Abstract
Introduction
Mutations in ABCC9 are associated with Cantú syndrome (CS), a very rare genetic disorder characterized by congenital hypertrichosis, acromegaloid facial appearance (AFA), cardiomegaly, and skeletal anomalies.
Case report
We report an 8-year-old female patient with congenital generalized hypertrichosis and coarse facial appearance but without cardiovascular or skeletal compromise. Whole exome sequencing revealed a novel de novo heterozygous mutation in ABCC9. In addition, the genotype and phenotype of the patient were compared with those of the patients reported in the literature and with other related conditions that include AFA, hypertrichosis and AFA, and CS.
Conclusion
This is the first report of a South-American patient with mutation in ABCC9. We propose that her phenotype is a part of a spectrum of features associated with congenital hypertrichosis and mutations in ABCC9, which differs from CS and related disorders. Whole exome sequencing enabled the identification of the causality of this disease characterized by high clinical and genetic heterogeneity.
Introduction
Hypertrichosis is defined as increase in body hair (lanugo, vellus hair, or terminal hair) that is abnormal for a patient reference group. Hypertrichosis may be classified as congenital or acquired, with generalized or regional hair growth. Congenital hypertrichosis may be isolated or part of a syndrome that is associated with dysmorphic features or metabolic disorders. The genetic basis of hypertrichosis is not well understood. Non-androgen-related excessive growth of terminal hair is associated with several rare genetic conditions.Citation1 Three entities with clinical features of hypertrichosis and acromegaloid facial features have been published: 1) Acromegaloid facial appearance syndrome (AFA, MIM: 102150), 2) Hypertrichosis with acromegaloid facial appearance (HAFA, MIM: #135400), and 3) Cantú syndrome (CS), also known as hypertrichotic osteochondrodysplasia (MIM #239850). CS is the best known of these three genetic disorders. This condition was first described by Cantú et al,Citation2 in 1982, and is characterized by autosomal dominant inheritance, congenital hypertrichosis, distinctive coarse facial features, skeletal abnormalities, and cardiac defects.Citation2,Citation3
About 35 patients with CS have been reported in literature. CS appears to be prevalent among all populations, affecting males and females equally, and the disease seems to typically originate by de novo mutations.Citation4–Citation7 Reported patients with AFA and HAFA syndrome have been documented occasionally with cardiac involvement, like pericardial effusionCitation8 and skeletal malformation, like scoliosis,Citation9 suggesting that cardiovascular and skeletal anomalies are not exclusive to CS. Clinical features of AFA, HAFA and CS are summarized in . Clinical overlap between CS and other genetic syndromes with hypertrichosis, including lysosomal storage disease, and poor knowledge about this disease may lead to its underdiagnosis.
Table 1 Summary of clinical features of reported patients with AFA, HAFA, and CS
In 2012, Harakalova et alCitation5 performed exome sequencing in 16 individuals with clinical features of CS and described a novel dominant missense mutation in the ABCC9 gene in 14 of them. They concluded that heterozygous missense mutations in ABCC9 cause CS.Citation5 Deletion/duplication type mutations have not yet been reported, and only two cases have been associated with mutations in another gene (KCNJ8).Citation10,Citation11 ABCC9 gene (previously known as SUR2) encodes a transmembrane protein that is part of an ATP-dependent potassium (KATP) channel that couples the metabolic state of a cell with its electrical activity. Two spliced forms with tissue-specific expression have been reported, namely, SUR2A, expressed in cardiac and skeletal muscles, and SUR2B, expressed in vascular smooth muscle and hair follicles. Mutations in ABCC9 gene reduce ATP-mediated K channel inhibition, resulting in dominant channel opening. This effect of this mutation is similar to the side effects produced by treatment with KATP channel agonist (Minoxidil).Citation10 Only two patients with HAFA syndrome have been reported with mutations in ABCC9.Citation9
However, CS is a part of a wide phenotypic spectrum with variable severity including, AFA, HAFA syndromes, and skeletal dysplasia or CS, which is the most severe form. We report a case of an 8-year-old girl from Colombia, with clinical features of congenital HAFA without skeletal abnormalities or cardiac involvement. Whole exome sequencing (WES) was performed in the patient, and a novel heterozygous missense, likely pathogenic variant in ABCC9 was identified.
