Abstract
Age-related macular degeneration (AMD) is a devastating retinal disease that occurs in later life as the retinal pigment epithelium (RPE) cells die, with subsequent photoreceptor degeneration. In the past, RPE transplant surgeries gave evidence that AMD was potentially treatable, but it involved limited amounts of ocular tissue, and the complication rate was high. Then, stem cell transplants offered an unlimited supply of retinal precursors for endogenous repair and exogenous cell replacement. Debate continues as to which type of stem cell is most appropriate for treating AMD. The prospects include adult-derived progenitor stem cells (including progenitor cells from ocular tissues), hematopoietic stem cells, embryonic stem cells, and induced pluripotent stem cells. Now the therapy is expanding into phase I human trials. This review examines the collective research contributions toward a clinical model of AMD management with stem cells.
Age-related macular degeneration
“Difficult” is an apt word to describe age-related macular degeneration (AMD). The disease is difficult for patients because they lose central vision (only the macula is affected, and the rest of the retina is undamaged),Citation1 which is devastating. AMD is difficult for the eye care provider because few therapeutic treatments are available.Citation2 This disease is also a difficult challenge for those pursuing stem cell therapy because it involves not only the retinal pigmented epithelium (RPE), a monolayer of the retina, but also the complex photoreceptors and their myriad nervous connections.Citation3
The RPE has many functions, but the phagocytic role of the RPE is one that is essential for the renewal of photoreceptors.Citation3,Citation4 However, after decades of phagocytic activity, accumulation of the immense amount of damaging metabolic waste becomes an ever-increasing burden on RPE cells. As RPE cells are injured and die in the macula, AMD progresses, resulting in central vision loss. Usually, the first visible sign on the retina is a concentration of yellowish, globular deposits called drusen at the macula. Drusen are hyalinized material containing membrane-bound bodiesCitation5 in Bruch’s membrane, which is situated between the RPE and the choriocapillaris (a choroidal structure that supplies blood for the outer layers of the retina).
In addition to advancing age, there are other factors that are related to AMD. One of these is genetics, which is a well-known influence in this disease.Citation6 AMD has been shown to be a polygenic disorder, and more than half a dozen chromosomesCitation7,Citation8 and numerous proteins are associated with it.Citation9 The Y402H sequence in complement factor H has a strong association with AMD. However, several different alleles appear to increase the risk of AMD.Citation10 Other contributors are lifestyle factors such as diet, smoking, and ultraviolet ray exposure.Citation3
There are two forms of AMD. Dry AMD is evidenced by drusen deposition and degeneration of the outer retina, RPE, and choriocapillaris. Wet AMD is characterized by widespread atrophy and choroidal neovascularization (CNV) formation, in which blood vessels from the choriocapillaris grow into the normally avascular subretinal space. Formation of this membrane can cause hemorrhage, RPE detachment, scarring, and profound vision loss.
In a normal retina, growth factors secreted by the RPE are involved in inhibiting the abnormal growth of vessels (angiogenesis) in the choroid. These growth factors also support the RPE and choroid, whereas brain-derived neurotrophic growth factor may support differentiation of the RPE.Citation11,Citation12 Pigment epithelium-derived growth factor may maintain angiogenic balance by prohibiting angiogenesis.Citation12,Citation13 Proangiogenic vascular endothelial growth factor-A (VEGF-A) promotes survival of choriocapillaris endothelial cells,Citation14 and angiopoietin 1 and 2 may stabilize new blood vessels and regulate vascular permeability.Citation15 Thus, damage to the RPE causes choroidal changes such as angiogenesis and ultimately the degeneration of photoreceptors.Citation16
The mechanism for CNV formation appears to be multifaceted. Usually, it is associated with a defect in Bruch’s membrane caused by atrophy or macrophage activity.Citation17 One plausible explanation for CNV formation is that the age-related lipid drusen deposits and inflammation of Bruch’s membrane cause decreased diffusion of oxygen and growth factors.Citation18 Deterioration and disorganization of Bruch’s membrane contribute to the breakdown of the blood–retinal barrier between the choroid and retina.Citation19 Hypoxia and decreased permeability lead to the overexpression of growth factors and the accumulation of VEGFs, which stimulates angiogenesis.Citation20 Hypoxia also attracts macrophages and promotes choriocapillaris atrophy.Citation14,Citation17 Bone marrow-derived endothelial precursor cells are recruited to the region and contribute to CNV formation as well.Citation21
In a diseased eye, oxidative stress may trigger changes in cell surface molecules that functionally impair circulating hematopoietic stem cells (HSCs) derived from bone marrow. Decreased HSC function has been linked to CNV development.Citation22 Bone marrow-derived mesenchymal stem cells express matrix metalloproteinase (MMP13). Increased expression of proangiogenic MMP13 has also been linked to CNV development. In both cases, the molecular mechanisms involved appear to be multifaceted and are not fully understood. If these responses can be therapeutically controlled, CNV formation might be subdued.Citation23
