Abstract
Background
Allopurinol, a common anti-hyperuricemia drug, is well known as an inducer of severe cutaneous adverse drug reactions (SCARs). One of the most well-defined risk factors of allopurinol-induced SCARs is the presence of polymorphic alleles of human leukocyte antigen (HLA) genes, such as HLA-B*58:01 and HLA-C*03:02 alleles. There is no commercial test or published in-house protocol for the specific detection of the HLA-C*03:02 allele. In this article, we established for the first time a simple allele-specific (AS) PCR method to identify HLA-C*03:02 allele carriers, and at the same time, determine their zygosities.
Methods
A two-step AS-PCR protocol, using four primer sets, was designed to specifically amplify and differentiate the HLA-C*03:02 allele from 17 other HLA-C alleles found in Vietnamese people. The protocol was validated with PCR-sequencing-based typing (SBT) of 100 samples of unknown genotypes.
Results
The PCR protocol can detect the HLA-C*03:02 allele and determine the zygosity. The results of this protocol were highly consistent with those of the SBT (ĸ = 0.98, p < 0.001). Regarding the specific detection of the HLA-C*03:02 allele, the PCR protocol had a sensitivity of 100% (95% CI: 91.61–100%) and specificity of 98.3% (95% CI: 90.9–99.7%). The protocol was used to determine the distribution of the HLA-C*03:02 allele in 810 unrelated Vietnamese Kinh people, 14.2% of which were HLA-C*03:02 carriers, the allele frequency was 7.5%.
Conclusion
A novel AS-PCR protocol with a sensitivity of 100% for the detection of the HLA-C*03:02 allele was established. The protocol can be used for personalized treatment with allopurinol in order to minimize the risk of SCARs in Vietnamese people as well as in other Asian populations with similar genetic characteristics.
Introduction
Allopurinol is a common hyperuricemia drug and one of the top inducers of severe cutaneous drug reactions (SCARs), especially in Asian patients.Citation1,Citation2 One of the most well-defined risk factors for allopurinol-induced SCARs is the presence of polymorphic human leukocyte antigen (HLA) alleles such as the HLA-B*58:01 allele,Citation3–Citation6 and to a lesser extent, the HLA-C*03:02 allele.Citation7,Citation8 The HLA-C*03:02 allele was found at 94% and 92%, respectively, of Han Chinese and Korean patients with allopurinol-induced SCARs. This allele was significantly associated with allopurinol-induced SCARs (OR = 97.7, p=1.4x10−9 in Han Chinese patients,Citation7 OR = 82.1, p = 9.39x10−11 in Korean patients).Citation8 These studies implicated that the HLA-C*03:02 allele might be another pharmacogenomic marker together with the HLA-B*58:01 allele in allopurinol personalized treatment. Notably, the frequencies of the HLA-C*03:02 allele in several Asian populations including Vietnamese people are as high as that of the HLA-B*58:01 allele.Citation9
A number of HLA-B*58:01 allele-specific detection methods have been commercialized to identify the patients at risk, change the prescription and therefore, minimize the SCARs risk. However, there is no protocol for specific detection of the HLA-C*03:02 allele. HLA-C*03:02 genotyping methods for research purposes include saturated tiling capture sequencing,Citation10 next-generation sequencing,Citation11 whole-genome sequencing,Citation12 multiplex sequencing-based typing (SBT),Citation13,Citation14 sequence-specific oligonucleotides (SSO),Citation9 and multiplex real-time PCRCitation15 which uses series of primer sets for analysis of multiple HLA loci. All of those methods are very costly and would be difficult to be applied in clinical settings for allopurinol personalized medicine. There is a need for a simple and specific HLA-C*03:02 detection method for allopurinol personalized therapy, in order to avoid SCAR risk.
In this study, we established, for the first time, a simple allele-specific (AS) PCR method to detect HLA-C*03:02 allele carriers in Vietnamese Kinh people and identify their zygosities. This protocol was applied to determine the frequency of the HLA-C*03:02 allele in 810 unrelated Vietnamese Kinh people.