Case report
We report a case of an 8-year-old girl, from southwest Colombia, who was the first child of a 29-year-old mother and nonconsanguineous 34-year-old father. Pregnancy was uncomplicated, and prenatal ultrasounds were normal, without history of polyhydramnios. Vaginal delivery at the 37th week of gestation was without complications, and the birth weight was 3,650 g (94th centile) and length was 50 cm (83th centile). At birth, the proband presented neonatal respiratory distress, which required monitoring in the neonatal intensive care unit for 8 days. Clinical findings at birth included excessively thick facial hair, mainly in the forehead region, broad nose, wide mouth, full lips, umbilical hernia, and general hypertrichosis moderately distributed on the trunk and limbs. No history of patent ductus arteriosus or another congenital cardiomyopathy was detected.
The patient experienced multiple episodes of respiratory infection during childhood, which improved after turbinoplasty and adenoidectomy at 4 years of age. At 2 years of age, pubic hair appeared, and follow-up was started with a pediatric endocrinologist who ruled out androgenic hormonal disorder (with normal levels of testosterone, α-OH-progesterone, and somatomedin C). Psychomotor development was unaffected. Although she presented language development delay, she exhibited a dysarthria-like speech and mild learning disabilities; however, IQ test results were within the normal range, and she had no motor developmental concerns. Similar clinical pictures were negative in her family history.
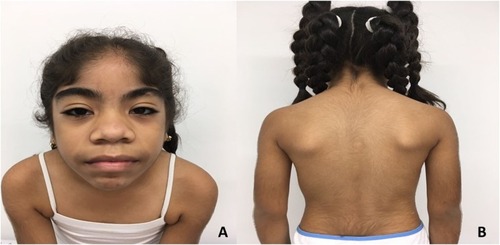
At 8 years of age, she was assessed medically. Her weight was 27.6 kg (48th centile) and height was 128 cm (27th centile). Physical examination revealed generalized hypertrichosis mainly on the face, limbs, back region, and genitals (). Other findings included low anterior hairline, synophrys, long eyelashes, dolichocephaly, hypoplastic nasal bones, broad nose and lips, dental malocclusion with inferior wide-spaced teeth, bilateral epicanthic folds, and AFA without corneal opacity. Osteomuscular examination revealed right fifth finger clinodactyly, bilateral sandal gap, and dorsal scoliosis.
Figure 1 Patient phenotype.
Notes: (A) Coarse facial features, AFA, low anterior hairline, synophrys, long eyelashes, epicanthic folds, broad lips, bulbous nose, and broad mouth. Phenotypic similarities with CS. (B) Generalized hypertrichosis predominantly on back and extremities.
At 7 years of age, radiological findings did not reveal any alterations of the extremities, hips, and spine. Radiography of the hand bone age correlated with her biological age. In addition, endocrinological laboratory test results were normal (thyroid-stimulating hormone, free T4, growth hormone, insulin-like growth factor-1, follicle-stimulating hormone, and leutinizing hormone). Echocardiography showed normal biventricular function, normal left ventricular size, no pericardial effusion, no pulmonary hypertension or signs of cardiomyopathy, and electrocardiogram (ECG) was within normal range. Audiometry reported mild hearing loss in the left ear. Metabolic impairment screening studies for mucopolysaccharides (mucopolysaccharide electrophoresis, enzyme activity of α-L-iduronidase, and arylsulfatase B) and quantitative chromatography of amino acids using plasma and urine revealed normal results.
Further investigation was performed using WES by trio approach with massive sequencing platform with Ion Proton™ technology. The library preparation was designed with Ion AmpliSeq Exome technology (Life Technologies, Carlsbad, CA, USA) which captures >97% of Consensus coding DNA sequencing (CCDS) (>19,000 genes and >198,000 exons) and flanking intronic regions (±5 bp). Only variants in the coding region and flanking intronic regions with a minor allele frequency <1.5% were evaluated. Minor allele frequencies were based on the following databases: 1,000 Genomes, dbSNP, Exome Variant Server (ESVor in-house), and Exome Aggregation Consortium (ExAc). A novel missense, likely pathogenic variant in heterozygous state in ABCC9 (NM_005691.3) was identified: c.3625T>C (p.Tyr1209Hys) (). This variant has not been previously reported in the literature, and the finding was confirmed by Sanger sequencing () and was compatible with the diagnosis of CS. Variant functional prediction software tools Mutation taster, Condel, SIFT, and FATHMM classified it as a Damaging variant (disease causing) (). Protein localization of the change is in the transmembrane domain 2 of ABCC9 (). No additional variants were identified, including in genes associated with lysosomal storage disease, like mucopolysaccharidoses.