Surgical removal of CNV is possible, but it may recur. Common surgical complications include hemorrhage and retinal detachment. The use of photodynamic therapy to treat CNV may actually trigger recurrence of CNV by promoting the hypoxic state.Citation19,Citation24 Anti-VEGF treatments have proven useful in inhibiting neovascularization and increasing visual acuity by 25%–40%.Citation25 However, anti-VEGF injections are costly and must be given every 4–6 weeks throughout the life of the patient.Citation26
Retinal transplantation
Macular translocation
Researchers reasoned that by relocating the fovea (the center of the macula) to a healthier area of RPE, visual function might be restored to AMD patients. This highly technical surgery requires vitrectomy, induced retinal detachment, retinotomy, excision of the neovascular complex, translocation of the fovea, retinal reattachment, and silicone tamponade. Since the original study by Machemer and Steinhorst in 1993,Citation27 the procedure has been performed on hundreds of AMD patients. Between 25% and 66% of macular translocation patients had improved visual acuity.Citation27–Citation29 A long-term follow-up of 40 patients found that 25% retained a significant improvement in visual acuity 3 years postoperatively,Citation30 thus proving that AMD is potentially treatable. However, macular translocation patients suffered a high complication rate, including proliferative vitreoretinopathy, recurrent CNV, hemorrhage, macular hole, low intraocular pressure, macular edema, intraocular lens dislocation, and double vision resulting from image rotation.Citation27–Citation31
Autologous RPE translocation
Grafts of RPE and choroid from the periphery of the retina have been proposed because this tissue source is more abundant, larger grafts can be harvested, and peripheral retinal regions are generally unaffected by AMD.Citation31 Because equatorial grafts are predominantly composed of rods, there is doubt as to how long transplanted rods can maintain foveal cones.Citation32 Hundreds of AMD patients have undergone RPE translocation. Between 25% and 57% experienced some visual improvement.Citation32–Citation36 One study of 84 patients found a visual acuity improvement at 1–2 years, with further improvement at 3 years, indicating that, in some cases, long-term visual improvement was sustainable.Citation33
However, RPE/choroid transplants were plagued by a similar high complication rate. Complications included hemorrhage, recurrent CNV, epiretinal membrane, intraretinal cysts, retinal detachment, macular hole, RPE damage, and proliferative vitreoretinopathy due to efflux of RPE cells into the intravitreal cavity.Citation36 There were also reports of difficulty in accurate graft placement and graft failure due to nonadhesion or nonperfusion of the transplant.Citation32–Citation36 Concerns were also raised that autologous RPE transplants may have impaired function due to inherent disease processes.Citation37
Iris pigmented epithelium transplantation
The iris pigmented epithelium (IPE) has the same embryonic origin as the RPE. In rabbit studies, subretinally transplanted autologous IPE grafts formed a monolayer on the RPE and phagocytosed rod outer segments (a critical function in the renewal of photoreceptors).Citation38 In comparison with the complexity of obtaining RPE transplants, harvesting IPE required a simple procedure on the iris. In a study of 19 patients (17 with AMD), autologous IPE grafts were transplanted subretinally. Five patients showed visual improvement of three to four lines on a visual acuity chart. The complication rate was reduced to two patients who suffered retinal detachment and proliferative vitreopathy. None of the patients had a recurrence of CNV at 11 months postoperatively.Citation39
In all of these transplantation studies, early intervention was deemed critical because damage to Bruch’s membrane interferes with RPE cell adhesion, which can result in graft failure,Citation40 a glial scar barrier in a diseased retina, which interferes with graft integration,Citation41 and disruption of the blood–retinal barrier, which can compromise graft survival.Citation42 Furthermore, in late stages of AMD, secondary retinal degeneration in the inner retina and retrograde neuronal degeneration result in reorganization of synaptic connections in higher visual pathways and may ultimately limit potential functional improvement.Citation43 These studies pointed to the need for an abundant RPE source for the millions who are affected by AMD and a less complicated surgical approach with sustainable results.
Stem cell therapy
Selecting a therapy
Stem cell therapy was proposed as a means to provide a limitless source of retinal precursors for endogenous repair and exogenous cell replacement of the RPE and photoreceptors. However, selecting the most appropriate stem cell for AMD treatment has been a challenge (see ).
Figure 1 Summary table and comparison of four promising stem cell classifications and their characteristics. The first line is the classification. The second line is the derivation of the stem cells. The third and fourth lines are general characteristics.
