Abstract
Objective
To describe a novel KAL1 mutation in patients affected by Kallmann syndrome.
Setting
Endocrinology Clinic of the João de Barros Barreto University Hospital – Federal University of Pará, Brazil.
Methods
Clinical examination, hormone assays and sequencing of exons 5, 6 and 9 of the KAL1 gene in four Brazilian brothers with Kallmann syndrome.
Results
Detected a novel KAL1 mutation, c.612G.A/p.Trp204*, in four hemizygous brothers with Kallmann syndrome, and five heterozygous female family members.
Conclusion
The novel p.Trp204* mutation of the KAL1 gene results in the production of a truncated anosmin-1 enzyme in patients with Kallmann syndrome. This finding broadens the spectrum of pathogenic mutations for this disease.
Introduction
Deficiencies in gonadotropins and sex hormones are detrimental for various stages of sexual maturation and lead to hypogonadotropic hypogonadism (HH).Citation1 The isolated form of HH is rare; however, its association with certain olfactory deficits (anosmia/hyposmia) that are part of Kallmann syndrome (KS) – OMIM 300836 – makes it more common.
KS can occur sporadically or be hereditary, and its incidence is 1:8,000 in males and 1:40,000 in females.Citation2,Citation3 Hereditary cases have been well documented but sporadic cases are more common, which suggests that de novo mutations are an important cause.Citation4–Citation6
There are at least six well-known genes involved in the etiology of KS: the KAL1 gene, which encodes the anosmin-1 glycoprotein; the FGFR1 (KAL2) gene, which encodes fibroblast growth factor receptor 1; the PROKR2 (KAL3) and PROK2 (KAL4) genes, which encode the prokineticin-2 receptor and prokineticin-2, respectively; and the CDH7 (KAL5) and FGF8 (KAL6) genes. Other genes have been reported to be associated with KS: NELF, WDR11, HS6ST1, SEMA3A, HESX1.Citation7,Citation8
X-linked KS (X-KS) typically involves mutations in the KAL1 gene, which is found on chromosome Xq22.3; such mutations can alter the form and function of the anosmin-1 protein, which is involved in the migration of olfactory neurons and neurons that produce gonadotropin-releasing hormone. Without anosmin-1, migration is impaired, which affects the patient’s olfaction and gonadotropic hormone production.Citation6,Citation9,Citation10
In combination with the profile of HH and anosmia/hyposmia, a patient may present with various other alterations; the following are some common findings: unilateral renal agenesis; cavus foot; cleft lip and palate; synkinesis; and auditory, visual or other sensory disturbances.Citation7 In any case, great phenotypic heterogeneity has been demonstrated in various studies, especially those of Quinton et al,Citation11 Oliveira et alCitation12 and Hardelin and Dodé,Citation13 suggesting that the activity of epigenetic factors or modifying genes COULD determine phenotypic variability.
Detailed clinical assessment of patients who are affected by HH and have anosmia/hyposmia has led to the discovery of other significant changes in this group, and adding genetic analysis permits appropriate genetic counseling and helps molecular understanding of KS.
Materials and methods
Ethical issues
Ethical consent was obtained according to the Declaration of Helsinki. Ethical approval was obtained from the Brazilian National Committee on Research Ethics (CEP/HUJBB – Protocol Number 142/2008). All of the patients assessed agreed to participate in the study by signing an informed consent form.
Description of the samples
A clinical and molecular study was conducted with four KS patients and 12 of their family members, for a total of 16 individuals. The clinical study of those affected assessed the following parameters: 1) pattern of family inheritance; 2) stage of sexual maturation; 3) anthropometric data; 4) hormone levels (follicle-stimulating hormone [FSH], luteinizing hormone [LH], testosterone, estradiol, thyroid-stimulating hormone [TSH], free T4); 5) other associated conditions including cryptorchidism, renal malformation, synkinesis, and others; and 6) molecular analysis of the KAL1 gene (NM_000216.2).
The family members underwent an assessment focused on the investigation of anosmia, identification of associated malformations and genetic study.
The olfactory assessment was conducted using the semiological test of the first cranial nerve based on the presentation of test tubes containing ten different common everyday odors to be identified by the patients and their family members. Chloroform was used to test for false positives because the olfactory nerve is not required to detect it.
