Abstract
Background
Lung nodules are being detected at an increasing rate year by year with high-resolution computed tomography (HRCT) being widely used. Ground-glass opacity nodule is one of the special types of pulmonary nodules that is confirmed to be closely associated with early stage of lung cancer. Very little is known about solitary ground-glass opacity nodules (SGGNs). In this study, we analyzed the clinical, pathological, and radiological characteristics of SGGNs on HRCT.
Methods
A total of 95 resected SGGNs were evaluated with HRCT scan. The clinical, pathological, and radiological characteristics of these cases were analyzed.
Results
Eighty-one adenocarcinoma and 14 benign nodules were observed. The nodules included 12 (15%) adenocarcinoma in situ (AIS), 14 (17%) minimally invasive adenocarcinoma (MIA), and 55 (68%) invasive adenocarcinoma (IA). No patients with recurrence till date have been identified. The positive expression rates of anaplastic lymphoma kinase and ROS-1 (proto-oncogene tyrosine-protein kinase ROS) were only 2.5% and 8.6%, respectively. The specificity and accuracy of HRCT of invasive lung adenocarcinoma were 85.2% and 87.4%. The standard uptake values of only two patients determined by 18F-FDG positron emission tomography/computed tomography (PET/CT) were above 2.5. The size, density, shape, and pleural tag of nodules were significant factors that differentiated IA from AIS and MIA. Moreover, the size, shape, margin, pleural tag, vascular cluster, bubble-like sign, and air bronchogram of nodules were significant determinants for mixed ground-glass opacity nodules (all P<0.05).
Conclusion
We analyzed the clinical, pathological, and radiological characteristics of SGGNs on HRCT and found that the size, density, shape, and pleural tag of SGGNs on HRCT are found to be the determinant factors of IA. In conclusion, detection of anaplastic lymphoma kinase expression and performance of PET/CT scan are not routinely recommended.
Introduction
With the increased use of high-resolution computed tomography (HRCT) for screening lung diseases, very faint and smaller lesions known as ground-glass opacity nodules (GGNs) are being encountered more frequently. GGNs are characterized as lesions of homogenous density and with hazy increase in density in the lung field that does not obscure the bronchovascular structure.Citation1–Citation3 They were classified into pure GGNs (pGGNs) and mixed GGNs (mGGNs), and the frequency of malignancy was higher for mGGNs than for pGGNs among patients who underwent low-dose CT scan.Citation4
Although some studies have reported the characteristics of pGGNs and their history,Citation5,Citation6 there is no report about solitary GGNs (SGGNs) on HRCT scan. Therefore, the purpose of our research was to compare the clinical pathology with morphologic features of patients with SGGNs ≤30 mm in diameter on HRCT.
Materials and methods
Subjects
We retrospectively reviewed all HRCT scan reports for SGGNs that were taken from January 2008 to March 2015 in the radiology department of West China Hospital and identified patients who underwent pathological biopsy after the surgery. One thoracic radiologist reviewed all the HRCT images again to identify the selected patients with persistent SGGNs before surgically removed, and collected all the preoperative HRCT images at the same time. Our inclusion criteria were as follows: 1) lesions with a thickness of ≤1 mm on HRCT scan, 2) SGGNs with a maximum diameter of ≤30 mm, and 3) patients who had their lesions surgically removed and had pathological diagnosis. Ethical approval was obtained from the Ethics Committee of West China Hospital, patients’ confidentiality was maintained. The Ethics Committee of West China Hospital deemed patient consent not necessary.
Evaluation of nodule morphology using HRCT scan
CT scans were obtained with SIEMENS SOMATOM® Definition Flash scanners (Munich, Germany). The following parameters were used to obtain HRCT images: collimator with 64×0.6 mm, section thickness of 1 mm, reorganization interval of 0.66 mm, and tube voltage of 120 kV. Tube current is calculated according to an individual’s weight, height, and body mass index. The tube current was 220 mAs for body mass index ≤25 kg/m2 and 330 mAs for body mass index >25 kg/m2. Two chest radiologists, each with more than 2 years of experience in diagnosing thoracic disease, assessed nodule morphology blindly and independently. Morphology included density, size (the maximum diameter), shape (round, oval, or irregular), margin (smooth, lobular, spiculated, or lobular and spiculated), pleural tag, vascular cluster, bubble lucency, and air bronchogram. The average values of size were obtained by two observers independently.
