Abstract
A myriad of locally produced factors into the microenvironment of the reproductive tract is regulated, not one-way but rather, through embryonic–maternal cross-talk. In this mini-review, we focused on the exosomes, which are cell-derived vesicles of 30–100 nm in diameter, as a communicating language facilitating this dialog. These nanovesicles are secreted from pre-implantation embryos, oviduct epithelium, and endometrium as well as from the placenta, and contain proteins, messenger RNA (mRNA), microRNA, and DNA cargoes, and have pleiotropic effects on both embryonic and maternal environments. A better understanding of the molecular mechanisms mediating this cross-talk will lead to the development of new regulating agents, with novel diagnostic, biological, and therapeutic potential for either supporting or hindering the normal reproductive functions.
Keywords:
Exosomes
An ever growing number of studies worldwide have helped to substantiate the essential functions of the cell-secreted, membrane-derived vesicles, particularly exosomes, and provided new dimensions for the concept of intercellular signaling. Exosomes are nanosized vesicles (30–100 nm in diameter) () that contain, not only proteins, but also, messenger RNAs (mRNAs), microRNAs (miRNAs), and double-strand or genomic DNA.Citation1–Citation6 The molecular cargoes carried by exosomes affect cellular activity via ready-made proteins and miRNA or by translation of transferred mRNAs (). The term “exosomal shuttle RNA (esRNA)” was proposed for those transferred RNAs.Citation3
Figure 1 Embryo-derived exosomes as seen by transmission electron microscope.
Abbreviations: mRNA, messenger RNA; miRNA, microRNA; TEM, transmission electron microscope.
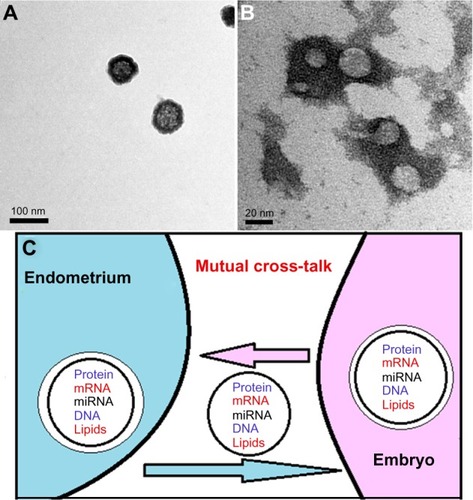
Exosomes, as cell membrane-derived nanovesicles, are specifically equipped to mediate intercellular communication, via the transfer of genetic information to recipient cells. As a result, exosomes play a fundamental biological role in the regulation of normal physiological as well as aberrant pathological processes, via altered gene regulatory networks and via epigenetic programming.Citation2 For example, exosome-mediated genetic transfer can regulate the maintenance of stem cell plasticity and induce beneficial cell phenotype modulation.Citation7 Alternatively, such vesicles play a role in tumor pathogenesisCitation8 and the spread of neurodegenerative diseases, via transfer of specific miRNAs and pathogenic proteins.Citation9 In addition, these cell membrane-derived vesicles are involved in cell adhesion and signal transfer, and provide an important means of cell communication.Citation10 Evidence of secretion of exosomes has been reported in most cell types, including embryonic stem cells and in vitro–produced embryos.Citation11–Citation16
Because of the ever increasing discoveries in the field of extracellular vesicles, Vesiclepedia (http://microvesicles.org) has been established as a compendium database for extracellular vesicles and exosomal components, including proteins and RNAs.Citation17
The early stages for pregnancy require preparatory cross-talk and signals. Of interest are the exosomes from both embryonic and maternal sides, by which the maternal recognition of pregnancy will be achieved successfully.Citation18 In the coming section, we give a brief description about the probable and/or proposed involvement of exosomes in mediating the embryonic–maternal cross-talk.
Embryo-derived exosomes
In study of the physical properties, including size and concentration, of in vitro fertilized (IVF) embryos-derived exosomes, it was revealed that their numbers increase with developmental stage and further, that their size correlates with embryo quality and may predict recovery from apparent growth-arrested embryos.Citation13
Moreover, in previous work, we showed the uptake of embryo-derived exosomes by cultured embryos. These exosomes were shown to act as a mediator, carrying early reprogramming mRNAs, such as Oct4, Sox2, cMyc, and Klf4, which improved the development of the cocultured embryos in group culture system.Citation14 We suggest that the continuous transfer of mRNA cargoes among cultured embryos via exosomes is advantageous over the acute transfer of mRNA by the conditioned medium – this confirms the concept of the “dynamic microenvironment” or “niche” among the cultured embryos. It was suggested that the stability of foreign mRNAs in cells is often tightly and intricately regulated with low transcriptional rates.Citation19 The foreign transferred mRNAs are rapidly turned over with half-lives of 20–40 minutes.Citation20 In our preliminary work, we found expression of sex determining mRNAs, Xist and Sry, in the conditioned medium of in vitro–derived embryos cultured individually (unpublished data), which could be used for sexing of in vitro–produced embryos.