Figure 2 ABCC9 protein structure and Sanger sequencing results.
Notes: (A) ABCC9 protein structure: mutation is located on TMD2 domain. Mutations at this point result in gain of function in the KATP channel resulting in the opening of the potassium channel. The arrow indicates the novel variant that was present in the patient. (B) Sanger sequencing results: The box frames the affected codon and the arrow indicates the heterozygous state of the identified variant.
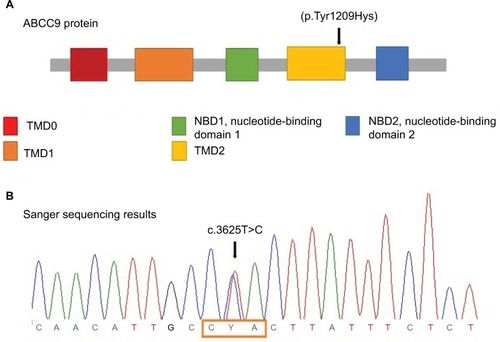
Table 2 Prediction of novel variant in ABCC9
Ethics and consent to participate
Written informed consent was obtained from patient’s parents for publication of her images and clinical data for scientific purposes. Data were collected in the context of studies performed in accordance with the Declaration of Helsinki Good Clinical Guidelines and protocol #509 “registry of surveil lance and survival of congenital defects of the Colombian South-West” approved by the Ethics Committee of Univer-sidad Icesi (Act 192/2011).
Discussion
An important feature of CS is high phenotypic variability. Neonatal characteristics including macrosomia, observed in our patient, soft tissue swelling, and edema have been reported in approximately 50% of the affected individuals.Citation2,Citation3,Citation13,Citation14 During childhood, patients usually have low subcutaneous fat and muscular appearance,Citation4 but our patient did not manifest these features. The phenotypic characteristics in our proband, including coarse facial features, low nasal bridge, epicanthic folds, synophris, and broad mouth and lips, are similar to those described in previously reported cases. In addition, coarse facial features and hypertrichosis are also associated with certain lysosomal storage diseases, such as Hurler and Hunter syndromes. Therefore, a clinical diagnostic workup to rule out these pathologies is necessary.
Cardiac manifestations, including patent ductus arteriosus, ventricular hypertrophy, pulmonary hypertension, pericardial effusion, and increased vascular tortuosity, have been reported in 80% of the patients.Citation4,Citation12–Citation14 Transthoracic echocardiography and electrocardiography results were within the normal range. These findings may suggest HAFA (MIM #135400) as a diagnosis. However, only one individual with HAFA has been identified with a mutation in ABCC9.Citation9 Mutations in this gene are associated with isolated atrial fibrillation, dilated cardiomyopathy,Citation15 myocardial infarction,Citation16 and repolarization syndrome.Citation17 This indicates that ABCC9 plays a key role in KATP channels, which function primarily in the heart and smooth muscles. This phenomenon may explain that mutations could greatly affect the cardiovascular system. Previous reports have described that cardiac manifestations appear early in life, including congenital presentation of hypertrophic and/or dilated cardiomiopathy.Citation4,Citation5 The absence of this feature in our proband was definitive; however, clinical follow-up is necessary regarding possible progressive behavior not previously reported.
Affected individuals with CS have skeletal abnormalities and craniofacial features including thickening of cranial vault, narrow thorax, broad ribs, long bones with metaphy-seal widening of long bones, flat-ovoid vertebral bodies, enlarged medullary canals, and coxa valga.Citation4,Citation13,Citation14 Skeletal abnormalities were not found by X-ray imaging. However, clinically, the patient exhibited scoliosis. Similar to cardiac manifestations, absence of skeletal abnormalities in X-ray studies suggest that they are unlikely to appear later in life.Citation4,Citation5 Other neurologic manifestations may include hypotonia, language and motor delays, and light to severe intellectual impairment.Citation4 In our proband, we observed mild dysarthria, without other neurological compromise. Recurrent infections in lower and upper respiratory tract appear in reported patients and in our patient.