Materials and Methods
Human Genomic DNA Samples
For protocol optimization, 10 DNA samples of known HLA-C genotype were provided by the Division of Pharmacogenomics and Personalized Medicine, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Thailand. The HLA genotypes of those samples were determined using the SSO method.Citation16
For protocol validation, 100 DNA samples were prepared from the whole blood of unrelated Vietnamese Kinh people, including allopurinol-induced SCAR patients (48) and healthy volunteers (52).
For the HLA-C*03:02 allele frequency identification, 810 DNA samples were prepared from the whole blood of unrelated Vietnamese Kinh people evenly distributed in the North, the Centre and the South of Vietnam.
The study complied with the Declaration of Helsinki and was approved by the Ethics Committee of the Vietnam National Institute of Hygiene and Epidemiology (IRB-VN01057-6/2018). All of the participants provided their informed written consents.
DNA Isolation
Whole blood was collected into EDTA anticoagulant tubes and stored at −20°C until DNA extraction. Genomic DNA was isolated using the E.Z.N.A.® Tissue DNA Kit (Omega Bio-tek, Atlanta, USA). The isolated DNA quantity and quality were assessed using Nanodrop 2000 (Thermo Fisher, Waltham, USA). The samples at the concentration of 35–250 ng/µL and the A260/280 of 1.65–1.95 were qualified for further experiments.
Detection of the HLA-C*03:02 Allele by the AS-PCR Method
The PCR protocol consisted of two steps (). The PCR primers were designed based on the alignment of 18 HLA-C alleles in the Vietnamese population reported by Hoa et al.Citation9 The sequences of the 18 alleles were obtained from the IPD-IMGT/HLA database.Citation17
Figure 1 Strategy for detecting and distinguishing homozygous/heterozygous genotypes of the HLA-C*03:02 allele. (A) PCR procedures: Step 1. The primer set HLACB1F/HLACB1R specifically amplified the exon 2–3 sequence of the HLA-C gene. Step 2. The 912 bp PCR product from step 1 was then used as a template for the step 2 PCR reactions, which used three primer sets. (B) Different patterns can be obtained with the three primer sets in the second PCR step according to the HLA-C*03:02 zygosity. (*): allele number.
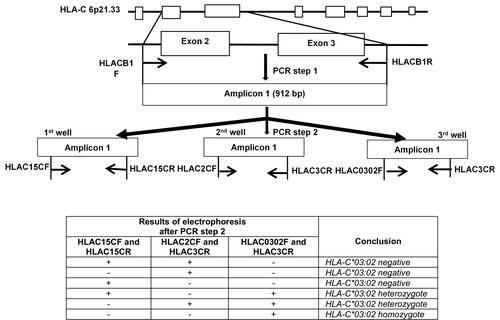
In step 1, the primer set (HLACB1F/HLACB1R) was used to amplify specifically the exon 2–3 of the HLA-C locus. The first PCR was performed in a reaction mixture of 20 µL containing 40 ng of genomic DNA, 0.5 pM of each primer (Integrated DNA Technologies, Coralville, USA) and 10 µL of GoTaq® Green Master Mix 2x (Promega Corporation, Madison, USA). The PCR conditions for the first step were 95°C for 3 minutes, followed by 28 cycles of 95°C for 30 seconds, and 65°C for 30 seconds, 72°C for 60 seconds; and finally 72°C for 7 minutes. The first step PCR products were visualized by ethidium bromide under UV with 1% agarose gel electrophoresis. 1 µL of the first PCR product was diluted 100-fold with distilled sterilized water and used as a template for step 2 PCR.
After the amplification of the exon 2–3 of the HLA-C locus, the HLA-C*03 alleles were amplified specifically in step 2. In step 2, the protocol can be flexibly used for two different purposes – determination of zygosity and screening the allele HLA-C*03:02. For the differentiation of homozygous and heterozygous genotypes of the HLA-C*03:02 alleles, three PCR reactions were performed with three primer sets. Each PCR reaction mixture of 20 µL contained 1 µL of the step 1 PCR diluted product, 0.5 pM of each primer (Integrated DNA Technologies, Coralville, USA) and 10 µL of GoTaq® Green Master Mix 2x (Promega Corporation, Madison, USA). For the purpose of HLA-C*03:02 screening only, one PCR reaction with the primer set (HLAC0302F/HLAC3CR) was needed. Touchdown PCR cycles were used in the second PCR step to increase specificity: 95°C for 3 minutes, followed by 5 cycles of 95°C for 30 seconds, and 70°C for 30 seconds, 72°C for 30 seconds; 5 cycles of 95°C for 30 seconds, 67°C for 30 seconds, 72°C for 30 seconds, 10 cycles of 95°C for 30 seconds, 65°C for 30 seconds, 72°C for 30 seconds, 20 cycles of 95°C for 30 seconds, 58°C for 30 seconds, 72°C for 30 seconds, and finally 72°C for 7 minutes. The PCR products were visualized by ethidium bromide under UV with 1% agarose gel electrophoresis.