In equines, a previous in vitro study suggested that exosomes can be secreted by day 8 embryos, which can modulate the functions of the oviduct epithelium through transfer of early pregnancy factor (HSP10) and miRNAs.Citation21
miRNAs, small (~22 nucleotides) noncoding RNAs that regulate gene expression, have been implicated in a wide array of biologic processes, including early embryo development and stem cell differentiation.Citation22–Citation25 Human blastocysts express miRNAs, which may be important to their survival. Differential miRNA expression between euploid and aneuploid embryos may be an early indicator of their prognosis or a mechanism behind their eventual fate.Citation23 Interestingly, miRNAs have also been found to exist in embryo culture medium, in both human and domestic animals.Citation15,Citation16
The expression of miRNA in culture medium has been correlated to fertilization method, chromosomal status, and pregnancy outcome, which makes miRNA a potential biomarker for predicting IVF success.Citation16 Some miRNAs, including miRNA-191, have been shown to be putative noninvasive candidates for predicting aneuploidy, while miR-191, miR-372, and miR-645 were found to be more highly concentrated in media from failed IVF/non–intracytoplasmic sperm injection (ICSI) cycles.Citation16 Additionally, miRNAs were found to be more highly concentrated in ICSI and day 5 media samples when compared to regularly inseminated and day 4 samples, respectively.Citation16 Furthermore, it was shown that differential miRNA gene expression analysis could be used to early discriminate between embryos that developed to the blastocyst stage and those that failed to develop from the morula to blastocyst stage, deemed degenerate embryos. miR-25, miR-302c, miR-196a2, and miR-181a expression was found to be higher in degenerate embryos compared to blastocyst embryos.Citation15 Therefore, expression of miRNAs in in vitro culture media could allow for the development of biological markers for selection of better quality embryos and for subsequent successful pregnancy.
Furthermore, embryonic and fetal genomic DNA was also found to circulate in the maternal blood.Citation26,Citation27 This raises many questions about the involvement of exosomes/microvesicles in transportation of embryonic and fetal genomic DNACitation4,Citation28,Citation29 and may mean that these exosomes can act as potent markers, specifically, for the Y-chromosome specific DNA; for embryo/fetal sexing as early as 6 weeks of gestation;Citation26,Citation30–Citation32 or as a noninvasive prenatal genetic diagnostic method to detect aneuploidy in the embryo and fetus.Citation6,Citation33–Citation35
Maternal-derived exosomes
On the other hand, exosomes can be secreted from maternal side; bioinformatic analysis of endometrium-derived exosome miRNAs revealed that these miRNAs have potential targets in biological pathways highly relevant for embryo implantation.Citation36 Western blot analysis demonstrated that in vivo–derived exosomes contain HSP70 and OVGP, which have roles in fertilization and early pregnancy; however, in vitro–derived exosomes contain only HSPA8 and MYH9.Citation37 They are also secreted from the endometrium and have been found to contain mRNAs for ovine enJSRV-envelope, HSP70, interleukins, and IFN-regulatory factors, thereby stimulating trophectoderm cells to proliferate and secrete IFNT for successful pregnancy recognition in sheep. These results would explain that free and/or exosomal enJSRV acts on the trophectoderm, via TLRs, to induce the secretion of IFNT.Citation38
Placenta-derived exosomes
A previous study unraveled the important role of placenta-derived exosomes; the syncytiotrophoblast constitutively and throughout the pregnancy secretes exosomes.Citation39 More recently, exosomes have been shown to be released by trophoblasts and to carry molecules involved in placental physiology. This involves immunomodulator proteins such as fibronectin and syncytin, Wnt/βcatenin-related molecules, galectin-3, and HLA-G; but also bioactive lipids, such as immunosuppressive PGE2; PPARγ ligand 15d-PGJ2; and miRNAs.Citation40
