Abstract
Mesenchymal stem cells have awakened a great deal of interest in regenerative medicine due to their plasticity, and immunomodulatory and anti-inflammatory properties. They are high-yield and can be acquired through noninvasive methods from adult tissues. Moreover, they are nontumorigenic and are the most widely studied. On the other hand, induced pluripotent stem (iPS) cells can be derived directly from adult cells through gene reprogramming. The new iPS technology avoids the embryo destruction or manipulation to generate pluripotent cells, therefore, are exempt from ethical implication surrounding embryonic stem cell use. The pre-differentiation of iPS cells ensures the safety of future approaches. Both mesenchymal stem cells and iPS cells can be used for autologous cell transplantations without the risk of immune rejection and represent a great opportunity for future alternative therapies. In this review we discussed the therapeutic perspectives using mesenchymal and iPS cells.
Keywords:
Introduction
The “stemness” of a stem cell can be defined by two important properties: the ability of self-renewal and the capacity to differentiate into mature cell types.Citation1 The ability of stem cells to differentiate into specific mature lineages is called plasticity and this is the most important property in the content of cell-based therapy.
Various cell types can potentially be used for clinical studies, including embryonic stem cells (ESC), isolated from the inner cell mass of blastocysts;Citation2 stem cells isolated from adult tissues like the mesenchymal stem cells (MSC); and induced pluripotent stem (iPS) cells which are adult somatic cells reprogrammed to pluripotency.Citation3 Several studies have been conducted to identify, characterize, and differentiate stem cells from various sources.Citation4,Citation5 From stem cells’ isolation, quantification, and expansion, their future application in human and animal cell therapy is expected.Citation6
MSC are multipotent stem cells present in adult tissues, such as bone marrow, muscle, liver, and adipose tissue. These cells are highlighted by their abundance and easy collection. iPS cells are the most promising among those classified as pluripotent because of their high plasticity, similar to ESC, without its controversial origin. This review is aimed to discuss and compare the general insights and clinical applications of MSC and iPS cells. The interest in these two distinct cell types comes from their high potential therapeutic associated to the numerous advantages over the other cell lineages, such as easy harvest and high yield, greater proliferation capacity, and high plasticity. Moreover, the iPS cells can be easily differentiated into MSC with similar properties than traditional MSC.
General characteristics of stem cells
In general, a stem cell is defined as a cell with the ability to divide for an indefinite period of time throughout the life of an individual (self-renewal) and, under appropriate conditions and specific signals, can differentiate into a variety of lineages, with different characteristics and specialized functions (differentiation). According to the differentiation potential, stem cells are classified as totipotent, pluripotent, multipotent, oligopotent, and unipotent.Citation1
Totipotent and pluripotent stem cells correspond to ESC. Totipotent cells are found in the zygote in early stage of development (up to 32-cell embryo) and pluripotent cells are found in the inner cell mass of the blastocyst (between 32–64 cells).Citation7 Totipotent cells have the capacity to generate all cell types, including embryonic and extra embryonic tissues. Pluripotent stem cells can give rise to the three germ layer: endoderm, mesoderm and ectoderm, but not the extra embryonic tissues.Citation8,Citation9 Such differentiation can generate, for example, myocytes, hepatocytes, and neurons.Citation8–Citation10
Multipotent stem cells are present in various adult organs and can differentiate into many cell types, usually from the same embryonic germ layer as MSC and hematopoietic stem cells.Citation5 Oligopotent cells have less ability to differentiate and unipotent stem cells can only generate one mature cell type. Therefore, oligopotent and unipotent stem cells are called progenitor cells.Citation7
The ESC are able to form spontaneous multicellular structures in vitro known as embryonic body. These structures have elements of all three germ layers and can give rise to many types of specialized cells such as cardiomyocytes, neurons, and other hematopoietic progenitors.Citation10,Citation11 ESC can be extensively expanded in culture without losing their pluripotency and self-renewing capacity, when factors to prevent their differentiation are used. Therefore, the advantage of using ESC is the ability to proliferate indefinitely and to generate a wide variety of cell groups. These features allow the manipulation in vitro in order to produce specific precursor cell lines for the treatment of various diseases.Citation10,Citation12 Despite the high plasticity, the use of ESC entails ethical implications due to blastocyst destruction for their isolation.
Adult stem cells have lower plasticity than ESC; however, they stand out in terms of their abundance, easy access, and high yield. These cells can be acquired through noninvasive methods from adult tissues and therefore are exempt from the typical ethical limitations.Citation13 In the body, they are tissue specific, and respond to specific stimuli to regulate the homeostasis and replacement of dead cells.Citation5,Citation14
It is known that pluripotent cells express a unique set of factors responsible for the state of pluripotency, and an interconnected network of regulatory genes is responsible for the development and maintenance of pluripotency in embryos.Citation15,Citation16 Recently, Takahashi and Yamanaka generated a new technology to achieve pluripotent stem cells from adult somatic cells. By the integration of pluripotent transcription factors into the genome of the cells, totally differentiated cells can be reprogrammed to acquire an induced pluripotent state. These cells are called iPS cells.Citation17
MSC
MSC are a type of multipotent stem cell and can be isolated from various adult or fetal tissues and membranes,Citation18,Citation19 including fat, bone marrow, umbilical cord blood,Citation20–Citation22 dental pulp,Citation23 placenta, and muscle.Citation24
In vivo, MSC provide structural support in different organs and regulate the flow of some substances. The stromal origin is characterized by their quick adhesion in culture surface as well as their fibroblastic-like morphology. In addition, they present a high and fast proliferation in simple and accessible culture medium and can be maintained in vitro without karyotype alterations for several passages.Citation25
MSC have the ability to differentiate into several cell types such as adipocytes, osteocytes, and chondrocytes, from the mesodermal germ layer.Citation14,Citation26 This plasticity depends on the extra-cellular matrix environment and soluble growth factors.Citation27 Some authors could induce the differentiation of MSC in cells of other embryonic germ layers, such as neurons,Citation28 which are originated in ectoderm, and hepatocytes, derived from endoderm.Citation29 However, the differentiation into nonmesodermal tissues is still controversial due to a lack of in vivo results.Citation22
Due to their plasticity, the MSC are considered the most important cell type for regenerative medicine, and are the most widely studied in preclinical and clinical trials. Their advantages for clinical application include the easy isolation and high yield, high plasticity, and the ability to mediate inflammation and to promote cell growth, cell differentiation, and tissue repair by immunomodulation and immunosuppression, and are exempt from ethical implications.Citation30–Citation32 Besides, MSC do not form teratomas after transplantation, ensuring safety to the host.