PCR-SBT
DNA sequences were determined by the PCR-SBT method using the BigDyeTM Terminator v3.1 Cycle Sequencing Kit (ThermoFisher Scientific, Waltham, USA) and an ABITM 3500 analyzer (Applied Biosystems, Massachusetts, USA). The primer sets and sequencing procedures have been previously described.Citation14
Data Analysis
The sensitivity and specificity of the AS-PCR protocol were determined using MedCalc v19.2.3 (MedCalc Software, Ostend, Belgium). Cohen’s Kappa coefficient for the comparison between the in-house protocol and the PCR-SBT method as well as the allele frequency was determined using SPSS 20 (Chicago, IL, USA). Raw data from direct sequencing were analyzed using Bioedit 7.0.5.3 (Informer Technologies, Inc).
Results
The strategy for the HLA-C*03:02 allele detection is described in . The first PCR step with the primer set HLACB1F/HLACB1R was performed to selectively amplify the exon 2–3 which is the most polymorphic region containing most of the SNPs of the HLA-C locus. Three primer sets were used in the second PCR to differentiate the HLA-C*03:02 allele from the other known HLA-C alleles in the Vietnamese population, especially the two highly homologous alleles HLA-C*03:03 and HLA-C*03:049. Results of the three parallel PCR reactions enabled the conclusion of either homozygous or heterozygous genotypes of the HLA-C*03:02 allele. Alternatively, only one PCR reaction with the primer set HLAC0302F/HLAC3CR is needed for the detection of HLA-C*03:02 carriers. The sequences of the primer sets designed for these purposes are shown in . Their binding sites are described in and .
Table 1 Sequences of Primer Sets Used for the Two PCR Steps
Figure 2 Binding sites of the primer set used in the step 1 PCR. (A) Forward primer HLACB1F: a mismatch (replacement of G with T) at the penultimate position of the 3′ terminus is shown in grey. (B) Reverse primer HLACB1R. The reference sequences were obtained from https://www.ebi.ac.uk/ipd/imgt/hla/.Citation17
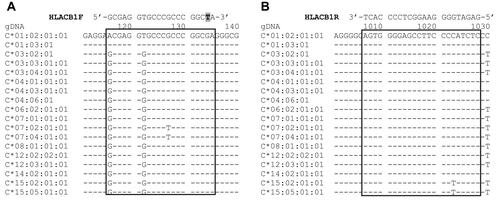
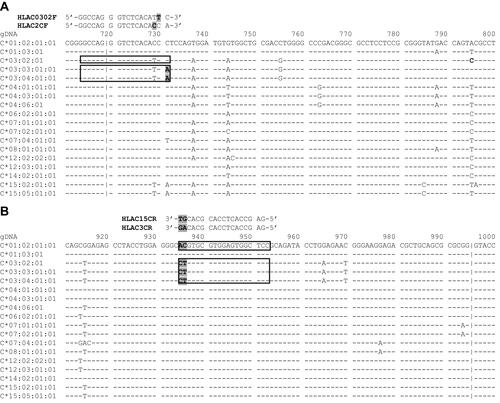
Figure 3 Binding sites of the primer sets used in the step 2 PCR. (A) HLAC0302F has one mismatch (replacement of C with T) at the penultimate position of the 3′ terminus; HLAC2CF has one mismatch (replacement of T with C) at the third position from the 3′ terminus. The mismatches are highlighted in grey; (B) HLAC3CR and HLAC15CR have two different nucleotides (highlighted in grey) at the 3′ terminus that ensure the specificity of the primers. The reference sequences were obtained from https://www.ebi.ac.uk/ipd/imgt/hla/.Citation17
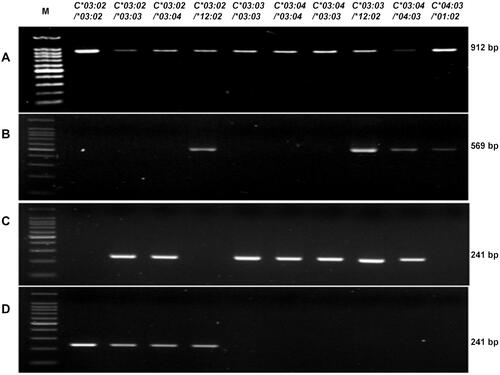