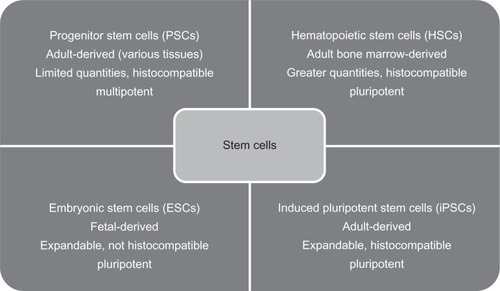
In general terms, a stem cell is an undifferentiated cell capable of self-renewal and giving rise to one or more differentiated cell types. Stem cells can migrate extensively and possess varying degrees of plasticity, meaning they can form cells in areas where they are not normally found. For example, when transplanted into the olfactory bulb, adult hippocampal progenitor cells generated site-specific olfactory cells with phenotypes not found in the hippocampus.Citation44
Adult-derived progenitor stem cells
Multipotent progenitor stem cells (PSCs) are derived from adult tissue and give rise to a limited array of cell types.Citation45 Because PSC differentiation is limited, the generated cell types are more predictable. Use of autologous PSCs obviates the need for immunosuppressive therapy, and their use is not prone to ethical debate. However, PSCs have more limited self-renewal potential, and because they do not always expand well in culture,Citation46 controlling PSC differentiation is difficult.Citation47,Citation48 Additionally, the available quantities of PSCs are more limited.Citation49
PSCs differentiate into a wide variety of retinal cell types, including photoreceptors.Citation50 Retinal injury stimulates signaling mechanisms and the release of growth factors that guide the migration, proliferation, and differentiation of PSCs in damaged areas. PSCs implanted subretinally have been shown to rescue photoreceptors, integrate with the outer nuclear layer of the retina, and express photoreceptor-specific markers such as recoverin, rhodopsin, and cone opsin.Citation51 When progenitor or precursor cells are precommitted to a rod lineage prior to transplantation, they produce rod receptors with a mature phenotype.Citation52 In rodent studies, a bypass nerve graft from the optic nerve head to the pretectal region of the brain resulted in a restored pupillary response.Citation53 A bypass graft from the optic nerve head to the superior colliculus produced positive visual-evoked potentials and restored the ability of rodents to visually identify simple linear patterns.Citation54–Citation56 Neural PSCs have been induced to differentiate into cortical neurons that survived many months and formed long-distance connections in the rodent brain.Citation44,Citation57 Taken together, these studies suggest that PSCs might repair the retina and damaged brain pathways and succeed in restoring some degree of visual function.
Limbal epithelial progenitor cells
Although available in limited quantities, epithelial cells from the limbus at the periphery of the cornea can acquire properties of neural progenitor cells and could potentially be expanded in vitro. These cells can seemingly differentiate into rod photoreceptors, albeit with a low (20%) in vitro efficiency.Citation48 Activation of Shh and Wnt pathways may increase their efficiency.Citation48
Müller cells
Müller cells, which serve a supportive function in the retina, are also progenitor-like cells. Müller cells may be responsible for retinal regeneration in ratsCitation58 and chicks.Citation59 Although the human retina does not regenerate, humans have gametes for organism regeneration, which are ostensibly inhibited to prevent uncontrolled cell division.Citation60 Human-derived Müller cells express markers for retinal neurons, which raises the possibility that if retinal regeneration could be activated in humans, it might allow for endogenous retinal repair.Citation61
Mesenchymal stromal stem cells
Mesenchymal stromal cells derived from human adipose tissue have been induced to differentiate into cells expressing RPE markers. The differentiated cells synthesize pigment, which may help offset the early pigmentary changes characteristic of dry AMD. Further research is needed to assess the functional capacity of these cells.Citation62
Bone marrow-derived stem cells
In rodent studies, bone marrow-derived stem cells differentiated into neural retinal cells. When injected into the vitreous of eyes with damaged retinas, they incorporated mainly into the outer nuclear layer of the retina and expressed rhodopsin.Citation63 In a separate study involving mice with RPE degeneration, HSCs were aspirated from the bone marrow of mouse femurs and injected systemically into the tail vein. Within 4–6 weeks, 90% of the cells in the subretinal space were bone marrow-derived RPE cells expressing RPE65. This demonstrated the ability of these cells to migrate extensively and ‘home’ to damaged subretinal tissues.Citation64
Encouraging results such as these prompted a phase I human trial in India in which autologous bone marrow-derived stem cells were harvested, isolated, and injected into the vitreous of 25 AMD and 25 retinitis pigmentosa patients. Visual acuity improved at 1 month and improved further at 3 months, expanding the visual range from near zero to a distance of a few meters.Citation65,Citation66 A similar experiment on one retinitis pigmentosa patient yielded comparable results, with the patient reporting improved vision, color discrimination, and reduced photosensitivity.Citation67
The advantages of using bone marrow-derived stem cells are their availability, ease of isolation, and the potential for autologous (histocompatible) transplants that would not require immunosuppression.Citation68 However, hematopoietic cells can migrate to other organs as well, such as the lungs, and it is unknown what complications might arise as a result.Citation64
Embryonic stem cells
Pluripotent embryonic stem cells (ESCs) are derived from a blastocyst inner cell mass, and they can give rise to almost all of the 220 cell types in the human body except the trophoblast.Citation46 ESCs can be grown in culture for extended periods and can differentiate into all three germ layers, but there are ethical concerns surrounding the use of fetal-derived ESCs. In addition, the mechanisms of differentiation are poorly understood and difficult to control. Researchers have encountered problems with differentiation into undesired or multiple cell types, mutations, and tumor formation.Citation69 A further problem is that in the absence of immunosuppression, an adult ESC recipient risks transplant rejection in response to ESC surface antigens.Citation45,Citation69,Citation70
Phagocytosis of rod outer segments is an important function because it permits continual renewal of the disks containing rhodopsin, the visual pigment of rods. In Royal College of Surgeons (RCS) rats, phagocytosis of rod outer segments is impaired, resulting in degeneration of the photreceptors in the first month of life and serious visual impairment within 2–3 months. ESCs from a mouse blastocyst were differentiated in vitro into neural precursors and were transplanted subretinally into 20-day-old RCS rats. Up to eight rows of photoreceptor cells were observed at 2 months in the eyes of the rats that received the transplants.Citation71 Researchers have wondered whether human embryonic stem cells (hESCs) would respond similarly.