The MSC derived from bone marrow have been the most intensively studied; however, invasive procedures are required for their isolation and the quantity and quality of isolated cells vary according to the donor age. Low frequencies of MSC are found in bone marrow aspirates compared to the total cells compounding the bone marrow stroma.Citation33 Due to cell population heterogeneity, their immunogenic properties depend on numerous settings such as isolation methods, surface and culture medium, plating density, and chemical products supplementation.Citation25
Therefore, the identification of alternative sources of MSC has been the focal point of recent researches. Between different sources of MSC, the adipose tissue is highlighted for their accessibility and the abundance of isolated cells.Citation13,Citation26,Citation34–Citation36 Each isolation results in approximately 100-fold more cells than the bone morrow isolation,Citation37 and the process is less invasive.Citation14
MSC are heterogeneous; therefore, their immunophenotypic profile and plasticity varies among species, source, and passage.Citation37 However, MSC positively express a combination of surface markers: CD29, CD73, CD90, CD105, CD44 and CD166 and are negative to CD14, CD31, CD34 and CD45. The expression pattern of some surface markers is controversial, for example, CD34 in humans,Citation14,Citation38 CD44 in ovines,Citation39,Citation40 and CD44 and CD105 in rabbits.Citation41,Citation42
Besides classified as multipotents, MSC express a relatively high level of pluripotent markers related to ESC, such as OCT4, NANOG, and SOX2.Citation14,Citation21,Citation24 These transcription factors are involved in the regulation of the multipotency, self-renewal, and proliferation of MSC.Citation21,Citation24 The OCT4 is evolved in the initial development of mammals and is essential for the formation of embryos’ inner cell mass and ESC maintenance.Citation15 SOX2 regulates the expression of OCT4 and maintains the pluripotent state of ESC, and NANOG is required for the maintenance of nondifferentiated state and self-renewal of stem cells.Citation21 As described eariler, these factors also play a key role in the pluripotency state of iPS cells.
iPS cells
The iPS cells are generated from the induction of expression of transcription factors associated with pluripotency, allowing a differentiated somatic cell to reverse its condition to the embryonic stage. Takahashi and Yamanaka developed this technique where four transcription factors, OCT4, SOX2, KLF4, and C-MYC (shown by the acronym OSKM), were incorporated into the genome of mouseCitation17 and human somatic cells.Citation43 The discovery of such technology was based on the hypothesis that nuclear reprogramming is a process driven by factors that play a critical role in maintaining the pluripotency of ESC.Citation17,Citation44 iPS cells could imply the elimination of ethical issues and problems of rejection after transplantation, as they can be collected from the patient (autologous), expanding the possibilities of research.Citation13,Citation17 It is well known that one or several transcriptional factors can convert one cell to another. Although, the mechanisms whereby exogenous factors change the epigenetic state remains unknown. Although Yamanaka factors are the most used, other combinations of factors were tested successfully, such as the replacement of C-MYC and KLF4 by NANOG and LIN28Citation45 or excluding the factor C-MYC.Citation46
The field of induced pluripotency has been growing exponentially in the last years. The efficiency, reliability, and security are crucial to the success of reprogramming and the method for introduction of transcription factors in the cells is a very significant step. Conventional reprogramming techniques depend on the stable integration of transgenes, but it can introduce the current risk of insertional mutagenesis. Thus, several nonintegrative reprogramming techniques have been developed to improve the quality of the generated MSC.Citation47
The integrative systems consist of viral vectors, such as retrovirusesCitation17 and lentiviruses.Citation48 Nonintegrative vectors, such as adenovirusCitation49 or nonviral systems, plasmids,Citation50 proteins,Citation51 and mRNA, do not promote the integration of OSKM factors’ cDNA into the cell genome.Citation50,Citation52,Citation53 Recently, new approaches were tested to induce the pluripotency, by using chemical exogenous moleculesCitation54 or episomal vectors.Citation53 Episomal reprogramming seems particularly well-suited for clinical translation because it is integration-free, works reliably with patient fibroblasts and blood cells, and is based on a very simple reagent (plasmid DNA).Citation47 However, it has shown lower efficiency than integrative vectors.Citation53
In the somatic cells, promoters of pluripotency genes are highly methylated, reflecting a repressed transcriptional state. The generation of iPS cells involves the activation of these genes, and their demethylation is used to determine the success of reprogramming.Citation55 When exogenous pluripotency genes are introduced into the cell, they induce the expression of endogenous pluripotency genes.Citation56 In turn, the upregulation of endogenous factors induces the silencing of exogenous genes by methylation of the promoters.Citation57
The pluripotency state of iPS cells can be attested by the ability to form teratomas in vivo and the formation of embryonic bodies in vitro. Moreover, they have the ESC morphology, such as round shape, large nucleolus, and scarce cytoplasm. The molecular profile of iPS cells is very similar to ESC, expressing the pluripotency markers OCT4, NANOG, SOX2, SSEA1, SSEA3, SSEA4, TRA1-60, TRA1-81, and ALP activity.Citation58–Citation60 Despite these characteristics, Takahashi and YamanakaCitation17 found that iPS cells are very similar but not identical to ESC.