First, the AS-PCR protocol was tested on 10 samples of known genotypes. After the first PCR, a single band of 912 bp was obtained in all of the 10 samples (). After the second PCR with the primer set HLAC15CF/HLC15CR, a single band of 569 bp was obtained with 4 samples (numbered 4, 8, 9, 10) (). The PCR with the primer set HLAC2CF/HLAC3CR resulted in a single band of 241 bp with samples numbered 2, 3, 5, 6, 7 8, 9 (). The specific amplification of the HLA-C*03:02 allele with the primer set HLAC0302F/HLAC3CR resulted in a single band of 241 bp with samples numbered 1, 2, 3, and 4 (). The comparison in shows a hundred-percent agreement.
Table 2 AS-PCR Results of 10 Samples of Known Genotypes
Figure 4 The detection of the HLA-C*03:02 allele in 10 samples of known genotype. (A) Step 1: HLA-C exon 2–3 amplicon, 912 bp; (B) Step 2: amplicon from the primer set HLAC15CF and HLAC15CR, 569 bp; (C) Step 2 amplicon from the primer set HLAC2CF and HLAC3CR, 241 bp; (D) Step 2: amplicon from the primer set HLAC0302F and HLAC3CR, 241 bp. (*): allele number.
This protocol was used to genotype 100 samples of unknown HLA-C genotype, of which, we detected seven samples of homozygous HLA-C*03:02 carriers, 36 heterozygous HLA-C*03:02 carriers, and 57 HLA-C*03:02-negative samples. For validation, we used PCR-SBT with all the 100 samples. The results of the protocol highly agreed with the SBT results (ĸ=0.98, p < 0.001). For specific detection of the HLA-C*03:02 allele, the PCR protocol had a sensitivity of 100% (95% CI: 91.6–100%) and specificity of 98.3% (95% CI: 90.9–99.7%) ().
Table 3 Comparison of the AS-PCR Method with PCR-SBT
This protocol was applied to determine the frequency of the HLA-C*03:02 allele in 810 unrelated Vietnamese Kinh people, 14.2% of which were HLA-C*03:02 carriers, the allele frequency was 7.5% ().
Table 4 HLA-C*03:02 Distributions in Vietnamese Kinh People
Discussion
The HLA-C gene is a polymorphic region of the human genome. According to the IPD-IMGT/HLA database, 6223 HLA-C alleles and 1540 distinct variant positions had been discovered.Citation18 These SNPs are located mostly in the exon 2–3 region, and approximately one SNP is present every 20–30 nucleotides.Citation19 To date, few PCR-based methods for the specific detection of HLA-A or HLA-B alleles at the two-field classification have been publishedCitation20,Citation21 and there have been no reports on the detection protocol of HLA-C alleles in general or the specific detection of the HLA-C*03:02 allele.
Due to the polymorphic characteristic of the HLA-C gene, it is difficult to design specific primers for direct amplification of each allele of this locus, it is necessary to cluster the alleles before a specific detection of each target allele. The primer set in the first step PCR was designed for specific amplification of the exon 2–3 region of the HLA-C gene. A mismatch (replacement of G with T) was placed at the second nucleotide from the 3′ terminus of the forward primer (HLACB1F) () in order to avoid non-specific amplification of other class I HLA loci such as HLA-A, B, E, F, G.
