Abstract
Gaucher disease is the most common sphingolipid storage disease and is present in all ethnic groups. Its symptoms span all systems including the cardiovascular system. The health care provider should be vigilant regarding this potentially fatal complication. Gaucher disease type IIIC has been linked to causing oculomotor apraxia and cardiac calcification. We report a Saudi girl who developed valvular and aortic calcification in late childhood and died as a result of her cardiovascular complications. This report further strengthens the association and reminds the clinicians that patients with D409H mutation need echocardiographic evaluation annually.
Keywords:
Introduction
Gaucher disease (GD) is the prototypical and the most known among storage diseases, and one of the earliest lysosomal diseases to be described. It is called after Dr Gaucher, a French dermatologist who described a 32-year-old lady with splenic enlargement in 1882. It was not until 1962 when the biochemical abnormalities in GD were unpuzzled. GD is caused by deficient activity of lysosomal β-glucocerebrosidase (encoded by GBA gene), an enzyme that converts glucocerebroside to ceramide and glucose.Citation1 GD is multisystem autosomal recessive disease, and it has been classified into three known clinical phenotypes based on the age of onset, progression, and neurological involvement. GD type I (OMIM #230800) is the nonneuronopathic type, and it is character-ized by the presence of bone disease, hepatosplenomegaly, anemia, thrombocytopenia, and, to a lesser extent, pulmonary and renal involvement.Citation2 Neurological symptoms are present in type II and III. GD type II (OMIM #230900) is the acute neuronopathic form, and it is characterized by hepatosplenomegaly and extensive central nervous system damage in infancy. Type III GD (OMIM #231000) has a later onset and less severe course. Patterson et alCitation3 divided type III into type IIIA, which is characterized by myoclonus and dementia, and type IIIB, characterized by early onset of isolated horizontal supranuclear gaze palsy and aggressive systemic disease. Type IIIC (OMIM #231005) has been proposed recently and was discovered in patients harboring the homozygous missense variant (D409H) in GBA gene. They present with cardiac valvular calcifications and neurological manifestations.Citation4 It is believed that other genetic and environmental factors play a role in the final encountered phenotype.Citation5 GD shows poor genotype–phenotype correlation, and it has been found that the phenotype differs even between monozygotic twins.Citation6,Citation7
Case report
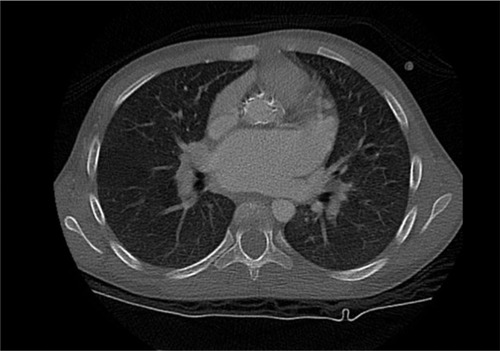
The patient was an 11-year-old Saudi girl who was referred to our center from a local hospital at age of 4 years when she was discovered to have hepatosplenomegaly and pancytopenia. She was born at full term to a healthy Saudi mother following an uneventful pregnancy. Her perinatal history as well as developmental history were unremarkable. Family history was significant for consanguinity and a history of an older brother deceased at age of 12 years with GD type III based on molecular and enzymatic testing with a similar clinical picture of his sister. On physical examination, her weight was in the 10th percentile and height was in the 3rd percentile. Her heart rate and blood pressure were within normal range. The liver and spleen were palpable 5 and 7 cm below the costal margin, respectively. Cardiac examination was normal at the time of presentation. Neurological examination was normal apart from oculomotor apraxia. Examinations of other systems were unremarkable. Complete blood count showed pancytopenia as follows: white blood cells: 4×109/L (5–15), hemoglobin: 11.7 g/dL (11.5–13.5), platelets: 106×109/L (140–350). Echocardiography was normal. β-glucocerebrosidase activity was low (0.02 U/g protein). Abdominal ultrasound showed mild enlargement of the liver with a measurement of 13.5 cm and diffuse increased echogenicity. Also, there was moderate splenomegaly, with a splenic measurement of 14.2 cm. She was subsequently diagnosed with GD, which was confirmed by genetic testing that showed a homozygous mutation in the GBA gene (NM_000157.3(GBA):c.1342G>C: p.