Many studies have confirmed the repeatability of the iPS cell process in different species such as humans, mice,Citation17,Citation45 rhesus monkeys,Citation61 pigs,Citation62 cattle,Citation63 horses,Citation64 rabbits,Citation65 sheep,Citation66 large cats such as the leopard,Citation67 and canids,Citation68–Citation72 most of them being made from fibroblasts. Honda et alCitation65 were not able to generate iPS cells from rabbit fibroblasts, probably due to the exceptional speed of proliferation of these specific cells, which quickly reach confluence, discontinuing the differentiation. In fact, the high proliferation of donor cells seems to be detrimental to reprogramming.Citation46
As an alternative source, MSC derived from adipose tissue were used to generate iPS cells in mice and humans. Adipose-derived stem cells are naturally multipotent and acquire pluripotency after induction. It is described that the reprogramming of MSC into iPS cells can be achieved 200-fold more efficiently and rapidly than from fibroblasts.Citation73,Citation74
The cellular reprogramming is desired in many different biotechnology areas; therefore, many authors strive to elucidate the mechanisms involved in cell pluripotency. However, the exact mechanism remains unclear and the efficacy is very low. Two issues appear to limit the application of iPS cells: the low efficiency of transgene integration in the somatic genome and the low efficiency of the reprogramming process.Citation75 These factors have imposed significant limitations on their biomedical and therapeutic applications. In this context, considerable effort has been made to identify compounds that can improve the efficiency of reprogramming.Citation76,Citation77
Small molecules able to remodel chromatin and epigenetic control are being actively investigated due to their effect on reprogramming. It has been demonstrated that inhibitors of methyltransferase, histone deacetylase, and histone demethylase may increase the reprogramming efficiency rate.Citation76,Citation78,Citation79 In fact, it is known that inhibitors can induce partial reprogramming colonies to achieve the complete reprogramming state.Citation80,Citation81
Some molecules acting on the signaling pathways involved in self-renewal and pluripotency, such as Wnt, TGFb, and MEK, also increase such rates.Citation80–Citation84 In addition, Esteban et alCitation62 showed that vitamin C, a common nutrient vital to human health, enhances the reprogramming of somatic cells to pluripotent stem cells. The addition of vitamin C to the culture medium resulted in high-quality iPS cells from mouse and human cells. This can be explained by the suppression of reactive oxygen species production, normally accumulated by somatic cells undergoing senescence.
Other strategies to increase efficiency include the reduction of transcription factors, like SOX2 and C-MYCCitation85 or C-MYC and KLF4, and the addition of valproic acidCitation74 or inhibitor of GSK-3 signaling cascade, which is a known facilitator of complete reprogramming in partially reprogrammed colonies.Citation78 Interestingly, Wang et alCitation86 enhanced the generation of iPS cells by the addition of lithium, an antipsychotic drug. This drug interacts metabolically with many pathways and promotes reprogramming by acting on GSK3β. Besides, lithium increases the expression of NANOG and facilitates iPS cell generation with just one (OCT4) or two factors (OCT4 and SOX2 or OCT4 and KLF4).
Even with the advent of new techniques, the transcriptional factor OCT4 remains a key player in the reprogramming process. In fact, OCT4 alone seems to be sufficient to induce pluripotency.Citation87 However, OCT4 could be replaced by nuclear receptors such as NR5a1 and NR5a2 or by a combination of microRNAs such as miR-200c, miR-302s, and miR-369s.Citation88,Citation89 Nevertheless, increasing the efficiency of iPS cell generation is crucial for future therapeutic use.
Clinical perspectives of mesenchymal and iPS cells
Stem cells normally present in the adult organism contribute to the postnatal development by replacement of lost cells due to injury, apoptosis, or physiological programmed turnover.Citation14 When therapeutically applied, stem cells secrete factors and promote physical repair in injured tissues.Citation5
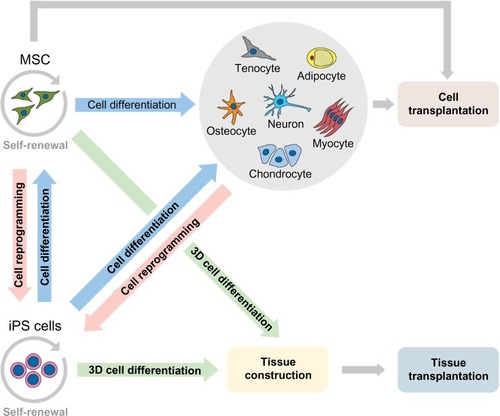
MSC and iPS cells have particular characteristics (). These features reflect the wide therapeutic potential of both cell types, each possessing its pros and cons. The ideal stem cell for transplantation should be immunologically inert, derived from sources easily accessible, with high and fast expansion in vitro, long-term survival, ability to provide integration into the host site, and able to transfect and express exogenous genes.Citation90 The autologous transplantation, available for MSC and iPS cell strategies, is preferred in regenerative medicine since the rejection risks are avoided.Citation14
Table 1 General characteristics of mesenchymal and induced pluripotent stem cells
The sources of both MSC and iPS cells are diverse. While MSC can be readily isolated from adult tissues and easily expanded in vitro, the iPS cell technology is slightly more complex. However, pluripotency can be achieved from virtually any cell type after several days in vitro, reaching a large amount of iPS cell colonies with great therapeutic potential. Cells can be systematically transplanted by intravenous injection either by direct application at the injury site or by scaffolds, a pre-cultivated structure that keeps the cells attached to the target site. Through tissue engineering, cells cultured in scaffolds can be induced to form tissues before transplantation. There is no agreement on the most effective mode of implementation. Several authors have succeeded using stem cells intravenouslyCitation91 and through local administration.Citation36,Citation92 In these studies, authors reported a rapid migration and homing of stem cells to the injured tissues,Citation93 attracted by extracellular matrix signals and soluble growth factors.Citation27 However, Lam and LongakerCitation13 argue that injected cells dissipate or die in the body, and the adhesion of cells is directly related to their growth and differentiation. In this context, MSC showed better ability to migrate and engraft more easily than the iPS cells in different biomaterial models.