hESCs
In multiple experiments, hESCs were transplanted subretinally into mice and RCS rats. They differentiated into retinal progenitors and gave rise to photoreceptor precursors at 20% efficiency,Citation72 and the rodents showed improved responses to light, orientation patterns, and spatial frequencies.Citation73,Citation74 hESCs have also been shown to restore electroretinogram responses, which is evidence that nervous signals are reaching the visual cortex.Citation75 However, in one study, the electroretinogram response subsequently disappeared at 19 weeks, suggesting that visual rescue was not sustainable.Citation76 Use of hESCs has shown other characteristics of usable cells as they differentiated into RPE. They formed tight junctions (which are important for maintaining the blood–brain barrier), expressed RPE markers, and survived up to 9 months long term.Citation43,Citation77,Citation78 The embryonic stem cell-derived pigmented epithelial cells have shown functionality by demonstrating phagocytosis of latex beads simulating the rod outer segment disks.Citation79
Various culturing techniques have been tested to increase the efficiency of hESCs. Wnt and nodal antagonists improved hESCs efficiency,Citation80 whereas nicotinamide with activin A increased RPE differentiation from 13% to 73% in 8 weeks.Citation76,Citation78 Further, noggin or dkk1 with IGF 1 increased retinal progenitors expressing Crx, rhodopsin, s-opsin, and recoverin and accelerated development by 3–4 weeks.Citation72 Recently, three-dimensional constructs of RPE were generated from hESCs grown on a matrix. It is hoped that these young graft structures might integrate and adhere more readily because RPE cells are anchorage dependent.Citation81 If Bruch’s membrane and the extracellular matrix are not supportive, apoptosis and graft failure can occur. Adhesion molecules like integrin and cadherin may aid binding, and various scaffolding matrices have been tried with varying success.Citation82
Potentially, hESCs seem to offer an unlimited source of RPE, but there are drawbacks to using them.Citation47 One argument against expanding RPE in this way is that after 40 passages, glial cells are preferentially produced.Citation60 RPE alters as early as two to three passages. Over time, RPE cell potency can decline and RPE65 (which is needed for photoisomerization of all trans-retinaldehyde) becomes undetectable.Citation82 In addition, pluripotent ESCs are known to cause tumor formation.Citation69 Epigenetic variation in hESC passages could result in safety issues, so a stable karyotype over multiple passages is needed.Citation83 In addition, cells must exit the active cycle prior to transplantation; otherwise, recoverin-positive cells can proliferate and form tumors.Citation84
hESCs of fetal origin have demonstrated potential because they can differentiate into specific cell types, but they have raised ethical concerns and are not widely available.Citation85 Additionally, fetal cells can be rejected in an adult recipient due to immunoincompatibility.Citation85 In rodent studies, cyclosporine has often been used as an immunosuppressant, but immunosuppressants can have serious side effects. It is unknown how much immunosuppression might be needed in correlating human studies.Citation47 In RCS rat studies, visual acuity was shown to decrease with or without immunosuppression, which may indicate the need for multiple transplants.Citation77 One proposed solution is to establish an hESC bank of pure, pathogen-free cells with minimal epigenetic variationsCitation26,Citation86 and with a human leukocyte antigen histocompatibility match.Citation47
Amniotic fluid-derived cells also express ESC markers and behave like pluripotent cells, but, unlike ESCs, they have not proven to be tumorigenic in vivo. They can migrate and integrate in mouse brains and are precursors to a broad array of cells, including cells expressing Pax 6, which characterizes a retinal progenitor. They retain a normal karyotype for 260 doublings and could also be human leukocyte antigen matched. Further, research is needed to determine whether they can be used to restore retinal function.Citation46
Induced pluripotent stem cells
To create a stem cell with the best attributes of both an adult progenitor stem cell and an embryonic stem cell, researchers tried a new approach by reprogramming somatic (adult) cells to behave like ESCs. Although induced pluripotent stem cells (iPSCs) were first derived from mouse fibroblasts,Citation87 a year later, human iPSCs were generated by overexpressing four transcription factors that were introduced via a viral vector and randomly integrated into the genome.Citation88,Citation89 This procedure was highly technical and time consuming. In addition, altering the genetic material raised the risk of mutations and tumor formation. There were other concerns that using patient-derived somatic cells might reintroduce the original genetic defects and contribute to the patient’s disease.Citation90 It is unclear as to how long the cells have been reprogrammed and whether cells derived from and transplanted into an aged eye will respond adequately.Citation26
Nevertheless, iPSCs appear to differentiate into a variety of retinal cells, including retinal ganglion cells, rods, and cones.Citation85 iPSCs derived from human fetal lung fibroblasts and transplanted subretinally in RCS rats differentiated into functional RPE capable of phagocytosing rod outer segments and preserving the outer nuclear layer of the retina. The rats showed improved visual acuity and light responses; however, at 13 weeks, the iPSCs were undetectable, perhaps due to macrophage activity. More research is needed to ensure long-term cell survival.Citation91
Nonviral induction methods were next explored, including plasmids,Citation92 nonintegrating episomal vectors,Citation93 and chemicals.Citation94 More recently, protein-induced pluripotent stem cells have been produced by direct delivery of recombinant (reprogramming) proteins.Citation95 The use of recombinant proteins eliminates the risk of genetic modification. It is simpler and more efficient than genetic manipulation. Recombinant proteins are readily available and cost-effective. In addition, over 30 passages, protein-induced pluripotent stem cells produced cells identical to ESCs, and they differentiated into all three germ layers.Citation96
The differentiation state of induced PSCs is unique, and genetic differences between stem cell-derived RPE cells and primary RPE have been noted. It is unclear how these variations may affect the function of stem cell-derived RPE cells.Citation97 Further research is needed to determine whether protein-induced PSCs or ESCs are ultimately the best cells for treating AMD.