Exosomes contain all the ingredients (ie, syncytin, phosphatidic acid, and endosomal phospholipid BMP) to promote intercellular fusion and might behave as initiators of syncythiotrophoblast formation.Citation41–Citation43 Moreover, the placenta-derived exosomes are immunosuppressive and carry proteins and RNA molecules that, in a redundant manner, influence a number of mechanisms and promote the fetal allograft survival.Citation39,Citation44,Citation45 Specifically, isolated human placental exosomes carry NKG2D ligands, ULBPs (ULBP1–5), and MIC proteins on their surface by which they induce downregulation of the NKG2D receptor on NK, CD8(+), and gamma delta T cells. This, in turn, leads to reduction of their in vitro cytotoxicity without affecting the perforin-mediated lytic pathway.Citation44 Moreover, bioactive FasL- and TRAIL-carrying exosomes, which are able to convey apoptosis, are secreted by the placenta and responsible for the immunomodulatory and protective role of the human placenta.Citation45 Exosomes can also be used for prediction of preeclampsia: Villous cytotrophoblast exosomes have been found to be positive for HERV envelope gene proteins and to be rapidly taken up by BeWo choriocarcinoma cells in a syncytin 1- and syncytin 2- dependent manner. Syncytin 2 has also been found to be reduced in women with preeclampsia.Citation43
Furthermore, it has been shown that trophoblast-derived exosomes containing miRNAs could mediate cross-talk between the fetoplacental unit and the mother during pregnancy. The function of placenta-derived miRNAs, particularly from the primate-specific chromosome 19 miRNA clusters, which are highly expressed in human placentas and in the serum of pregnant women, has been reviewed.Citation46
Synthetic and semisynthetic exosomes
The application of synthetic or semisynthetic exosomes for therapeutic drug delivery is still in its infancy. Issues regarding the understanding of exosomes biogenesis, large-scale production, and in vivo interactions need to be addressed to develop successful and cost-effective exosome-based drug delivery systems.Citation47 Few investigations have explored their potential to serve as a platform for the development of semisynthetic nanovesicles. Given their nanoscale size, potential to express targeting ligands in native conformations, and their deformable structure, exosomes offer a logical biological vesicle platform for adapting and producing semisynthetic vesicles with excellent potential for nanomedicine applications.Citation48 No doubt, progress in synthetic biology to harnessing exosomes for therapeutic nucleic acid delivery is crucial to mimic the physicochemical properties of naturally occurring exosomes to overcome delivery challenges and to establish robust technology platforms.Citation49 If successful, the exosomes could then transport the nucleic acid into a subsequent population of cells, including embryos, that manifest the desired effect. This piggy-back mechanism could be responsible for reported deep-tissue RNA or DNA effects with certain carriers, and offers novel and promising roles in interfering with or supporting the dialog between the embryos and the mother.Citation50
Conclusion
From these novel results, we may suggest a dynamic mutual paracrine communication between both the embryonic and the maternal environments, through the exosomes, at the early stages of preimplantation embryo development. Anticipating the potential implications of the functions of the embryonic-and maternal-derived exosomes is essential. Particularly, the detection and characterization of embryo-derived exosomes could be a noninvasive method for: 1) the judgment of embryo quality and implantation capability; 2) the sexing of the embryos; and 3) the prediction of abnormal genetic constituents of embryos, as an alternative to the invasive prenatal genetic diagnosis (PGD) method. Therefore, advancement in the physicochemical analysis of small quantities of embryo culture medium, including analysis of exosomes size (such as nanoparticle tracking analysis)Citation51 and content of mRNA, miRNA and DNA, is crucial to substantiate these targets.
Finally, much remains to be learned concerning the communicating signals comprising the embryonic–maternal cross-talk through the exosomes, especially considering that their effects are often redundant and pleiotropic. Deepening our understanding of exosomes will be critical in understanding the physiology and the pathophysiology of the early stages of pregnancy recognition. Moreover, this study will enable the development of novel nanodiagnostics and nanotherapeutics, to maximize pregnancy rates during IVF treatment; to improve the treatment of disorders such as infertility, pregnancy loss, and preeclampsia; and possibly even to offer new methods for locally acting contraceptives.
Acknowledgments
This study was supported by IPET (grant numbers 311011-05-4-SB010 and 114059-3), MI (grant number 10048948), the Research Institute for Veterinary Science, TS Corporation, and the BK21 plus program.
Disclosure
The authors declare that there is no conflict of interest.