It is known that pluripotent stem cells require specific culture conditions to maintain an undifferentiated state. iPS cells have been cultured in 2D feeder cells (eg, mouse embryonic fibroblasts); however, these methods require extensive culture time and have high related labor cost.Citation94 The development of biomaterials assembling suitable culture conditions can support large-scale pluripotent cells’ proliferation or differentiation not involving feeder cells. Biomaterials designed for culture or to improve self-renewal capability or cell differentiation for iPS cells and MSC have been investigated.Citation95 Synthetic or natural polymers and hydrogels mimicking specific 2D or 3D extracellular matrix have been used to support guided differentiation of iPS cells into specific cell lineages.Citation94,Citation96–Citation98 These biomaterials are biologically inert and are therefore suitable to prevent allograft rejections and are the key tool for tissue engineering. In addition, the use of biomaterials colonized with pre-differentiated cells accelerates and improves the tissue regeneration.
The wide differentiation potential of the stem cells is essential for their use in multiple applications. MSC are multipotent stem cells with proven capacity to generate mesodermal cells, such as hepatocytes, myocytes, and osteocytes. iPS cells are able to generate cells from the three germ layers. In this context, the iPS cells represent a new possibility of using pluripotent stem cells, exempt from ethical implications surrounding ESC use. The capacity of teratoma formation of iPS cells can be avoided by the pre-differentiation in committed lineages.Citation25
The therapeutic potential of MSC is unquestionably promising as a result of their advantageous effects and safety. These cells have been studied for many human and animal diseases. They exert a paracrine effect by the secretion of growth factors such as BGF, EGF, and BDNF and work by directly differentiating into specific somatic cells.Citation99
In recent years, many preclinical studies have been carried out to investigate the application of stem cells for human disease, especially (neurodegenerative diseases) in animal models.Citation100 Stem cells improved neuron replacement and healing in animal models for Parkinson’s disease,Citation101,Citation102 Alzheimer’s disease,Citation103 epilepsy,Citation104 sclerosis,Citation105 ischemic stroke,Citation106 and spinal cord injury.Citation107 Although promising results were achieved, the mechanisms underlying cell survival, migration, homing, and differentiation in the pathological environment must be investigated before these results can be translated to humans.Citation100
In wound healing, MSC induces the inhibition of the inflammatory response, differentiation into skin cells, stimulation of angiogenesis, and secretion of growth factors.Citation35,Citation108 The beneficial effects of MSC were observed in cancer immunosuppression;Citation109,Citation110 in the formation of new vessels;Citation111 and in cardiac,Citation112 liver,Citation113 and kidneyCitation114,Citation115 regeneration. In fact, MSC are extensively studied and tested in various affections, diseases, and even for cosmetic purposes.Citation36
Despite their valuable application for regenerating tissues, the MSC have limitations such as quick loss of plasticity during expansion. Furthermore; the MSC can be isolated from numerous adult or fetal tissues; the isolation procedures are mostly invasive, and the harvested cells are limited in number.Citation116 The iPS cells are obtained through noninvasive methods and can differentiate into all body cell types. Therefore, iPS cells are the most attractive stem cell source for cell therapy.Citation117 Due to rapid growth and high plasticity, direct transplantation of iPS cells can result in in vivo teratoma formation. The differentiation of pluripotent cells into multipotent cells prior to transplantation arises as a promising tool for safe use of iPS cells. Multipotent-like cells derived from pluripotent cells have been investigated as well as effective methods and strategies for iPS cell derived MSC establishment.Citation118
In recent years, the MSC derived from diverse iPS cell lines represent the effective source of multipotent cells, incorporating the advantages of both iPS cells and traditional MSC cells.Citation118,Citation119 The iPS cell-MSC have a greater proliferation capacity in vitro with no time limit.Citation111 They also have immunomodulatory properties similar to traditional MSC lines, and it was reported recently that they are capable of impairing NK-cells’ function to prevent graft rejection.Citation119 Despite their long-term survival after transplantation,Citation111 the iPS cell-MSC are nontumorigenic and are safe and effective for cell-based therapy.
Among the therapeutic progress of iPS cells, Christoforou et alCitation120 generated cardiac progenitors and cardiomyocytes capable of forming biosynthetic tissues and produced an in vitro cellular model of amyotrophic lateral sclerosis.Citation121 Currently, the investigation of pathophysiology, drug development, and toxicology studies are the major applications of these cells.Citation122
Several preclinical trials have been carried out evaluating the in vitro pre-differentiation of iPS cells for regeneration, as in nerve functionCitation123 and periodontal regeneration.Citation124 Despite its clinical potential and the possibility to avoid rejection, immunogenic issues were present in previous attempts.Citation45,Citation125 Recent studies have demonstrated that the rejection is related to gene expression and epigenetic inheritance of reprogramming process, and not to specific characteristics of iPS cells. Therefore, increasing the production efficiency and reducing chromosomal and epigenetic alterations, could lead to the use of iPS cells in therapy without rejection issues.Citation126,Citation127
Regarding the clinical approaches, the American government recognizes more clinical trials with MSC, involving neoplasias, immunodeficiency, syndromes and others, however, a few clinical studies are recognized with iPS cells, such as hypertension and fibromuscular dysplasia.Citation128 Clearly, we still have a long way to go regarding iPS cell therapy, but research is advancing rapidly and is heading for satisfactory results. The potential and various possibilities of clinical applications of MSC and iPS cells are summarized in .