Molecular analysis
DNA was extracted from peripheral blood samples taken from each individual by the phenol-chloroform method.Citation14 Exons 5, 6 and 9 of the KAL1 gene (NM_000216.2) were selected for amplification by polymerase chain reaction (PCR) because they contained the greatest number of mutations described at the time of the present study.Citation15 The primers used for amplification reaction are shown in Table S1. The final reaction volume was 25 μL and included 100 ng of template DNA, 0.1 μM dNTPs, 0.1 μM of each primer, 1.5 μM MgCl 2, 1× PCR buffer (10 mM Tris-HCl, pH 8.4, and 50 mM KCl) and 1 U Platinum® Taq DNA Polymerase (Invitrogen). After initial denaturing for 10 min at 95°C, the samples underwent 30 cycles under the following parameters: denaturing at 94°C for 1 minute, annealing at 54°C for 1 minute for exon 5, and at 56°C for 1 minute for exons 6 and 9, and extension at 72°C for 1 minute. The samples were checked by electrophoresis on a 1.5% agarose gel.
The sequencing reaction was performed using 1 μL of purified PCR product, 0.5 μL reverse primer, 2.0 μL of Big Dye Terminator V3.1 Cycle Sequencing Kit (Applied Biosystems®) and sterile deionized water to a final volume of 20 μL. The reaction was performed on a thermocycler with an initial denaturing at 96°C for 2 minutes, followed by 36 cycles of 96°C for 30 seconds, 54/56°C for 20 seconds (for exon 5, and exons 6 and 9, respectively), and 60°C for 4 minutes. The samples were treated and sent to an ABI 3110 sequencer for electrophoresis on 4.5% acrylamide gel (10 mL of 18.5 acrylamide:1 bis-acrylamide, 36 g of urea and 60 mL of 1× TBE). The sequencing results were interpreted using the ABI Analysis Software®.
Results
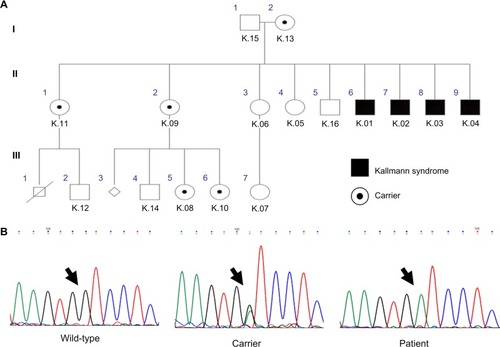
Four male patients in the third decade of life presented with the clinical and laboratory profile of HH () combined with anosmia confirmed by the semiological test. Four other features were observed that are common in KS (): unilateral renal agenesis, cryptorchidism, synkinesis and dental agenesis. Nystagmus, ptosis, auricular dysgenesis and cleft lip/palate were not found in any patient.
Table 1 Summary of laboratorial tests in the first assessment
Table 2 Summary of clinical findings in Kallmann syndrome patients
The molecular analysis detected the transversion of G to A at position 612 on exon 5 of the KAL1 gene. This mutation was hemizygous in the four affected brothers. Five females heterozygous for this mutation were identified in the family, including the patients’ mother (). The same mutation was not found in the remaining seven family members. This novel mutation generates a stop codon instead of a tryptophan amino acid at position 204 of the anosmin-1 protein.
Figure 1 The inheritance pattern for the family studied shows the hemizygous affected individuals with Kallmann syndrome, and heterozygous carriers who were detected by genetic analysis (A). The chromatogram patterns found in the study are shown above with mutations indicated by an arrow (B).
Discussion
KS is one of the most common causes of HH.Citation4 In male patients (who have a greater incidence) who present with a clear picture including delayed puberty and, therefore, likely hypogonadism, it is appropriate to question or investigate their olfactory function with possible investigation oriented toward KS. In the cases studied, the complaint of difficulty perceiving odors aided the clinical study.
X-linked inheritance is the best clinically characterized form of the disease.Citation11 Some associated conditions are more common in this type of inheritance, such as dental agenesis, synkinesis and renal agenesis.Citation16 All of these features were observed in the probands, reinforcing the suspicion for X-linked inheritance.