This PCR protocol was customized for the Vietnamese population, with the 18 known HLA-C alleles.Citation9 Therefore, an approach to differentiate the HLA-C*03:02 allele (presenting in 6.8% of the Vietnamese populationCitation9) from the other 17 alleles was designed. Three primer sets were used in the second PCR for differentiation purposes. The exon 2–3 sequences of HLA-C*03:02, *03:03, and *03:04 alleles are highly homologous. Moreover, they all have dinucleotide polymorphisms (at position 935–936) that are different from those of the other 15 HLA-C alleles reported by Hoa et al.Citation9 This is the favorable position for designing the reverse primer (HLAC3CR) which is specific for the three homologous alleles, and the reverse primer (HLAC15CR), which is specific for the other 15 HLA-C alleles ().
For the cluster of the three homologous alleles including HLA-C*03:02, *03:03 and *03:04, there are only two SNPs (at position 731 and 795) within the exon 2–3 sequence, that can be used to distinguish the HLA-C*03:02 allele from the other two alleles (). The SNP at position 731 was used to design the forward primer (HLAC2CF) which was specific to the HLA-C*03:03 and HLA-C*03:04 alleles and the forward primer (HLAC0302F) which was specific to the HLA-C*03:02 allele. The PCR reaction using these primers resulted in a longer PCR product which is more favorable for detection by electrophoresis. Additionally, a mismatch (replacement of C with T) was placed at the penultimate position of the 3′ terminus of the HLAC0302F primer and another mismatch (replacement of T with C) was placed at the third nucleotide from the 3′ terminus of the HLAC2CF primer (). The protocol was tested on 10 samples of known genotypes, resulting in a hundred-percent agreement, indicating the efficacy of the PCR strategy mentioned above. The validation by PCR-SBT of 100 samples of unknown genotypes showed a sensitivity of 100%, assuring no false negatives, which means that no patients at risk for SCARs due to the HLA-C*03:02 genotype would be missed if this test is applied in clinical settings.
The HLA-B*58:01 allele has been reported to be a predominant allele,Citation22 for this reason, most of HLA-B*58:01 specific genotyping methods only aim to determine the presence or absence of the allele in the genotype. Meanwhile, there has not been any report on the difference between homozygous and heterozygous genotypes of the HLA-C*03:02 allele in the association with allopurinol-induced SCARs. Therefore, we established a flexible protocol that can either determine the presence or absence of the HLA-C*03:02 allele in the genotype or determine zygosity. For the first aim, only one primer set (HLAC0302F/HLAC3CR) is needed in the second PCR. This protocol thus can be used for both research and clinical purposes.
A limitation of this protocol is time-consuming in comparison with other methods such as real-time PCR which requires approximately two hours.Citation23 Nevertheless, the total time for the test including DNA extraction is four hours, enabling to return genotyping results much earlier than sequencing by an outsourcing unit. In addition, this protocol does not require specially trained workers or expensive reagents and equipment; thus, it can be used for screening patients at risk of allopurinol-induced SCARs in local hospitals in developing countries. The total cost for reagents in this method is less than $2, while the costs for high-throughput methods are usually higher.Citation23
Another limitation of this PCR-based protocol is the probability of false-positive results due to cross-contamination during electrophoresis or preparation of DNA template. The validation on 100 samples showed one sample with false-positive result (). For a screening test, sensitivity is more important than specificity. However, a validation on a larger sample size is needed for a comprehensive evaluation of the protocol.
To date, there has been a report on the HLA-C*03:02 frequency in 170 unrelated Vietnamese Kinh people in Hanoi (the North of Vietnam).Citation9 The present study on 810 unrelated Vietnamese Kinh people evenly distributed in the North, the South and the Centre of Vietnam had a significantly bigger and more representative sample of Vietnamese population. The allele frequency (AF) of the HLA-C*03:02 allele in this study was 7.5%, higher than the AF in the North of Vietnam (6.8%). The HLA-C*03:02 AF of the Vietnamese Kinh people in our study was the same as that of the Korean people (7.42%)Citation24 and the Thai people (7.77%),Citation16 more than that of the Chinese people (5.9%)Citation25 and much more than that of the Japanese people (0.6%),Citation26 the Italian people (0.6%),Citation27 the Swiss people (0.54–0.72%),Citation28 the African American people (0.975%)Citation29 or the European American people (0.358%).Citation30 The frequency of HLA-C*03:02 carriers was notably high (14.2%), which was similar to that of the Thai people (14.68%).Citation16 These comparisons indicate a diversity of the HLA-C*03:02 allele distribution among various populations and explain a significant association of the HLA-C*03:02 allele and the risk of allopurinol-induced SCARs in certain Asian populations with high HLA-C*03:02 AF such as the Koreans.Citation8 This AS-PCR protocol can be used for the HLA-C*03:02 allele detection in not only Vietnamese people but some other Asian populations with similar genetic characteristics as well.