Figure 1 Potential application of MSC and iPS cells in preclinical and clinical transplantation.
Abbreviations: MSC, mesenchymal stem cells; iPS, induced pluripotent stem.
Conclusion
MSC are easily collected and maintained in culture, show a high proliferation in vitro and are nontumorigenic when transplanted in vivo. They can differentiate into several mesodermal cell types and can be used for cell transplantation or tissue engineering. The therapeutic utilization of MSC is advantageous because they are easy to collect and maintain, and a short period of time is needed between the culture establishment and clinical application. On the other hand, the pluripotency state of iPS cells can mean a wide possibility of disease treatment, and their pre-differentiation in vitro can guarantee the safeness of their utilization. However, iPS cell research is still beginning to reach the preclinical and clinical stage, and much more studies are necessary to determine their therapeutic applications.
Acknowledgments
The authors appreciate the grant support of FAPESP (São Paulo Research Foundation) (grant numbers 2012/04196-4 and 2013/09392-9).
Disclosure
The authors report no conflicts of interest in this work.
References
- Malaver-OrtegaLFSumerHLiuJVermaPJThe state of the art for pluripotent stem cells derivation in domestic ungulatesTheriogenology20127881749176222578625
- KeeferCLPantaDBlombergLTalbotNCChallenges and prospects for the establishment of embryonic stem cell lines of domesticated ungulatesAnim Reprod Sci2007981–214716817097839
- HardingJRobertsRMMirochnitchenkoOLarge animal models for stem cell therapyStem Cell Res Ther2013422323672797
- De BariCDell’AccioFTylzanowskiPLuytenFMultipotent mesenchymal stem cells from adult human synovial membraneArthritis Rheum20014481928194211508446
- NardiNBda Silva MeirellesLMesenchymal stem cells: isolation, in vitro expansion and characterizationHandb Exp Pharmacol2006174249282
- BarkerJNWagnerJEUmbilical cord transplantation: current practice and future innovationsCrit Rev Oncol Hematol2003481354314585482
- MitalipovSWolfDTotipotency, pluripotency and nuclear reprogrammingAdv Biochem Eng Biotechnol200911418519919343304
- BreviniTATosettiVCrestanMAntoniniSGandolfiFDerivation and characterization of pluripotent cell lines from pig embryos of different originsTheriogenology2007671546317055567
- KuijkEWChuva de Sousa LopesSMGeijsenNMacklonNRoelenBAThe different shades of mammalian pluripotent stem cellsHum Reprod Update201017225422720705693
- VerfaillieCMPeraMFLansdorpPMStem cells: hype and realityHematology Am Soc Hematol Educ Program200236939112446433
- EvansMJNotarianniELaurieSMoorRMDerivation and preliminary characterization of pluripotent cell lines from porcine and bovine blastocystsTheriogenology1990331125128
- WeissmanILTranslating stem and progenitor cell biology to the clinic: barriers and opportunitiesScience200028754571442144610688785
- LamMTLongakerMTComparison of several attachment methods for human iPS, embryonic and adipose-derived stem cells for tissue engineeringJ Tissue Eng Regen Med20126Suppl 3s80s8622610948
- SachsPCFrancisMPZhaoMDefining essential stem cell characteristics in adipose-derived stromal cells extracted from distinct anatomical sitesCell Tissue Res2012349250551522628159
- NicholsJZevnikBAnastassiadisKFormation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4Cell19989533793919814708
- MitsuiKTokuzawaYItohHThe homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cellsCell200330563164212787504
- TakahashiKYamanakaSInduction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factorsCell2006126466367616904174
- BianchiGMuragliaADagaACorteGCanceddaRQuartoRMicroenvironment and stem properties of bone marrow-derived mesenchymal cellsWound Repair Regen20019646046611896988
- Filioli UranioMValentiniLLange-ConsiglioAIsolation, proliferation, and pharacterization of mesenchymal stem cells from amniotic fluid, amnion, and umbilical cord matrix in the DogMol Reprod Dev201178536137321491540
- KernSEichlerHStoeveJKlüterHBiebackKComparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissueStem Cells20062451294130116410387
- TakemitsuHZhaoDYamamotoIHaradaYMichishitaMAraiTComparison of bone marrow and adipose tissue-derived canine mesenchymal stem cellsBMC Vet Res201231815022937862
- StriogaMViswanathanSDarinskasASlabyOMichalekJSame or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cellsStem Cells Dev201221142724275222468918
- XiaoLTsutsuiTCharacterization of human dental pulp cells-derived spheroids in serum-free medium: Stem cells in the coreJ Cell Biochem2013114112624263623794488
- KisielAHMcDuffeeLAMasaoudEBaileyTREsparza GonzalezBPNino-FongRIsolation, characterization, and in vitro proliferation of canine mesenchymal stem cells derived from bone marrow, adipose tissue, muscle, and periosteumAm J Vet Res20127381305131722849692
- WebsterRABlaberSPHerbertBRWilkinsMRVeseyGThe role of mesenchymal stem cells in veterinary therapeutics – a reviewN Z Vet J201260526527222646715
- DuYRohDSFunderburghMLAdipose-derived stem cells differentiate to keratocytes in vitroMol Vis2010162680268921179234
- VidaneASZomerHDOliveiraBMReproductive stem cell differentiation: extracellular matrix, tissue microenvironment, and growth factors direct the mesenchymal stem cell lineage commitmentReprod Sci201320101137114323420825