The cryptorchidism found in the four patients should be of high importance because it is often the most evident clinical feature, especially in pediatric patients when it is not possible to apply olfactory tests and hormone levels are of little value. In such cases, it is important to look for other signs such as the presence of micropenis. Additionally, a family history of the characteristic signs of KS should be investigated.Citation17
Renal agenesis is present in 35%–40% of X-linked KS cases.Citation18 Other malformations of the urinary tract can also be found in such patients, including horseshoe kidney, kidney rotation, hydronephrosis, duplicated ureter, etc.Citation19 The importance of imaging exams, especially abdominal ultrasound, is clear because they can show renal abnormalities that are not clinically evident. Therefore, such exams should be considered imperative for KS patients.
Georgopoulos et alCitation20 and Sato et alCitation16 reported a greater incidence of right renal agenesis with a tendency for KS patients to present with this side affected, especially those with a KAL1 mutation. Left renal agenesis is more commonly found in individuals without KS. The mechanisms that explain these findings are still unknown.
Synkinesis, which is considered the marker of X-linked inheritance and was present in the probands in this study, can be explained by an absence of inhibitory fibers that connect the two motor cortices, resulting in fewer fibers in the corpus callosum or even agenesis.Citation11 These alterations were not found in the studied patients with magnetic resonance imaging.
An analysis of the type of mutation that generates a protein truncated at codon 204 revealed interrupted synthesis at the first fibronectin type III-like domain. This finding agrees with the analysis by Georgopoulos et al,Citation20 who found many mutations in this domain related to renal alterations. A more recent study could not specifically attribute this renal phenotype to any of the genes known to cause the syndrome.Citation7
Most cases require lifelong sex steroid hormone replacement to maintain sexual features and bone density, although there have been cases in which the profile of hypogonadism reversed after a period of hormone replacementCitation21 in patients who had not been genetically screened and in patients with detected mutations in the KAL1Citation22 and KAL2/FGFR1 genes.Citation23
Reversal of the disease is achieved when the individual maintains normal serum levels of sex hormones after a long period without hormone replacement, allowing the possibility of endogenous maintenance of complete virilization and fertility.Citation22 Although the estimated number of reversible cases is low, Quinton et alCitation21 have suggested that they are underestimated due to interruption of treatment by the patient, now eugonadal, without any effect on their clinical progression. Additionally, there may be a lack of follow-up by specialized professionals. Therefore, there may be reversible cases that remain unidentified.
Advances in the understanding of the genetic bases of KS have allowed increasingly complex molecular studies that can provide additional information on the pathogenesis of the disease and the genotype–phenotype associations.Citation7 Although it has been widely studied, novel recessive mutations of the KAL1 gene are still being described in the literature, as in the present study and in a recent analysis of Chinese probands.Citation24
In conclusion, the clinical and molecular studies conducted herein broadened the spectrum of the known pathogenic recessive mutations related to KS. The results will serve as the basis for appropriate genetic counseling to inform patients and their families about the disease and its repercussions, especially reproductive issues. Additionally, counselors can warn them about the risk of recurrence in the family and suggest early diagnosis of future patients so that treatment can be initiated at the ideal time.
Acknowledgments
The authors thank all of the patients and the Endocrinology team from João de Barros Barreto University Hospital – UFPA and the Genetics team from the Laboratory of Human and Medical Genetics – UFPA. Financial support was received from UFPA(PROPESP)/FADESP and CAPES.
Supplementary material
Table S1 Polymerase chain reaction primers used for amplification of KAL1 exons 5, 6 and 9, their annealing temperature and product size
Disclosure
The authors have no conflicts of interest in this work.