ABCC9 gene (also known as SUR2) is located on locus 12p12.1 of human chromosomes and encodes a transmembrane protein of 1,549 amino acids. SUR2 forms the regulatory part of an ATP-sensitive potassium complex that consists of four subunits of a transmembrane pore (KCNJ8). The protein has two splice variants SUR2A (expressed in cardiac and skeletal muscle) and SUR2B (expressed in smooth muscle), which contains three transmembrane cytoplasmic domains (TMD0, TMD1, TMD2), and two nucleotide binding folds (NFB1 and NFB2) (). Mutations in CS located in ABCC9 transmembrane domain result from a gain of function in the KATP channel regulated by these subunits.Citation4–Citation6,Citation9 Therefore, missense mutations are believed to cause the opening of the potassium channel, causing metabolic and electric disequilibrium essentially in cardiomyocytes.Citation5,Citation18 We identified a de novo pathogenic missense mutation c.3625T>C (p.Tyr1209Hys), which was not found in the 1,000 Genomes Project, ExAC, EVSor in-house database, and so it was considered as previously not reported. Based on the American College of Medical Genetics and Genomics (AMCG) variant interpretation guideline,Citation19 this variant is classified as likely pathogenic (), and was validated by Mutation taster, Condel, SIFT, and FATHMM; it is predicted to have deleterious effect. This change occurs in a genomic position highly evolutionarily conserved in vertebrates.
Two similar mutations have been reported in the TMD2 domain, which were associated with AFA and HAFA syndrome. The phenotype of these patients included AFA, gingival hyperplasia, enlarged hands and foot, and arched eyebrows, in combination with congenital severe generalized hypertrichosis.Citation9 In addition, the HAFA phenotype is described as having AFA, congenital hypertrichosis terminalis, and gingival hyperplasia; although the gene is unknown, it has been demonstrated that this condition is a contiguous gene syndrome, comprised on chromosomic region 17q (q24.2-q24.3).Citation20 The data suggest that HAFA might be related with less severe manifestations of CS, with a different genetic cause than ABCC9 and KCNJ8.Citation20 Despite some authors distinguishing AFA, HAFA, and CS, we suggest they should be considered as the same disorder with a variable severity in phenotype rather than as separate conditions.
Furthermore, it is important to mention that WES is an effective diagnostic tool that is useful for a geneticist to identify monogenic conditions. Exome sequencing has greatly influenced the timeframe within which new disease genes are identified. In the last decade, exome sequencing has improved diagnostic performance by more than 25%, leading to identification of disease-causing genes in cases where the diagnosis was previously unknown, and allows a complete characterization of genetic variations.Citation21 Confirmatory identification of the underlying genetic cause of CS mutations in ABCC9 by WES technology has been done in about 28 of 35 patients. This technology helps to clarify the spectrum of this condition and its overlap with other similar syndromes mentioned previously.Citation22
Conclusion
In summary, we report on a Colombian patient with congenital hypertrichosis, acromegaloid facial features, and no skeletal abnormalities or cardiac manifestations, with a novel missense mutation in ABCC9 gene, which may suggest a wide spectrum of phenotypes associated with mutations in this gene. We propose that her mild phenotype could be similar to the patient reported by Czeschik et alCitation9 in 2013, and hence is suggestive of a new genetic condition. However, additional reports are needed to confirm this observation and experimental studies are necessary to demonstrate those findings.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
We would like to thank the family of the patient for their participation in this study.
Disclosure
The authors report no conflicts of interest in this work.