Higher visual pathways
All of these approaches rely on early intervention, ie, before secondary degeneration, including retrograde neuronal degeneration, has occurred. As discussed in our previous article,Citation98 retinal progenitor cells can migrate extensively throughout the retina,Citation99 and growth factors promote axonal growth of retinal ganglion cells. In response to environmental cues, axons can grow and extend into the optic nerve head of the retina,Citation100 but gliosis or scarring and inhibitory proteins in the myelin of the optic nerve sheath prevent further axonal nerve growth beyond this point.Citation101–Citation103 In rodent studies, peripheral nerve grafts from the optic nerve head to the superior colliculus or pretectal regions of the brain were used to bypass this roadblock. The axons extended into the grafts and reached the brain, where they apparently formed functional synapses and resulted in improved responses to light and striped visual patterns, restored pupil reactivity, and increased visual evoked potentials in the visual cortex.Citation52–Citation55,Citation104
In a human model, however, it might be expected that bypassing the lateral geniculate nucleus (which mediates stereoscopic vision) could disrupt correspondence to the retina and result in visual confusion. A lack of knowledge regarding the lateral geniculate nucleus leaves this question unanswered. More research is needed to determine whether such a bypass graft is feasible in a human and whether it might succeed in restoring function to the higher visual pathways. Rodent studies suggest that significant visual improvement may be achieved even if a small population (about 15%) of axons reach the brain,Citation52,Citation104 but it is unknown whether this may hold true for humans. For stem cell therapy to succeed in AMD patients with advanced degeneration, it is critical to develop a means of restoring these higher neuronal connections and pathways. This will perhaps pose the greatest challenge to stem cell researchers in this area.
Conclusion
Research on stem cell therapy for AMD ranges from the level of molecular mechanics to clinical trials on human subjects (see ). The treatment model seems simple and supplants the damaged or dead RPE and photoreceptor cells with viable cells. Stem cell therapy provides the potential to supply functioning cells, but it is evident that it is far from simple to accomplish this and restore vision to AMD patients.
Figure 2 Milestones in the development of options for the management of AMD.
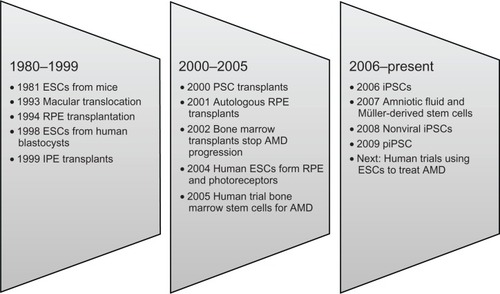
In preliminary human pilot projects, stem cell therapy has shown promise in restoring some visual function to AMD patients. But lingering questions remain to be answered. What type of stem cell is best suited to treating AMD patients? How much visual function can be restored? Are the visual acuity improvements sustainable? Will stem cell therapy prove safe and effective? How might stem cells be used to restore function to higher visual pathways?
Several phase I human trials to treat AMD with stem cells are poised to begin in the next several years. Results from these trials should add considerably to our knowledge about the above questions and hopefully point the way to a future treatment for AMD.
Disclosure
The authors report no conflicts of interest in this work.
References
- FineSLBergerJWMaguireMGHoACAge-related macular degenerationN Engl J Med2000342748349210675430
- FletcherELMechanisms of photoreceptor death during retinal degenerationOptom Vis Sci201087426927520019644
- de JongPTAge-related macular degenerationN Engl J Med2006355141474148517021323
- YoungRWBokDParticipation of the retinal pigment epithelium in the rod outer segment renewal processJ Cell Biol19694223924035792328
- AbdelsalamADel PrioreLZarbinMADrusen in age-related macular degeneration: pathogenesis, natural course, and laser photocoagulation-induced regressionSurv Ophthalmol199944112910466585
- SeddonJMAjaniUAMitchellBDFamilial aggregation of age-related maculopathyAm J Ophthalmol199712321992069186125
- WeeksDEConleyYPTsaiHJAge-related maculopathy: an expanded genome-wide scan with evidence of susceptibility loci within the 1q31 and 17q25 regionsAm J Ophthalmol2001132568269211704029
- KleinMLSchultzDWEdwardsAAge-related macular degeneration. Clinical features in a large family and linkage to chromosome 1qArch Ophthalmol19981168108210889715689
- CrabbJWMiyagiMGuXDrusen proteome analysis: an approach to the etiology of age-related macular degenerationProc Natl Acad Sci U S A20029923146821468712391305
- LiMAtmaca-SonmezPOthmanMCFH haplotypes without the Y402H coding variant show strong association with susceptibility to age-related macular degenerationNat Genet20063891049105416936733
- HackettSFFriedmanZFreundJA splice variant of trkB and brain-derived neurotrophic factor are co-expressed in retinal pigmented epithelial cells and promote differentiated characteristicsBrain Res199878922012129573364