Abbreviations
AF, allele frequency; AS, allele specific; DRESS, drug reactions with eosinophilia and systemic symptoms; HLA, human leukocyte antigen; SBT, sequencing-based typing; SCAR, severe cutaneous adverse drug reactions; SJS, Steven-Johnson syndrome; SNP, Single nucleotide polymorphism; SSO, sequence specific oligonucleotide; TEN, toxic epidermal necrolysis.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Acknowledgments
The authors would like to thank Dr. Duong Tuan Linh (National Institute of Nutrition) for his valuable supports and comments on the research.
Disclosure
The authors report no conflicts of interest for this work.
Additional information
Funding
References
- Campochiaro C. Allopurinol-induced severe cutaneous adverse reactions. Ann Rheum Dis. 2016;75(4):e20. doi:10.1136/annrheumdis-2015-209108
- Nguyen KD, Tran TN, Nguyen MT, et al. Drug-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in vietnamese spontaneous adverse drug reaction database: a subgroup approach to disproportionality analysis. J Clin Pharm Ther. 2019;44(1):69–77. doi:10.1111/jcpt.12754
- Saksit N, Tassaneeyakul W, Nakkam N, et al. Risk factors of allopurinol-induced severe cutaneous adverse reactions in a Thai population. Pharmacogenet Genomics. 2017;27(7):255–263. doi:10.1097/FPC.0000000000000285
- Jung JW, Kim DK, Park HW, et al. An effective strategy to prevent allopurinol-induced hypersensitivity by HLA typing. Genet Med. 2015;17(10):807–814. doi:10.1038/gim.2014.195
- Hershfield MS, Callaghan JT, Tassaneeyakul W, et al. Clinical pharmacogenetics implementation consortium guidelines for human leukocyte antigen-B genotype and allopurinol dosing. Clin Pharmacol Ther. 2013;93(2):153–158. doi:10.1038/clpt.2012.209
- Sukasem C, Jantararoungtong T, Kuntawong P, et al. HLA-B (*) 58:01 for allopurinol-induced cutaneous adverse drug reactions: implication for clinical interpretation in Thailand. Front Pharmacol. 2016;7:186. doi:10.3389/fphar.2016.00186
- Hung SI, Chung WH, Liou LB, et al. HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci U S A. 2005;102(11):4134–4139. doi:10.1073/pnas.0409500102
- Kang HR, Jee YK, Kim YS, et al. Positive and negative associations of HLA class I alleles with allopurinol-induced SCARs in Koreans. Pharmacogenet Genomics. 2011;21(5):303–307. doi:10.1097/FPC.0b013e32834282b8
- Hoa BK, Hang NT, Kashiwase K, et al. HLA-A, -B, -C, -DRB1 and -DQB1 alleles and haplotypes in the Kinh population in Vietnam. Tissue Antigens. 2008;71(2):127–134. doi:10.1111/j.1399-0039.2007.00982.x
- Jiao Y, Li R, Wu C, et al. High-sensitivity HLA typing by saturated tiling capture sequencing (STC-Seq). BMC Genom. 2018;19(1):50. doi:10.1186/s12864-018-4431-5
- Hajeer AH, Al Balwi MA, Aytül Uyar F, et al. HLA-A, -B, -C, -DRB1 and -DQB1 allele and haplotype frequencies in Saudis using next generation sequencing technique. Tissue Antigens. 2013;82(4):252–258. doi:10.1111/tan.12200
- Juhos S, Vágó T, Ferriola D, et al. Deriving HLA genotyping from whole genome sequencing data using omixon HLA twin(tm) in G3’s global clinical study. Hum Immunol. 2015;76(Suppl):131. doi:10.1016/j.humimm.2015.07.183
- Hwang S, Oh HB, Yang JH, et al. Distribution of HLA-A, B, C allele and haplotype frequencies in Koreans. Korean J Lab Med. 2004;24(6):396–404.
