes polymorphonuclear neutrophils and hematopoietic progenitor cells into peripheral blood and synergizes with granulocyte colony-stimulating factorExp Hematol200533329530715730853
- ShenHChengTOlszakICXCR-4 desensitization is associated with tissue localization of hemopoietic progenitor cellsJ Immunol200116685027503311290783
- BroxmeyerHEOrschellCMClappDWRapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonistJ Exp Med200520181307131815837815
- LilesWCRodgerEBroxmeyerHEAugmented mobilization and collection of CD34+ hematopoietic cells from normal human volunteers stimulated with granulocyte-colony-stimulating factor by single-dose administration of AMD3100, a CXCR4 antagonistTransfusion200545329530015752146
- BurroughsLMielcarekMLittleMTDurable engraftment of AMD3100-mobilized autologous and allogeneic peripheral-blood mononuclear cells in a canine transplantation modelBlood2005106124002400816105977
- LarochelleAKrouseAMetzgerMAMD3100 mobilizes hematopoietic stem cells with long-term repopulating capacity in nonhuman primatesBlood200610793772377816439684
- LilesWCBroxmeyerHERodgerEMobilization of hematopoietic progenitor cells in healthy volunteers by AMD3100, a CXCR4 antagonistBlood200310282728273012855591
- DevineSMFlomenbergNVesoleDHRapid mobilization of CD34+ cells following administration of the CXCR4 antagonist AMD3100 to patients with multiple myeloma and non-Hodgkin’s lymphomaJ Clin Oncol20042261095110215020611
- GrignaniGPerissinottoECavalloniGCarnevale SchiancaFAgliettaMClinical use of AMD3100 to mobilize CD34+ cells in patients affected by non-Hodgkin’s lymphoma or multiple myelomaJ Clin Oncol2005231638713872 author reply 3872–387315923599
- PelusLMFukudaSChemokine-mobilized adult stem cells; defining a better hematopoietic graftLeukemia200822346647317972941
- FlomenbergNDevineSMDipersioJFThe use of AMD3100 plus G-CSF for autologous hematopoietic progenitor cell mobilization is superior to G-CSF aloneBlood200510651867187415890685
- CashenALopezSGaoFA phase II study of plerixafor (AMD3100) plus G-CSF for autologous hematopoietic progenitor cell mobilization in patients with Hodgkin lymphomaBiol Blood Marrow Transplant200814111253126118940680
- BurgerJAPeledACXCR4 antagonists: targeting the microenvironment in leukemia and other cancersLeukemia2009231435218987663
- StewartDASmithCMacFarlandRCalandraGPharmacokinetics and pharmacodynamics of plerixafor in patients with non-Hodgkin lymphoma and multiple myelomaBiol Blood Marrow Transplant2009151394619135941
- MacFarlandRHardMLScarboroughRBadelKCalandraGA pharmacokinetic study of plerixafor in subjects with varying degrees of renal impairmentBiol Blood Marrow Transplant20101619510119748593
- CalandraGMcCartyJMcGuirkJAMD3100 plus G-CSF can successfully mobilize CD34+ cells from non-Hodgkin’s lymphoma, Hodgkin’s disease and multiple myeloma patients previously failing mobilization with chemotherapy and/or cytokine treatment: compassionate use dataBone Marrow Transplant200841433133817994119
- WorelNRosskopfKNeumeisterPPlerixafor and granulocyte-colony-stimulating factor (G-CSF) in patients with lymphoma and multiple myeloma previously failing mobilization with G-CSF with or without chemotherapy for autologous hematopoietic stem cell mobilization: the Austrian experience on a named patient programTransfusion9282010 [Epub ahead of print]
- D’AddioACurtiAWorelNThe addition of plerixafor is safe and allows adequate PBSC collection in multiple myeloma and lymphoma patients poor mobilizers after chemotherapy and G-CSFBone Marrow Transplant5312010 [Epub ahead of print]
- DuarteRFShawBEMarinPPlerixafor plus granulocyte CSF can mobilize hematopoietic stem cells from multiple myeloma and lymphoma patients failing previous mobilization attempts: EU compassionate use dataBone Marrow Transplant3222010 [Epub ahead of print]
- TricotGCottler-FoxMHCalandraGSafety and efficacy assessment of plerixafor in patients with multiple myeloma proven or predicted to be poor mobilizers, including assessment of tumor cell mobilizationBone Marrow Transplant2010451636819543330
- HubelKFresenMMBasaraNPlerixafor with and without chemotherapy in poor mobilizers: results from the German compassionate use programBone Marrow Transplant10252010 [Epub ahead of print]
- BasakGWKnopinska-PuslusznyWMatuszakMHematopoietic stem cell mobilization with the reversible CXCR4 receptor inhibitor plerixafor (AMD3100): Polish compassionate use programAnn Hematol20101546167
- DiPersioJFStadtmauerEANademaneeAPlerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myelomaBlood2009113235720572619363221