- IshidaKYoshimuraNYoshidaMHondaYMuraseKHayashiKExpression of neurotrophic factors in cultured human retinal pigment epithelial cellsCurr Eye Res1997162961019068939
- DawsonDWVolpertOVGillisPPigment epithelium-derived factor: a potent inhibitor of angiogenesisScience1999285542524524810398599
- BlaauwgeersHGHoltkampGMRuttenHPolarized vascular endothelial growth factor secretion by human retinal pigment epithelium and localization of vascular endothelial growth factor receptors on the inner choriocapillaris. Evidence for a trophic paracrine relationAm J Pathol1999155242142810433935
- ThurstonGRole of Angiopoietins and Tie receptor tyrosine kinases in angiogenesis and lymphangiogenesisCell Tissue Res20033141616812915980
- WitmerANVrensenGFVan NoordenCJSchlingemannROVascular endothelial growth factors and angiogenesis in eye diseaseProg Retin Eye Res200322112912597922
- PenfoldPLMadiganMCGilliesMCProvisJMImmunological and aetiological aspects of macular degenerationProg Retin Eye Res200120338541411286898
- BirdACThe Bowman lecture. Towards an understanding of age-related macular diseaseEye (Lond)200317445746612802343
- CampochiaroPASolowayPRyanSJMillerJWThe pathogenesis of choroidal neovascularization in patients with age-related macular degenerationMol Vis199953410562658
- SpilsburyKGarrettKLShenWYConstableIJRakoczyPEOverexpression of vascular endothelial growth factor (VEGF) in the retinal pigment epithelium leads to the development of choroidal neovascularizationAm J Pathol2000157113514410880384
- SenguptaNCaballeroSMamesRNTimmersAMSabanDGrantMBPreventing stem cell incorporation into choroidal neovascularization by targeting homing and attachment factorsInvest Ophthalmol Vis Sci200546134334815623794
- SasaharaMOtaniAYodoiYYoshimuraNCirculating hematopoietic stem cells in patients with idiopathic choroidal neovascularizationInvest ophthalmol Vis Sci20095041575157918806291
- LecomteJLouisKDetryBBone marrow-derived mesenchymal cells and MMP13 contribute to experimental choroidal neovascularizationCell Mol Life Sci2010811 [Epub ahead of print]
- SchlingemannRORole of growth factors and the wound healing response in age-related macular degenerationGraefes Arch Clin Exp Ophthalmol200424219110114685874
- RosenfeldPJBrownDMHeierJSRanibizumab for neovascular age-related macular degenerationN Engl J Med2006355141419143117021318
- CoffeyPInterview: stemming vision loss with stem cells: seeing is believingRegen Med20094450550719580400
- MachemerRSteinhorstUHRetinal separation, retinotomy, and macular relocation: II. A surgical approach for age-related macular degeneration?Graefes Arch Clin Exp Ophthalmol1993231116356418258397
- AisenbreySLafautBASzurmanPMacular translocation with 360 degrees retinotomy for exudative age-related macular degenerationArch Ophthalmol2002120445145911934318
- PertileGClaesCMacular translocation with 360 degree retinotomy for management of age-related macular degeneration with subfoveal choroidal neovascularizationAm J Ophthalmol2002134456056512383813
- ChenFKPatelPJUppalGSTufailACoffeyPJDa CruzLLong-term outcomes following full macular translocation surgery in neovascular age-related macular degenerationBr J Ophthalmol201094101337134320494910
- MacLarenREBirdACSathiaPJAylwardGWLong-term results of submacular surgery combined with macular translocation of the retinal pigment epithelium in neovascular age-related macular degenerationOphthalmology2005112122081208716325706
- ChenFKUppalGSMacLarenRELong-term visual and microperimetry outcomes following autologous retinal pigment epithelium choroid graft for neovascular age-related macular degenerationClin Experiment Ophthalmol200937327528519459869
- MaaijweeKHeimannHMissottenTMulderPJoussenAvan MeursJRetinal pigment epithelium and choroid translocation in patients with exudative age-related macular degeneration: long-term resultsGraefes Arch Clin Exp Ophthalmol2007245111681168917562066
- BinderSStolbaUKrebsITransplantation of autologous retinal pigment epithelium in eyes with foveal neovascularization resulting from age-related macular degeneration: a pilot studyAm J Ophthalmol2002133221522511812425
- Van MeursJCVan Den BiesenPRAutologous retinal pigment epithelium and choroid translocation in patients with exudative age-related macular degeneration: short-term follow-upAm J Ophthalmol2003136468869514516809
- MacLarenREUppalGSBalagganKSAutologous transplantation of the retinal pigment epithelium and choroid in the treatment of neovascular age-related macular degenerationOphthalmology2007114356157017324698
- KlimanskayaIRetinal pigment epitheliumMethods Enzymol200641816919417141036
- ThumannGBartz-SchmidtKUEl BakriHTransplantation of autologous iris pigment epithelium to the subretinal space in rabbitsTransplantation199968219520110440387
- ThumannGAisenbreySSchraermeyerUTransplantation of autologous iris pigment epithelium after removal of choroidal neovascular membranesArch Ophthalmol2000118101350135511030816
- TezelTHDel PrioreLVRepopulation of different layers of host human Bruch’s membrane by retinal pigment epithelial cell graftsInvest Ophthalmol Vis Sci199940376777410067982