- KramperaMMarconiSPasiniAInduction of neural-like differentiation in human mesenchymal stem cells derived from bone marrow, fat, spleen and thymusBone200740238249017049329
- AurichHSgoddaMKaltwasserPHepatocyte differentiation of mesen chymal stem cells from human adipose tissue in vitro promotes hepatic integration in vivoGut200958457058119022918
- Lage-ConsiglioARossiDTassanSPeregoRCremonesiFParoliniOConditioned medium from horse amniotic membrane-derived multi-potent progenitor cells: immunomodulatory activity in vitro and first clinical application in tendon and ligament injuries in vivoStem Cells Dev201322223015302423795963
- PlockJASchniderJTSolariMGZhengXXGorantlaVSPerspectives on the use of mesenchymal stem cells in vascularized composite allotransplantationFront Immunol2013417523888159
- InsaustiCLBlanquerMGarcía-HernándezAMCastellanosGMoraledaJMAmniotic membrane-derived stem cells: immunomodulatory properties and potential clinical applicationStem Cells Cloning20147536324744610
- BydlowskiSPDebesAAMaselliLMFJanzFLCaracterísticas biológicas das células-tronco mesenquimais [Biological characteristics of the mesenchymal stem cells]Rev Bras Hematol Hemoter2009312535 Portuguese
- HousmanTSLawrenceNMellenBGThe safety of liposuction: results of a national surveyDermatol Surg2002281197197812460288
- CherubinoMRubinJPMiljkovicNKelmendi-DokoAMarraKGAdipose-derived stem cells for wound healing applicationsAnn Plast Surg201166221021521200308
- KimJHJungMKimHSKimYMChoiEHAdipose-derived stem cells as a new therapeutic modality for ageing skinExp Dermatol201120538338721355887
- DeyDEvansGRGeneration of induced pluripotent stem (iPS) cells by nuclear reprogrammingStem Cells Int2011201161958322007240
- YangXFHeXHeJHigh efficient isolation and systematic identification of human adipose-derived mesenchymal stem cellsJ Biomed Sci2011185921854621
- BarryFPBoyntonREHaynesworthSMurphyJMZaiaJThe monoclonal antibody SH-2, raised against human mesenchymal stem cells, recognizes an epitope on endoglin (CD105)Biochem Biophys Res Commun1999265113413910548503
- FadelLVianaBRFeitosaMLModels, Biological Protocols for obtainment and isolation of two mesenchymal stem cell sources in sheepActa Cir Bras201126426727321808838
- Martínez-LorenzoMJRoyo-CañasMAlegre-AguarónEPhenotype and chondrogenic differentiation of mesenchymal cells from adipose tissue of different speciesJ Orthop Res200927111499150719408284
- SunayOCanGCakirZAutologous rabbit adipose tissue-derived mesenchymal stromal cells for the treatment of bone injuries with distraction osteogenesisCytotherapy201315669070223522867
- TakahashiKTanabeKOhnukiMInduction of pluripotent stem cells from adult human fibroblasts by defined factorsCell2007131586187218035408
- YamanakaSPluripotency and nuclear reprogrammingPhilos Trans R Soc Lond B Biol Sci200836315002079208718375377
- YuJVodyanikMASmuga-OttoKInduced pluripotent stem cell lines derived from human somatic cellsScience200731858581917192018029452
- XuYWeiXWangMProliferation rate of somatic cells affects reprogramming efficiencyJ Biol Chem2013288149767977823439651
- DieckeSJungSMLeeJJuJHRecent technological updates and clinical applications of induced pluripotent stem cellsKorean J Intern Med201429554755725228828
- Picanço-CastroVRusso-CarbolanteEReisLCPluripotent reprogramming of fibroblasts by lentiviral mediated insertion of SOX2, C-MYC, and TCL-1AStem Cells Dev201120116918020504151
- StadtfeldMNagayaMUtikalJWeirGHochedlingerKInduced pluripotent stem cells generated without viral integrationScience2008322590394594918818365
- OkitaKYamanakaSIntracellular signaling pathways regulating pluripotency of embryonic stem cellsCurr Stem Cell Res Ther20061110311118220859
- ZhouHWuSJooJYGeneration of induced pluripotent stem cells using recombinant proteinsCell Stem Cell20094538138419398399
- WarrenLManosPDAhfeldtTHighly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNACell Stem Cell20107561863020888316
- YuJHuKSmuga-OttoKHuman induced pluripotent stem cells free of vector and transgene sequencesScience2009324592879780119325077
- HouPLiYZhangXPluripotent stem cells induced from mouse somatic cells by small-molecule compoundScience2013341614665165423868920
- MikkelsenTSHannaJZhangXDissecting direct reprogramming through integrative genomic analysisNature20084547200495518509334
- JaenischRYoungRStem cells, the molecular circuitry of pluripotency and nuclear reprogrammingCell2008132456758218295576
- HottaAEllisJRetroviral vector silencing during iPS cell induction: an epigenetic beacon that signals distinct pluripotent statesJ Cell Biochem2008105494094818773452
- LewitzkyMYamanakaSReprogramming somatic cells towards pluripotency by defined factorsCurr Opin Biotechnol200718546747318024106
- WangYMahNPrigioneAWolfrumKAndrade-NavarroMAAdjayeJA transcriptional roadmap to the induction of pluripotency in somatic cellsStem Cell Rev20106228229620336394
- ScheperWCopraySThe molecular mechanism of induced pluripotency: a two-stage switchStem Cell Rev20095320422319551525
- LiuHZhuFYongJGeneration of induced pluripotent stem cells from adult rhesus monkey fibroblastsCell Stem Cell20083658759019041774
- EstebanMAWangTQinBVitamin C enhances the generation of mouse and human induced pluripotent stem cellsCell Stem Cell201061717920036631
- SumerHLiuJMalaver OrtegaLFLimMLKhodadadiKVermaPJNANOG is a key factor for induction of pluripotency in bovine adult fibroblastsJ Anim Sci20118992708271621478453