References
- PavonePPraticòAFalsaperlaRCongenital generalized hypertrichosis: the skin as a clue to complex malformation syndromesItal J Pediatr20154115526242548
- CantúJMGarcia-CruzDSanchez-CoronaJHernandezANazaraZA Distinct osteochondrodysplasia with hypertrichosis – individualization of a probable autosomal recessive entityHum Genet19826036417076246
- Garcia-CruzDSánchez-CoronaJNazaráZCongenital hypertrichosis, osteochondrodysplasia, and cardiomegaly: further delineation of a new genetic syndromeAm J Med Genet1997691381519056550
- Van BonBWGilissenCGrangeDKCantú syndrome is caused by mutations in ABCC9Am J Hum Genet2012901094110122608503
- HarakalovaMvan HarsselJJTerhalPADominant missense mutations in ABCC9 cause Cantú syndromeNature Genet20124479379622610116
- HirakiYMiyatakeSHayashidaniMAortic aneurysm and craniosynostosis in a family with Cantú syndromeAm J Med Genet A2014164A23123624352916
- ParkJYKooSHJungYJLimYJChungMLA patient with Cantú syndrome associated with fatal bronchopulmonary dysplasia and pulmonary hypertensionAm J Med Genet2014164A2118212024715715
- ZalanteLGaspariniPSavoiaALomutoMPellicanoRA new case of acromegaloid facial appearance (AFA) síndrome with an expanden phenotypeClin Dysmorphol2000922122210955485
- CzeschikJCVoigtCGoeckeTOWide clinical variability in conditions with coarse facial features and hypertrichosis caused by mutations in ABCC9Am J Med Genet A2013161A29530023307537
- BrownsteinCATowneMCLuquetteLJMutation of KCNJ8 in a patient with Cantú syndrome with unique vascular abnormalities - support for the role of K(ATP) channels in this conditionEur J Med Genet20135667868224176758
- CooperPEReutterHWoelfleJCantú syndrome resulting from activating mutation in the KCNJ8 geneHum Mutat20143580981324700710
- GrangeDKLorchSMColePLSinghGKCantú syndrome in a woman and her two daughters: further confirmation of autosomal dominant inheritance and review of the cardiac manifestationsAm J Med Genet A20061401673168016835932
- NevinNCMulhollandHCThomasPSCongenital hypertrichosis, cardiomegaly and mild osteochondrodysplasiaAm J Med Genet19966633388957508
- RosserEMKaariainenHHurstJAThree patients with the osteochondrodysplasia and hypertrichosis syndrome – Cantú syndromeClin Dysmorphol1998779859571276
- BienengraeberMOlsonTMSelivanovVAABCC9 mutations identified in human dilated cardiomyopathy disrupt catalytic KATP channel gatingNat Genet20043638238715034580
- MinorettiPFalconeCAldeghiAA novel Val734Ile variant in the ABCC9 gene associated with myocardial infarctionClin Chim Acta20063701–212412816563363
- HuDBarajas-MartínezHTerzicAABCC9 is a novel Brugada and early repolarization syndrome susceptibility geneInt J Cardiol2014171343144224439875
- BryanJMuñozAZhangXABCC8 and ABCC9: ABC transporters that regulate K+ channelsPflugers Arch2007453570371816897043
- RichardsSAzizNBaleSStandards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular PathologyGenet Med20151740542425741868
- SunMLiNDongWCopy-number mutations on chromosome 17q24.2-q24.3 in congenital generalized hypertrichosis terminalis with or without gingival hyperplasiaAm J Hum Genet20098480781319463983
- BieseckerLGGreenRCDiagnostic clinical genome and exome sequencingN Engl J Med20143702418242524941179
- BramswigNCLüdeckeHJAlanayYExome sequencing unravels unexpected differential diagnoses in individuals with the tentative diagnosis of Coffin-Siris and Nicolaides-Baraitser syndromesHum Genet2015134655356825724810
- HughesHEMcAlpinePJCoxDWPhilippsSAn autosomal dominant syndrome with ‘acromegaloid’ features and thickened oral mucosaJ Med Genet19852221191253989825
- DallapiccolaBZelanteLAccadiaLMingarelliRAcromegaloid facial appearance (AFA) syndrome: report of a second familyJ Med Genet19922964194221619638
- KiniUClayton-SmithJAcromegaloid facial appearance syndrome: a further case reportClin Dysmorphol20041325125315365463
- CanúnSGuevara-SanginésEGElvira-MoralesASierra-Romero MdelCRodríguez-AsbunHHypertrichosis terminalis, gingival hyperplasia, and a characteristic face: a new distinct entityAm J Med Genet2003116A327828312503107
- IrvineADDolanOMHaddenDRStewartFJBinghamEANevinNCAn autosomal dominant syndrome of acromegaloid facial appearance and generalised hypertrichosis terminalisJ Med Genet1996339729748950682
- ScurrIWilsonLLeesMCantu syndrome: report of nine new cases and expansion of the clinical phenotypeAm J Med Genet2011155A50851821344641
- FryssiraHPsoniSAmentaSCantú Syndrome Associated with Ovarian AgenesisMol Syndromol20178420621028690487
- AfifiHHAbdel-HamidMSEidMMMostafaISAbdel-SalamGMDe Novo Mutation in ABCC9 Causes Hypertrichosis Acromegaloid Facial Features DisorderPediatr Dermatol2016332e109e11326871653