- KinouchiRTakedaMYangLRobust neural integration from retinal transplants in mice deficient in GFAP and vimentinNat Neurosci20036886386812845328
- WangSVillegas-PerezMPVidal-SunzMLundRDProgressive optic axon dystrophy and vascular changes in rd miceInvest Ophthalmol Vis Sci200041253754510670486
- LundRDOnoSJKeeganDJLawrenceJMRetinal transplantation: progress and problems in clinical applicationJ Leukoc Biol200374215116012885930
- FrickerRACarpenterMKWinklerCGrecoCGatesMABjorklundASite-specific migration and neuronal differentiation of human neural progenitor cells after transplantation in the adult rat brainJ Neurosci199919145990600510407037
- GageFHMammalian neural stem cellsScience200028754571433143810688783
- De CoppiPBartschGJrSiddiquiMMIsolation of amniotic stem cell lines with potential for therapyNat Biotechnol200725110010617206138
- HarutaMEmbryonic stem cells: potential source for ocular repairSemin Ophthalmol2005201172315804840
- ZhaoXDasAVBhattacharyaSDerivation of neurons with functional properties from adult limbal epithelium: implications in autologous cell therapy for photoreceptor degenerationStem Cells200826493994918203675
- ZhaoXLiuJAhmadIDifferentiation of embryonic stem cells to retinal cells in vitroMethods Mol Biol200633040141616846039
- TropepeVColesBLChiassonBJRetinal stem cells in the adult mammalian eyeScience200028754602032203610720333
- KlassenHJNgTFKurimotoYMultipotent retinal progenitors express developmental markers, differentiate into retinal neurons, and preserve light-mediated behaviorInvest Ophthalmol Vis Sci200445114167417215505071
- MacLarenREPearsonRAMacNeilARetinal repair by transplantation of photoreceptor precursorsNature2006444711620320717093405
- ThanosSAdult retinofugal axons regenerating through peripheral nerve grafts can restore the light-induced pupilloconstriction reflexEur J Neurosci19924869169912106313
- CarterDABrayGMAguayoAJLong-term growth and remodeling of regenerated retino-collicular connections in adult hamstersJ Neurosci19941425905987507980
- CarterDABrayGMAguayoAJRegenerated retinal ganglion cell axons can form well-differentiated synapses in the superior colliculus of adult hamstersJ Neurosci1989911404240502479728
- KeirsteadSARasminskyMFukudaYCarterDAAguayoAJVidal-SanzMElectrophysiologic responses in hamster superior colliculus evoked by regenerating retinal axonsScience198924649272552572799387
- EnglundUFricker-GatesRALundbergCBjorklundAWictorinKTransplantation of human neural progenitor cells into neonatal rat brain: extensive migration and differentiation with long-distance axonal projectionsExp Neurol2002173112111771935
- OotoSAkagiTKageyamaRPotential for neural regeneration after neurotoxic injury in the adult mammalian retinaProc Natl Acad Sci U S A200410137136541365915353594
- FischerAJRehTAMüller glia are a potential source of neural regeneration in the postnatal chicken retinaNat Neurosci20014324725211224540
- KlassenHSakaguchiDSYoungMJStem cells and retinal repairProg Retin Eye Res200423214918115094129
- LawrenceJMSinghalSBhatiaBMIO-M1 cells and similar müller glial cell lines derived from adult human retina exhibit neural stem cell characteristicsStem Cells20072582033204317525239
- VossmerbaeumerUOhnesorgeSKuehlSRetinal pigment epithelial phenotype induced in human adipose tissue-derived mesenchymal stromal cellsCytotherapy200911217718819241195
- TomitaMAdachiYYamadaHBone marrow-derived stem cells can differentiate into retinal cells in injured rat retinaStem Cells200220427928312110696
- Atmaca-SonmezPLiYYamauchiYSystemically transferred hematopoietic stem cells home to the subretinal space and express RPE-65 in a mouse model of retinal pigment epithelium damageExp Eye Res20068351295130216949576
- KumarAPahwaVKTandonRKumarLMohantySUse of autologous bone marrow derived stem cells for rehabilitation of patients with dry age related macular degeneration and retinitis pigmentosa: phase-1 clinical trialIndian J Med Paediatr Oncol200526Suppl 31214
- Blind World MagazineStem cell promise cure for vision loss2006419 Available from: http://www.home.earthlink.net/~blindworld2/MEDICAL/6-04-19-01.htmAccessed May 22, 2007
- Blind World MagazineRetinitis pigmentosa (RP) patient gets stem cell implant200655 Available from: http://www.home.earthlink.net/~blindworld2/MEIDCAL/6-05-05-02.htmAccessed May 22, 2007
- TrounsonANew perspectives in human stem cell therapeutic researchBMC Med200972919519878
- ArnholdSKleinHSemkovaIAddicksKSchraermeyerUNeurally selected embryonic stem cells induce tumor formation after long-term survival following engraftment into the subretinal spaceInvest Ophthalmol Vis Sci200445124251425515557428
- LanzaRRosenthalNThe stem cell challengeSci Am20042906929915195398
- SchraermeyerUThumannGLutherTSubretinally transplanted embryonic stem cells rescue photoreceptor cells from degeneration in the RCS ratsCell Transplant200110867368011814109