- NagyKSungHKZhangPInduced pluripotent stem cell lines derived from equine fibroblastsStem Cell Rev20117369370221347602
- HondaAHiroseMHatoriMGeneration of induced pluripotent stem cells in rabbits: potential experimental models for human regenerative medicineJ Bio Chem201028541313623136920670936
- BaoLHeLChenJReprogramming of ovine adult fibroblasts to pluripotency via drug-inducible expression of defined factorsCell Res201121460060821221129
- VermaRHollandMKTemple-SmithaPVermaPJInducing pluripotency in somatic cells from the snow leopard (Panthera uncia), an endangered felidTheriogenology201277122022822079579
- ShimadaHNakadaAHashimotoYShigenoKShionoyaYNakamuraTGeneration of canine-induced pluripotent stem cells by retroviral transduction and chemical inhibitorsMol Reprod Dev2010771219890968
- LuoJSuhrSTChangEAGeneration of leukemia inhibitory factor and basic fibroblast growth factor-dependent induced pluri-potent stem cells from canine adult somatic cellsStem Cells Dev201120101669167821495906
- WhitworthDJOvchinnikovDAWolvetangEJGeneration and characterization of LIF-dependent canine induced pluripotent stem cells from adult dermal fibroblastsStem Cells Dev201221122288229722221227
- GonçalvesNJBressanFFSouzaACanine fibroblasts expressing human transcription factors: what is in the route for the production of canine induced pluripotent stem cellsReprod Domest Anim201247Suppl 6848723279472
- KohSThomasRTsaiSGrowth requirements and chromosomal instability of induced pluripotent stem cells generated from adult canine fibroblastsStem Cells Dev201222695196323016947
- SugiiSKidaYBerggrenWTEvansRMFeeder-independent iPS cell derivation from human and mouse adipose stem cellsNat Protoc20116334635821372815
- SunNPanettaNJGuptaDMFeeder-free derivation of induced pluripotent stem cells from adult human adipose stem cellsProc Natl Acad Sci U S A200910637157201572519805220
- YamanakaSStrategies and new developments in the generation of patientspecific pluripotent stem cellsCell Stem Cell200711394918371333
- HuangfuDOsafuneKMaehrRInduction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2Nat Biotechnol200826111269127518849973
- LyssiotisCAForemanRKStaerkJReprogramming of murine fibroblasts to induced pluripotent stem cells with chemical complementation of Klf4Proc Natl Acad Sci U S A2009106228912891719447925
- ZhuSLiWZhouHReprogramming of human primary somatic cells by OCT4 and chemical compoundsCell Stem Cell20107665165521112560
- MaliPChouBKYenJButyrate greatly enhances derivation of human induced pluripotent stem cells by promoting epigenetic remodeling and the expression of pluripotency-associated genesStem Cells201028471372020201064
- SilvaJBarrandonONicholsJKawaguchiJTheunissenTWSmithAPromotion of reprogramming to ground state pluripotency by signal inhibitionPLoS Biol2008610e25318942890
- LinTAmbasudhanRYuanXA chemical platform for improved induction of human iPSCsNat Methods200961180580819838168
- IchidaJKBlanchardJLamKA small-molecule inhibitor of tgf-Beta signaling replaces sox2 in reprogramming by inducing nanogCell Stem Cell20095549150319818703
- MarsonAForemanRChevalierBWnt signaling promotes reprogramming of somatic cells to pluripotencyCell Stem Cell20083213213518682236
- MaheraliNHochedlingerKTgfβ Signal Inhibition Cooperates in the Induction of iPSCs and Replaces Sox2 and cMycCurr Biol200919201718172319765992
- NakagawaMKoyanagiMTanabeKGeneration Of Induced pluripotent stem cells without Myc from mouse and human fibroblastsNat Biotechnol200826110110618059259
- WangQXuXLiJLithium, an anti-psychotic drug, greatly enhances the generation of induced pluripotent stem cellsCell Res201121101424143521727907
- KimDKimCHMoonJGeneration of Human Induced Pluri-potent Stem Cells by Direct Delivery of Reprogramming ProteinsCell Stem Cell200954647247619481515
- HengJCFengBHanJThe nuclear receptor Nr5a2 can replace Oct4 in the reprogramming of murine somatic cells to pluripotent cellsCell Stem Cell20106216717420096661
- Anokye-DansoFTrivediCMJuhrDHighly efficient miRNA-mediated reprogramming of mouse and human somatic cells to pluripotencyCell Stem Cell20118437638821474102
- AziziSAStokesDAugelliBJDiGirolamoCProckopDJEngraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats – similarities to astrocyte graftsProc Natl Acad Sci U S A1998957390839139520466
- LeeRHSeoMJRegerRLMultipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid miceProc Natl Acad Sci U S A200610346174381744317088535
- KamadaYYoshidaYSajiYTransplantation of basic fibroblast growth factor-pretreated adipose tissue-derived stromal cells enhances regression of liver fibrosis in miceAm J Physiol Gastrointest Liver Physiol20092962157167
- SohniAVerfaillieCMMesenchymal Stem Cells Migration Homing and TrackingStem Cells Int2013201313076324194766
- HiguchiALingQDKumarSSExternal stimulus-responsive biomaterials designed for the culture and differentiation of ES, iPS, and adult stem cellsProg Polym Sci201439915851613
- HiguchiALingQDKumarSSDesign of polymeric materials for culturing human pluripotent stem cells: Progress toward feeder-free and xeno-free culturingProg Polym Sci201439713481374
- XieCHuJMaHThree-dimensional growth of iPS cell-derived smooth muscle cells on nanofibrous scaffoldsBiomaterials201132194369437521439638
- KuoYCChungCYTATVHL peptide-grafted alginate/poly(γ−glutamic acid) scaffolds with inverted colloidal crystal topology for neuronal differentiation of iPS cellsBiomaterials201233358955896622998813