- DiPersioJFMicallefINStiffPJPhase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for patients with non-Hodgkin’s lymphomaJ Clin Oncol200927284767477319720922
- StiffPJMicallefINNademaneeAPTransplanted CD34+ cell dose is associated with long-term platelet count following autologous hematopoietic stem cell transplant in patients with non-Hodgkin’s lymphoma and multiple myelomaASH Annual Meeting Abstracts2008112112175a
- JonesJAQazilbashMHShihYCCantorSBCooksleyCDEltingLSIn-hospital complications of autologous hematopoietic stem cell transplantation for lymphoid malignancies: clinical and economic outcomes from the Nationwide Inpatient SampleCancer200811251096110518286506
- PDRRed Book: Pharmacy’s Fundamental ReferenceMontvaleNJThompson Healthcare2009
- TanhehcoYCAdamskiJSellMPlerixafor mobilization leads to a lower ratio of CD34+ cells to total nucleated cells which results in greater storage costsJ Clin Apher201025420220820818715
- ShaughnessyPIslas-OhlmayerMMurphyJCost and clinical analysis of autologous hematopoietic stem cell mobilization with G-CSF and plerixafor compared to G-CSF and cyclophosphamideBiol Blood Marrow Transplant8302010 [Epub ahead of print]
- CostaLJAlexanderETHoganKRSchaubCFoutsTVStuartRKDevelopment and validation of a decision-making algorithm to guide the use of plerixafor for autologous hematopoietic stem cell mobilizationBone Marrow Transplant2011461646920383210
- CostaLJMillerANAlexanderETGrowth factor and patient-adapted use of plerixafor is superior to CY and growth factor for autologous hematopoietic stem cells mobilizationBone Marrow Transplant7122010 [Epub ahead of print]
- FruehaufSVeldwijkMRSeegerTA combination of granulocyte-colony-stimulating factor (G-CSF) and plerixafor mobilizes more primitive peripheral blood progenitor cells than G-CSF alone: results of a European phase II studyCytotherapy2009118992100119929463
- JinPWangERenJDifferentiation of two types of mobilized peripheral blood stem cells by microRNA and cDNA expression analysisJ Transl Med200863918647411
- DevineSMVijRRettigMRapid mobilization of functional donor hematopoietic cells without G-CSF using AMD3100, an antagonist of the CXCR4/SDF-1 interactionBlood2008112499099818426988
- GazittYFreytesCOAkayCBadelKCalandraGImproved mobilization of peripheral blood CD34+ cells and dendritic cells by AMD3100 plus granulocyte-colony-stimulating factor in non-Hodgkin’s lymphoma patientsStem Cells Dev200716465766617784839
- EinhornLHWilliamsSDChamnessABramesMJPerkinsSMAbonourRHigh-dose chemotherapy and stem-cell rescue for metastatic germ-cell tumorsN Engl J Med2007357434034817652649
- BurgerJAKippsTJCXCR4: a key receptor in the crosstalk between tumor cells and their microenvironmentBlood200610751761176716269611
- BurgerJABurkleAThe CXCR4 chemokine receptor in acute and chronic leukaemia: a marrow homing receptor and potential therapeutic targetBr J Haematol2007137428829617456052
- BurgerJATsukadaNBurgerMZvaiferNJDell’AquilaMKippsTJBlood-derived nurse-like cells protect chronic lymphocytic leukemia B cells from spontaneous apoptosis through stromal cell-derived factor-1Blood20009682655266311023495
- OrimoAGuptaPBSgroiDCStromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretionCell2005121333534815882617
- LiZWDaltonWSTumor microenvironment and drug resistance in hematologic malignanciesBlood Rev200620633334216920238
- JacobiAThiemeSLehmannRImpact of CXCR4 inhibition on FLT3-ITD-positive human AML blastsExp Hematol201038318019020035824
- LiesveldJLBechelliJRosellKEffects of AMD3100 on transmigration and survival of acute myelogenous leukemia cellsLeuk Res200731111553156317403536
- NerviBRamirezPRettigMPChemosensitization of acute myeloid leukemia (AML) following mobilization by the CXCR4 antagonist AMD3100Blood2009113246206621419050309
- TavorSEisenbachMJacob-HirschJThe CXCR4 antagonist AMD3100 impairs survival of human AML cells and induces their differentiationLeukemia200822122151515818769446
- DillmannFVeldwijkMRLaufsSPlerixafor inhibits chemotaxis toward SDF-1 and CXCR4-mediated stroma contact in a dose-dependent manner resulting in increased susceptibility of BCR-ABL+ cell to Imatinib and NilotinibLeuk Lymphoma200950101676168619657955
- KurtovaAVTamayoATFordRJBurgerJAMantle cell lymphoma cells express high levels of CXCR4, CXCR5, and VLA-4 (CD49d): importance for interactions with the stromal microenvironment and specifictargetingBlood2009113194604461319228923
- KatzBZTavorSThe stromal derived factor-1\CXCR4 axis–a legitimate therapeutic target in multiple myeloma?Leuk Lymphoma20095071067106819557626
- BurgerJAStewartDJCXCR4 chemokine receptor antagonists: perspectives in SCLCExpert Opin Investig Drugs2009184481490