- Peterson T, Bielawny T, Lacap P, et al. Diversity and frequencies of HLA class I and class II genes of an East African population. Open J Genet. 2014;04(2):99–124. doi:10.4236/ojgen.2014.42013
- Koehler RN, Walsh AM, Sanders-Buell EE, et al. High-throughput high-resolution class I HLA genotyping in East Africa. PLoS One. 2010;5(5):e10751. doi:10.1371/journal.pone.0010751
- Satapornpong P, Jinda P, Jantararoungtong T, et al. Genetic diversity of HLA class I and class II alleles in Thai populations: contribution to genotype-guided therapeutics. Front Pharmacol. 2020;11:78. doi:10.3389/fphar.2020.00078
- IPD-IMGT/HLA Database. Cambridgeshire: EMBL-EBI, The Wellcome Genome Campus; 2019. Available from: https://www.ebi.ac.uk/ipd/imgt/hla/. Accessed December 20, 2020.
- Robinson J, Barker DJ, Georgiou X, et al. IPD-IMGT/HLA database. Nucleic Acids Res. 2019;48(D1):D948–D955.
- Allcock RJ. The major histocompatibility complex: a paradigm for studies of the human genome. Methods Mol Biol. 2012;882:1–7.
- Uchiyama K, Kubota F, Ariyoshi N, et al. Development of a simple method for detection of HLA-A* 31: 01 allele. Drug Metab Pharmacokinet. 2013;28(5):435–438. doi:10.2133/dmpk.DMPK-12-NT-136
- Sita Virakul JN, Kupatawintu P, Kangwanshiratada O, et al. Detection of HLA-B*5801 by in-house PCR-SSP. Proceedings of the 11th Graduate Research Conference; 2010; Thailand: Khon Kaen University.
- Saksit N, Nakkam N, Konyoung P, et al. Comparison between the HLA-B(*)58: 01 allele and single-nucleotide polymorphisms in chromosome 6 for prediction of allopurinol-induced severe cutaneous adverse reactions. J Immunol Res. 2017;2017:Dec:2738784. doi:10.1155/2017/2738784
- Nguyen DV, Vidal C, Chu HC, et al. Developing pharmacogenetic screening methods for an emergent country: Vietnam. World Allergy Organ J. 2019;12(5):100037. doi:10.1016/j.waojou.2019.100037
- In JW, Roh EY, Oh S, et al. Allele and haplotype frequencies of human leukocyte antigen-A, -B, -C, -DRB1, and -DQB1 from sequence-based DNA typing data in Koreans. Ann Lab Med. 2015;35(4):429–435. doi:10.3343/alm.2015.35.4.429
- Shen J, Guo T, Wang T. HLA-B*07, HLA-DRB1*07, HLA-DRB1*12, and HLA-C*03:02 strongly associate with BMI: data from 1.3 million healthy chinese adults. Diabetes. 2018;67(5):861–871. doi:10.2337/db17-0852
- Itoh Y, Mizuki N, Shimada T, et al. High-throughput DNA typing of HLA-A, -B, -C, and -DRB1 loci by a PCR–SSOP–luminex method in the Japanese population. Immunogenetics. 2005;57(10):717–729. doi:10.1007/s00251-005-0048-3
- Guerini FR, Fusco C, Mazzi B, et al. HLA-Cw allele frequencies in northern and southern Italy. Transpl Immunol. 2008;18(3):286–289.
- Buhler S, Nunes JM, Nicoloso G, et al. The heterogeneous HLA genetic makeup of the Swiss population. PLoS One. 2012;7(7):e41400. doi:10.1371/journal.pone.0041400
- Tu B, Mack SJ, Lazaro A, et al. HLA-A, -B, -C, -DRB1 allele and haplotype frequencies in an African American population. Tissue Antigens. 2007;69(1):73–85. doi:10.1111/j.1399-0039.2006.00728.x
- Mack S, Tu B, Lazaro A, et al. HLA-A, -B, -C, and-DRB1 allele and haplotype frequencies distinguish Eastern European Americans from the general European American population. Tissue Antigens. 2008;73(1):17–32. doi:10.1111/j.1399-0039.2008.01151.x