References
- ThéryCExosomes: secreted vesicles and intercellular communicationsF1000 Biol Rep201131521876726
- LeeYEl AndaloussiSWoodMJExosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapyHum Mol Genet201221R1R125R13422872698
- ValadiHEkströmKBossiosASjöstrandMLeeJJLötvallJOExosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cellsNat Cell Biol20079665465917486113
- ThakurBKZhangHBeckerADouble-stranded DNA in exosomes: a novel biomarker in cancer detectionCell Res201424676676924710597
- CaiJHanYRenHExtracellular vesicle-mediated transfer of donor genomic DNA to recipient cells is a novel mechanism for genetic influence between cellsJ Mol Cell Biol20135422723823580760
- KahlertCMeloSAProtopopovAIdentification of double-stranded genomic DNA spanning all chromosomes with mutated KRAS and p53 DNA in the serum exosomes of patients with pancreatic cancerJ Biol Chem201428973869387524398677
- AliottaJMLeeDPuenteNProgenitor/stem cell fate determination: interactive dynamics of cell cycle and microvesiclesStem Cells Dev201221101627163822214238
- IeroMValentiRHuberVTumour-released exosomes and their implications in cancer immunityCell Death Differ2008151808817932500
- RajendranLHonshoMZahnTRAlzheimer’s disease beta-amyloid peptides are released in association with exosomesProc Natl Acad Sci U S A200610330111721117716837572
- van der PolEBöingANHarrisonPSturkANieuwlandRClassification, functions, and clinical relevance of extracellular vesiclesPharmacol Rev201264367670522722893
- RatajczakJMiekusKKuciaMEmbryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein deliveryLeukemia200620584785616453000
- KatsmanDStackpoleEJDominDRFarberDBEmbryonic stem cell-derived microvesicles induce gene expression changes in Müller cells of the retinaPLoS One2012711e5041723226281
- GardinerCFFerrieraJFPoliMTurnerKChildTSargentILIVF embryos release extracellular vesicles which may act as an indicator of embryo qualityJ Extracell Vesicles2013220826 Abstract26082317
- SaadeldinIMKimSJChoiYBLeeBCImprovement of cloned embryos development by co-culturing with parthenotes: a possible role of exosomes/microvesicles for embryos paracrine communicationCell Reprogram201416322323424773308
- KroppJSalihSMKhatibHExpression of microRNAs in bovine and human pre-implantation embryo culture mediaFront Genet201459124795753
- RosenbluthEMSheltonDNWellsLMSparksAEVan VoorhisBJHuman embryos secrete microRNAs into culture media – a potential biomarker for implantationFertil Steril201410151493150024786747
- KalraHSimpsonRJJiHVesiclepedia: a compendium for extracellular vesicles with continuous community annotationPLoS Biol20121012e100145023271954
- TannettaDDragovicRAlyahyaeiZSouthcombeJExtracellular vesicles and reproduction-promotion of successful pregnancyCell Mol Immunol201411654856324954226
- RajagopalanLEMalterJSTurnover and translation of in vitro synthesized messenger RNAs in transfected, normal cellsJ Biol Chem19962713319871198768702698
- WisdomRLeeWThe protein-coding region of c-myc mRNA contains a sequence that specifies rapid mRNA turnover and induction by protein synthesis inhibitorsGenes Dev1991522322431995415
- BemisLTMcCuePMHatzelJNBemisJFerrisRAEvidence for production of early pregnancy factor (Hsp10), microRNAs and exosomes by day 8 equine embryosJ Equine Vet Sci2012327398
- LaurentLCMicroRNAs in embryonic stem cells and early embryonic developmentJ Cell Mol Med2008126A2181218819120702
- RosenbluthEMSheltonDNSparksAEDevorEChristensonLVan VoorhisBJMicroRNA expression in the human blastocystFertil Sterility2013993855861.e3
- LingenfelterBMTripuraniSKTejomurtulaJSmithGWYaoJMolecular cloning and expression of bovine nucleoplasmin 2 (NPM2): a maternal effect gene regulated by miR-181aReprod Biol Endocrinol201194021447182
- TesfayeDWorkuDRingsFIdentification and expression profiling of microRNAs during bovine oocyte maturation using heterologous approachMol Reprod Dev200976766567719170227
- Al-YatamaMKMustafaASAliSAbrahamSKhanZKhajaNDetection of Y chromosome-specific DNA in the plasma and urine of pregnant women using nested polymerase chain reactionPrenat Diagn200121539940211360283