(Asp448His)). This variant is known as D409H, and we will use the old nomenclature for consistency. The family refused enzyme replacement therapy due to social reasons. Years later, she presented at 11 years of age with a history of syncope after exertion and dyspnea. On examination, her weight and height were below the 3rd percentile. She continued to have hepatosplenomegaly and pancytopenia and has normal neurological status. Complete blood count showed the following values: white blood cells: 9.6×109/L (5–15), hemoglobin: 9.7 g/dL (11.5–15.5), platelets: 109×109/L (140–350). Abdominal ultrasound showed mild hepatomegaly, with a measurement of 15.6 cm, and moderate splenomegaly, with a measurement of 17 cm. There was no focal lesion and normal parenchyma. A repeated echocardiography revealed thickened mitral valve leaflets with severe mitral regurgitation, thickened aortic valve leaflets, fixed right aortic cusp with mild regurgitation, severely dilated left atrium, depressed biventricular systolic function, right ventricular outflow tract obstruction, and peak end-diastolic gradient of 18 mmHg. Cardiac computed tomography scan () showed diffuse thickening of the aortic root extending along the ascending aorta and aortic arch with calcifications. There was minimal fluid surrounding the aorta, thickening of the aortic valve leaflets and mitral valve, and marked enlargement of the left atrium. She was started on antifailure medications without significant improvement, as observed on repeated echocardiography. She was scheduled for surgery, but unfortunately, she deteriorated rapidly and died before the surgery.
Discussion
We report a case of a Saudi girl who was homozygous for the D409H mutation and developed valvular and aortic calcifications. Aortic and valve calcification in GD has been previously reported in multiple ethnic groups. All the patients reported had the variant D409H, which is known to cause GD type III. This variant has been reported in most ethnic groups with most reports from the Middle East. Casta et alCitation8 was the first to report aortic calcification in GD. He described a 15-year-old boy with diffuse corneal deposits and calcification of ascending aorta and aortic and mitral valves.Citation8 Uyama et alCitation9 reported three Japanese siblings with supranuclear gaze palsies, corneal opacities, communicating hydrocephalus, deformed toes, deafness, calcified aortic valve, and mitral stenosis. Those siblings were homozygous for the D409H mutation.Citation10 Abrahamov et alCitation11 described 12 Arab patients with oculomotor apraxia, calcifications of the aortic or mitral valves, and corneal opacities. All of them were homozygous for the D409H mutation.Citation11 Chabás et alCitation12 has previously shown that three siblings with GD harboring the variant D409H had calcification of the ascending aorta and of the aortic and mitral valves. They also had neurological findings in the form of tonic–clonic seizures in one patient and ophthalmoplegia and saccadic eye movements in two patients.Citation12 Bohlega et alCitation13 reported four siblings with abnormal eye movements and calcification of the ascending aorta, aortic and mitral valves, and aortic root. George et alCitation14 reported a Palestinian boy with a severe mitral and aortic valves thickening and calcification as well as thickening and calcification of the ascending aorta, transverse arch, and isthmus.
Cindik et alCitation15 reported a 14-year-old Turkish girl with hydrocephalus, biatrial and left ventricular dilatation, severe aortic and mitral valve insufficiency, and aortic and mitral valve stenosis and thickening. Talluto and SilvermanCitation16 reported a 13-year-old Mexican–American girl with severe aortic valve stenosis and generalized calcification involving the aortic and coronary arteries and aortic and mitral valves. Altunbas et alCitation17 reported widespread calcification in the ascending aorta, left main coronary artery, and carotid arteries in a Turkish patient.Citation17 Saleh et alCitation19 and Abdelwahab et alCitation18 described three Egyptian patients with aortic calcification with severe stenosis and left ventricular hypertrophy. One patient expired due to sudden cardiac death.Citation19 Kör et alCitation20 reported a 15-year-old Turkish boy with aortic and mitral valves thickening with fibrosis, aortic and mitral valve regurgitation, aortic valve stenosis, and widespread calcification involving the ascending and transverse aorta, left main coronary artery, and the left carotid and subclavian artery. The main distinguishing feature of D409H mutation is that it leads to calcification of the aorta and mitral and aortic valves, as illustrated above. How the disease results in aortic and valvular calcification is poorly understood. Since the disease was acquired in almost all cases, it has been proposed that it is caused by valvulitis triggered by deposition of Gaucher cells or by the release of cytokines.Citation13,Citation21 This association between D409H mutation and cardiac calcification necessitates comprehensive cardiac evaluation in patients with GD, particularly those homozygous for the D409H mutation. Some of the reported patients received enzyme replacement therapy that did not seem to stop the calcification, which further supports the need for cardiac surveillance in those patients. We herein recommend annual echocardiogram for patients harboring the mutation D409H as a measure to detect the disease earlier.