- LambaDAKarlMOWareCBRehTAEfficient generation of retinal progenitor cells from human embryonic stem cellsProc Natl Acad Sci U S A200610334127691277416908856
- CoffeyPJGirmanSWangSMLong-term preservation of cortically dependent visual function in RCS rats by transplantationNat Neurosci200251535611753416
- GirmanSVWangSLundRDCortical visual functions can be preserved by subretinal RPE cell grafting in RCS ratsVision Res200343171817182712826105
- LambaDAGustJReyTATransplantation of human embryonic stem cell-derived photoreceptors restores some visual function in Crx-deficient miceCell Stem Cell200941737919128794
- CorneoBTempleSSense and serendipity aid RPE generationCell Stem Cell20095434734819796611
- LuBMalcuitCWangSLong-term safety and function of RPE from human embryonic stem cells in preclinical models of macular degenerationStem Cells20092792126213519521979
- IdelsonMAlperRObolenskyADirected differentiation of human embryonic stem cells into functional retinal pigment epithelium cellsCell Stem Cell20095439640819796620
- HarutaMSasaiYKawasakiHIn vitro and in vivo characterization of pigment epithelial cells differentiated from primate embryonic stem cellsInvest Ophthalmol Vis Sci20044531020102514985325
- OsakadaFIkedaHMandaiMToward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cellsNat Biotechnol200826221522418246062
- NistorGSeilerMJYanFFergusonDKeirsteadHSThree- dimensional early retinal progenitor 3D tissue constructs derived from human embryonic stem cellsJ Neurosci Methods20101901637020447416
- VuglerALawrenceJWalshJEmbryonic stem cells and retinal repairMech Dev200712411–1280782917881192
- AlgeCSSuppmannSPriglingerSGComparative proteome analysis of native differentiated and cultured human RPE cellsInvest Ophthalmol Vis Sci20034483629364112882817
- PolansASWitkowskaDHaleyTLAmundsonDBaizerLAdamusGRecoverin, a photoreceptor-specific calcium-binding protein, is expressed by the tumor of a patient with cancer-associated retinopathyProc Natl Acad Sci U S A19959220917691807568096
- ParameswaranSBalasubramanianSBabaiNInduced pluripotent stem cells generate both retinal ganglion cells and photoreceptors: therapeutic implications in degenerative changes in glaucoma and age-related macular degenerationStem Cells201028469570320166150
- The London Project to Cure BlindnessLondonUCL Institute of Ophthalmology2007 Available from: http://thelondonproject.org/aboutus/index.aspx?id=1258Accessed May 14, 2010
- TakahashiKYamanakaSInduction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factorsCell2006126466367616904174
- TakahashiKTanabeKOhnukiMInduction of pluripotent stem cells from adult human fibroblasts by defined factorsCell2007131586187218035408
- YuJVodyanikMASmuga-OttoKInduced pluripotent stem cell lines derived from human somatic cellsScience200731858581917192018029452
- BuchholzDEHikitaSTRowlandTJDerivation of functional retinal pigmented epithelium from induced pluripotent stem cellsStem Cells200927102427243419658190
- CarrAJVuglerAAHikitaSTProtective effects of human iPS-derived retinal pigment epithelium cell transplantation in the retinal dystrophic ratPLoS One2009412e815219997644
- OkitaKNakagawaMHyenjongHIchisakaTYamanakaSGeneration of mouse induced pluripotent stem cells without viral vectorsScience2008322590394995318845712
- YuJHuKSmuga-OttoKHuman induced pluripotent stem cells free of vector and transgene sequencesScience2009324592879780119325077
- LyssiotisCAForemanRKStaerkJReprogramming of murine fibroblasts to induced pluripotent stem cells with chemical complementation of Klf4Proc Natl Acad Sci U S A2009106228912891719447925
- KimDKimCHMoonJIGeneration of human induced pluripotent stem cells by direct delivery of reprogramming proteinsCell Stem Cell20094647247619481515
- ZhouHWuSJooJYGeneration of induced pluripotent stem cells using recombinant proteinsCell Stem Cell20094538138419398399
- LiaoJLYuJHuangKMolecular signature of primary retinal pigment epithelium and stem-cell-derived RPE cellsHum Mol Genet201019214229423820709808
- MooneyILaMotteJA review of the potential to restore vision with stem cellsClin Exp Optom2008911788418045253
- TakahashiMPalmerTDTakahashiJGageFHWidespread integration and survival of adult-derived neural progenitor cells in the developing optic retinaMol Cell Neurosci19981263403489888988
- StuermerCABastmeyerMThe retinal axon’s pathfinding to the optic diskProg Neurobiol200062219721410828383
- MacLarenRERegeneration and transplantation of the optic nerve: developing a clinical strategyBr J Ophthalmol19988255775839713068
- WeibelDCadelliDSchwabMERegeneration of lesioned rat optic nerve fibers is improved after neutralization of myelin-associated neurite growth inhibitorsBrain Res19946421–22592668032887
- SchwabMECaroniPOligodendrocytes and CNS myelin are nonpermissive substrates for neurite growth and fibroblast spreading in vitroJ Neurosci199881238123933074158
- ThanosSNaskarRHeiduschkaPRegenerating ganglion cell axons in the adult rat establish retinofugal topography and restore visual functionExp Brain Res199711434834919187284