- KuoYCChangYHDifferentiation of induced pluripotent stem cells toward neurons in hydrogel biomaterialsColloids Surf B Biointerfaces201310240541123010124
- BahnJJChungJYImWKimMKimSHSuitability of autologous serum for expanding rabbit adipose-derived stem cell populationsJ Vet Sci201213441341723271183
- LindvallOKokaiaZStem cells in human neurodegenerative disorders – time for clinical translation?J Clin Invest20101201294020051634
- WernigMZhaoJPPruszakJNeurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson’s diseaseProc Natl Acad Sci U S A2008105155856586118391196
- AcquaroneMde MeloTMMeirelesFMitomycin-treated undifferentiated embryonic stem cells as a safe and effective therapeutic strategy in a mouse model of Parkinson’s diseaseFront Cell Neurosci201599725904842
- KanamaruTKamimuraNYokotaTIntravenous transplantation of bone marrow-derived mononuclear cells prevents memory impairment in transgenic mouse models of Alzheimer’s diseaseBrain Res20151605495825698614
- AgadiSShettyAKProspects of bone marrow mononuclear cells and mesenchymal stem cells for treating status epilepticus and chronic epilepsyStem Cells20153372093210325851047
- FaghihiFMirzaeiEAiJDifferentiation potential of human chorion-derived mesenchymal stem cells into motor neuron-like cells in two- and three-dimensional culture systemsMol Neurobiol Epub2015320
- BacigaluppiMPluchinoSMartinoGKilicEHermannDMNeural stem/precursor cells for the treatment of ischemic strokeJ Neurol Sci20082651–2737717610905
- SareenDGowingGSahabianAHuman induced pluripotent stem cells are a novel source of neural progenitor cells (iNPCs) that migrate and integrate in the rodent spinal cordJ Comp Neurol2014522122707272824610630
- FalangaVIwamotoSChartierMAutologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous woundsTissue Eng20071361299131217518741
- StudenyMMariniFCChamplinREZompettaCFidlerIJAndreeffMBone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumorsCancer Res200262133603360812097260
- YangCLeiDOuyangWConditioned media from human adipose tissue-derived mesenchymal stem cells and umbilical cord-derived mesenchymal stem cells efficiently induced the apoptosis and differentiation in human glioma cell lines in vitroBiomed Res Int2014201410938924971310
- LianQZhangYZhangJFunctional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in miceCirculation201012191113112320176987
- GarikipatiVNJadhavSPalLPrakashPDikshitMNityanandSMesenchymal stem cells from fetal heart attenuate myocardial injury after infarction: an in vivo serial pinhole gated SPECT-CT study in ratsPLoS One201496e10098224971627
- TakebeTSekineKEnomuraMVascularized and functional human liver from an iPSC-derived organ bud transplantNature2013499745948148423823721
- VillanuevaSCarreñoJESalazarLHuman mesenchymal stem cells derived from adipose tissue reduce functional and tissue damage in a rat model of chronic renal failureClin Sci (Lond)2013125419921023480877
- WiseAFWilliamsTMKiewietMBHuman mesenchymal stem cells alter macrophage phenotype and promote regeneration via homing to the kidney following ischemia-reperfusion injuryAm J Physiol Renal Physiol201430610F1222F123524623144
- LiuYGoldbergAJDennisJEGronowiczGAKuhnLTOne-step derivation of mesenchymal stem cell (MSC)-like cells from human pluripotent stem cells on a fibrillar collagen coatingPLoS One201273e3322522457746
- Villa-DiazLGBrownSELiuYDerivation of mesenchymal stem cells from human induced pluripotent stem cells cultured on synthetic substratesStem Cells20123061174118122415987
- HynesKMenicaninDHanJMesenchymal stem cells from iPS cells facilitate periodontal regenerationJ Dent Res201392983383923884555
- GiulianiMOudrhiriNNomanZMHuman mesenchymal stem cells derived from induced pluripotent stem cells down-regulate NK-cell cytolytic machineryBlood2011118123254326221803852
- ChristoforouNLiauBChakrabortySChellapanMBursacNLeongKWInduced pluripotent stem cell-derived cardiac progenitors differentiate to cardiomyocytes and form biosynthetic tissuesPLoS One201386e6596323785459
- BurkgardtMFMartinezFJWrightSA cellular model for sporadic ALS using patient-derived induced pluripotent stem cellsMol Cell Neurosci20135635536423891805
- MeyerJRThe significance of induced pluripotent stem cells for basic research and clinical therapyJ Med Ethics2008341284985119043107
- YuanTLiaoWFengNHHuman induced pluripotent stem cell-derived neural stem cells survive, migrate, differentiate, and improve neurological function in a rat model of middle cerebral artery occlusionStem Cell Res Ther2013437323769173
- DuanXTuQZhangJApplication of induced pluripotent stem (iPS) cells in periodontal tissue regenerationJ Cell Physiol2011226115015720658533
- FairchildPJThe challenge of immunogenicity in the quest for induced pluripotencyNat Rev Immunol2010101286887521107347
- ArakiRUdaMHokiYNegligible immunogenicity of terminally differentiated cells derived from induced pluripotent or embryonic stem cellsNature2013494743510010423302801
- MorizaneADoiDKikuchiTDirect comparison of autologous and allogeneic transplantation of iPSC-derived neural cells in the brain of a nonhuman primateStem Cell Reports20131428329224319664
- clinicaltrialsgov [homepage on the Internet]A service of the US National Institutes of Health Available from: https://clinicaltrials.gov/Accessed August 16, 2015