- LoYMCorbettaNChamberlainPFPresence of fetal DNA in maternal plasma and serumLancet199735090764854879274585
- SarkerSScholz-RomeroKPerezAPlacenta-derived exosomes continuously increase in maternal circulation over the first trimester of pregnancyJ Transl Med20141220425104112
- SalomonCTorresMJKobayashiMA gestational profile of placental exosomes in maternal plasma and their effects on endothelial cell migrationPloS One201496e9866724905832
- YasminLTakanoJNagaiYOtsukiJSankaiTDetection and quantification of male-specific fetal DNA in the serum of pregnant cynomolgus monkeys (Macaca fascicularis)Comp Med2015651707625730760
- KolialexiATountaGApostolouPEarly non-invasive detection of fetal Y chromosome sequences in maternal plasma using multiplex PCREur J Obstet Gynecol Reprod Biol20121611343722261468
- DevaneySAPalomakiGEScottJABianchiDWNoninvasive fetal sex determination using cell-free fetal DNA: a systematic review and meta-analysisJAMA2011306662763621828326
- LiuFMWangXYFengXWangWYeYXChenHFeasibility study of using fetal DNA in maternal plasma for non-invasive prenatal diagnosisActa Obstet Gynecol Scand200786553554117464580
- SekizawaAPurwosunuYMatsuokaRRecent advances in noninvasive prenatal DNA diagnosis through analysis of maternal bloodJ Obstet Gynaecol Res200733674776418001438
- WrightCFBurtonHThe use of cell-free fetal nucleic acids in maternal blood for non-invasive prenatal diagnosisHum Reprod Update200915113915118945714
- NgYHRomeSJalabertAEndometrial exosomes/microvesicles in the uterine microenvironment: a new paradigm for embryo-endometrial cross talk at implantationPLoS One201383e5850223516492
- AlmiñanaCCorbinETsikisGCharacterization of bovine oviductal exosomes from in vivo and in vitro originReprod Fertil Dev2015271147
- Ruiz-GonzálezIXuJWangXBurghardtRCDunlapKABazerFWExosomes, endogenous retroviruses and toll-like receptors: pregnancy recognition in ewesReproduction2015149328129125526899
- Mincheva-NilssonLBaranovVThe role of placental exosomes in reproductionAm J Reprod Immunol201063652053320331583
- RecordMIntercellular communication by exosomes in placenta: a possible role in cell fusion?Placenta201435529730224661568
- RecordMCarayonKPoirotMSilvente-PoirotSExosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologiesBiochim Biophys Acta20141841110812024140720
- LaulagnierKMottaCHamdiSMast cell- and dendritic cell-derived exosomes display a specific lipid composition and an unusual membrane organizationBiochem J2004380Pt 116117114965343
- VargasAZhouSÉthier-ChiassonMSyncytin proteins incorporated in placenta exosomes are important for cell uptake and show variation in abundance in serum exosomes from patients with preeclampsiaFASEB J20142883703371924812088
- HedlundMStenqvistACNagaevaOHuman placenta expresses and secretes NKG2D ligands via exosomes that down-modulate the cognate receptor expression: evidence for immunosuppressive functionJ Immunol2009183134035119542445
- StenqvistACNagaevaOBaranovVMincheva-NilssonLExosomes secreted by human placenta carry functional Fas ligand and TRAIL molecules and convey apoptosis in activated immune cells, suggesting exosome-mediated immune privilege of the fetusJ Immunol2013191115515552324184557
- OuyangYMouilletJFCoyneCBSadovskyYReview: placenta-specific microRNAs in exosomes – good things come in nano-packagesPlacenta201435SupplS69S7324280233
- van der MeelRFensMHVaderPvan SolingeWWEniola-AdefesoOSchiffelersRMExtracellular vesicles as drug delivery systems: lessons from the liposome fieldJ Control Release2014195728525094032
- HoodJLWicklineSAA systematic approach to exosome-based translational nanomedicineWiley Interdiscip Rev Nanomed Nanobiotechnol20124445846722648975
- MarcusMELeonardJNFedExosomes: Engineering therapeutic biological nanoparticles that truly deliverPharmaceuticals (Basel)20136565968023894228
- NguyenJSzokaFCNucleic acid delivery: the missing pieces of the puzzle?Acc Chem Res20124571153116222428908
- DragovicRACollettGPHolePIsolation of syncytiotrophoblast microvesicles and exosomes and their characterisation by multicolour flow cytometry and fluorescence Nanoparticle Tracking AnalysisMethodsIn press2015