References
- TussetCTrarbachEBSilveiraLFGBeneduzziDMontenegroLRLatronicoACClinical and molecular aspects of congenital isolated hypogonadotropic hypogonadismArq Bras Endocrinol Metabol20115550151122218430
- DodéCHardelinJPClinical genetics of Kallmann syndromeAnnales d’Endocrinologie201071149157
- RibeiroRSAbuchamJKallmann syndrome: a historical, clinical and molecular reviewArq Bras Endocinol Metabol200852817
- JansenCHendricks-StegemanBIJansenMA novel nonsense mutation of the KAL gene in two brothers with Kallmann syndromeHorm Res20005320721211044805
- HardelinJPKallmann syndrome: towards molecular pathogenesisMol Cell Endocrinol2001179758111420131
- CadmanSMKimSHHuYGonzález-MartínezDBoulouxPMMolecular pathogenesis of Kallmann syndromeHorm Res20076723124217191030
- Costa-BarbosaFABalasubramanianRKeefeKWPrioritizing genetic testing in patients with Kallmann syndrome using clinical phenotypesJ Clin Endocrinol Metab2013985E943E95323533228
- NewbernKNatrajanNKimHGIdentification of HESX1 mutations in Kallmann syndromeFertil Steril20139971821183723357452
- CariboniAPimpinelliFColamarinoSThe product of X-linked Kallmann’s syndrome gene (KAL-1) affects the migratory activity of gonadotropin-releasing hormone (GnRH)-producing neuronsHum Mol Genet2004132781279115471890
- FechnerAFongSMcgovernPA review of Kallmann syndrome: genetics, pathophysiology and clinical managementObstet Gynecol Surv20086318919318279545
- QuintonRDukeVMDe ZoysaPAPlattsADThe Neuroradiology of Kallmann’s syndrome: a genotypic and phenotypic analysisJ Clin Endocrinol Metab1996818301030178768867
- OliveiraLMBSeminaraSBBeranovaMHayesFJValkenburghSBThe importance of autosomal genes in Kallmann syndrome: genotype-phenotype correlations and neuroendocrine characteristicsJ Clin Endocrinol Metab20018641532153811297579
- HardelinJPDodéCThe complex genetics of Kallmann syndrome: KAL1, FGFR1, FGF8, PROKR2, PROK2, et alSex Dev2008218119318987492
- SambrookJRusselDWMolecular Cloning: a Laboratory Manual3rd edNew YorkCold Spring Harbor2001
- TrarbachEBBaptistaMTMGarmesHMHackelCMolecular analysis of KAL1, GnRH-R, NELF and EBF2 genes in a series of Kallmann syndrome and normosmic hypogonadotropic hypogonadism patientsJ Endocrinol200518736136816423815
- SatoNKatsumataNKagamiMClinical assessment and mutation analisys of Kallmann syndrome 1 (KAL1) and fibroblast growth factor receptor 1 (FRFR1, or KAL2) in five families and 18 sporadic patientsJ Clin Endocrinol Metab2004891079108815001591
- ZenatyDBretonesPLambeCPaediatric phenotype of Kallmann syndrome due to mutations of fibroblast growth factor receptor 1 (FGFR1)Mol Cell Endocrinol20062542557883
- TrarbachEBMonlleoILPorciunculaCGGFontesMIBBaptistaMTMHackelCSimilar intersticial deletions of the KAL-1 gene in two Brazilian families with X-linked Kallmann syndromeGenet Mol Biol200427337341
- ZentenoJCMéndezJPMaya-NúñezGUlloa-AguirreAKofman-AlfaroSRenal abnormalities in patients with Kallmann syndromeBJU International19998338338610210557
- GeorgopoulosNAKoikaVGalli-TsinopoulouARenal dysgenesis and KAL1 gene defects in patients with sporadic Kallmann syndromeFertil Steril2007881311131717603054
- QuintonRCheowHKTymmsDJBoulouxPMGWuFCWJacobsHSKallmann’s syndrome: is it always for life?Clin Endocrinol199950481485
- RibeiroRSVieiraTCAbuchamJReversible Kallmann syndrome: report of the first case with a KAL1 mutation and literature reviewEur J Endocrinol200715628529017322486
- PitteloudNAcirenoJSMeysingAUReversible Kallmann syndrome, delayed puberty and isolated anosmia occurring in a single family with a mutation in the fibroblast growth factor receptor 1 geneJ Clin Endocrinol Metab20059031317132215613419
- ZhangSXuHWangTLiuGLiuJThe KAL1 pVal610Ile mutation is a recessive mutation causing Kallmann syndromeFertil Steril201399615201523