Informed consent
The patient’s guardian has provided written informed consent for publication of the case details.
Disclosure
The authors report no conflicts of interest in this work.
References
- FerreiraCRGahlWALysosomal storage diseasesTransl Sci Rare Dis201721–217129152458
- BradyROBartonNWGrabowskiGAThe role of neurogenetics in Gaucher diseaseArch Neurol19935011121212248215980
- PattersonMCHorowitzMAbelRBIsolated horizontal supranuclear gaze palsy as a marker of severe systemic involvement in Gaucher’s diseaseNeurology19934310199319978413956
- MirelesSASeyboldJWilliamsGUndiagnosed type IIIc Gaucher disease in a child with aortic and mitral valve calcification: perioperative complications after cardiac surgeryJ Cardiothorac Vasc Anesth201024347147419632857
- KoprivicaVStoneDLParkJKAnalysis and classification of 304 mutant alleles in patients with type 1 and type 3 Gaucher diseaseAm J Hum Genet20006661777178610796875
- LachmannRHGrantIRHalsallDCoxTMTwin pairs showing discordance of phenotype in adult Gaucher’s diseaseQJM200497419920415028849
- ElsteinDGellmanAAltarescuGDisease severity in sibling pairs with type 1 Gaucher diseaseJ Inherit Metab Dis2010331798320049528
- CastaAHaydenKWolfWJCalcification of the ascending aorta and aortic and mitral valves in Gaucher’s diseaseAm J Cardiol19845410139013916507325
- UyamaETakahashiKOwadaMHydrocephalus, corneal opacities, deafness, valvular heart disease, deformed toes and leptomeningeal fibrous thickening in adult siblings: a new syndrome associated with beta-glucocerebrosidase deficiency and a mosaic population of storage cellsActa Neurol Scand19928644074201333717
- UyamaEUchinoMIdaHEtoYOwadaMD409H/D409H genotype in Gaucher-like diseaseJ Med Genet19973421759040001
- AbrahamovAElsteinDGross-TsurVGaucher’s disease variant characterised by progressive calcification of heart valves and unique genotypeLancet19953468981100010037475546
- ChabásACormandBGrinbergDUnusual expression of Gaucher’s disease: cardiovascular calcifications in three sibs homozygous for the D409H mutationJ Med Genet19953297407428544197
- BohlegaSKambourisMShahidMAl HomsiMAl SousWGaucher disease with oculomotor apraxia and cardiovascular calcification (Gaucher type IIIC)Neurology200054126126310636167
- GeorgeRMcmahonJLytleBClarkBLichtinASevere valvular and aortic arch calcification in a patient with Gaucher’s disease homozygous for the D409H mutationClin Genet200159536036311359469
- CindikNOzcayFSürenDGaucher disease with communicating hydrocephalus and cardiac involvementClin Cardiol2010331E26E3019816973
- TallutoCJSilvermanNHAortic and mitral valve stenosis with regurgitation: not due to rheumatic heart diseaseEchocardiography2011282E24E2720718842
- AltunbasGErcanSInançIHOzerOKervancıoğluSDavutoğluVExtensive vascular and valvular involvement in Gaucher diseaseAsian Cardiovasc Thorac Ann201523444644824887908
- AbdelwahabMBlankenshipDSchiffmannRLong-term follow-up and sudden unexpected death in Gaucher disease type 3 in EgyptNeurol Genet201622e5527123474
- SalehYAlmaghrabyAHammadBMokhtarAAbdel-HayMAGaucher disease causing sudden cardiac deathEgypt Heart J2016683201204
- KörYKeskinMBaşpınarOSevere cardiac involvement in Gaucher type IIIC: a case report and review of the literatureCardiol Young20172771426142928393750
- VeinotJPElsteinDHananiaDAbrahamovASrivatsaSZimranAGaucher’s disease with valve calcification: possible role of Gaucher cells, bone matrix proteins and integrinsCan J Cardiol199915221121610079781