Surgery and histopathologic evaluation
All specimens were designated R0 (no residual tumor at the primary tumor site after surgical resection). Adenocarcinomas were histologically classified according to the criteria of Lung Cancer/American Thoracic Society/European Respiratory Society International Multidisciplinary Lung Adenocarcinoma ClassificationCitation7 by two lung pathologists (Wei-Ya Wang and Wei-Lu Wu, each with more than 5 years of experience in lung pathology).
Treatment and follow-up evaluation
A total of 81 adenocarcinoma patients were included for analysis. All the SGGNs were removed by lobectomy (n=46), sublobar resection (n=21), and wedge resection (n=14). Every patient was followed up for chest X-ray post-surgery, and some were regularly followed up for chest CT scanning at an interval of 3-months. We followed up all patients by telephone and based on outpatient records.
Statistical analysis
Statistical analysis was carried out by SPSS17.0 (Statistical Program for Social Sciences) software. Data were expressed as mean ± standard deviation, and group comparison was performed by one-way analysis of variance or Wilcoxon test. Count data were expressed as value or percentage, and comparison between groups was performed by chi-square test or Fisher’s exact test. P-values <0.05 were considered statistically significant.
Results
Patient demographics
There were total 95 (20.0%) SGGNs in 474 solitary nodules from January 2008 to March 2015. Among the patients with SGGNs, 81 had adenocarcinoma and 14 had benign nodules. The clinicopathologic characteristics of 81 (17.1%) patients with adenocarcinoma are summarized in . Among the 81 patients, 55 (67.9%) were females and 26 (32.1%) males, and the median age was 55.9 years (range 30–84 years). Seventeen (21%) patients were smokers and the rest nonsmokers. Four patients had a prior history of other tumors and eleven patients had complications such as COPD or asthma. Seventeen patients had a family history of cancer (such as liver cancer, gastric cancer, and colorectal cancer) and eight (9.9%) patients had a family history of lung cancer. The number of patients whose blood tumor markers such as CEA, CA19-1, and NSE have elevated were 15 (18.3%), 14 (17.1%), and 17 (20.7%) respectively. However, 15 (3.0%) benign nodules were also reported, and the data are shown in Table S1.
Table 1 Characteristics of patients with GGNs
Among the 81 patients with SGGNs, 12 (14.8%) had AIS (), 14 (17.3%) MIA (), and 55 (67.9%) IA (). The predominant histologic subtypes among 55 patients with IA were lepidic and acinar patterns (n=29, 52.7%) and five (9.1%) patients had papillary patterns. All patients had stage I lung cancer. However, the rate of expression of anaplastic lymphoma kinase (ALK-V) and ROS-1 performed by immunohistochemistry was positive in 2.5% (n=2) and 8.6% (n=7) patients, respectively. The specificity and accuracy of HRCT of invasive lung adenocarcinoma were 85.2% and 87.4%.
Figure 1 Adenocarcinoma in situ in a 44-year-old woman.
Abbreviations: ALK, anaplastic lymphoma kinase; HRCT, high-resolution computed tomography.
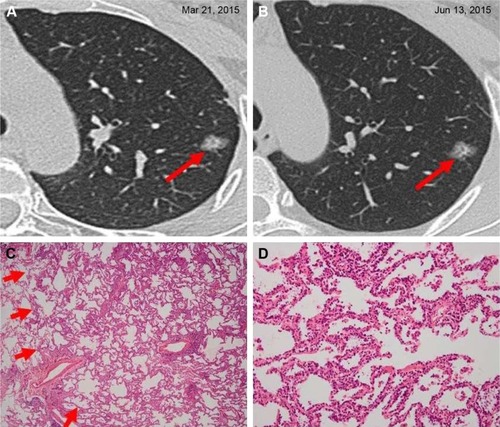
Figure 2 Minimally invasive adenocarcinoma in a 55-year-old woman.
Abbreviations: ALK, anaplastic lymphoma kinase; HRCT, high-resolution computed tomography.
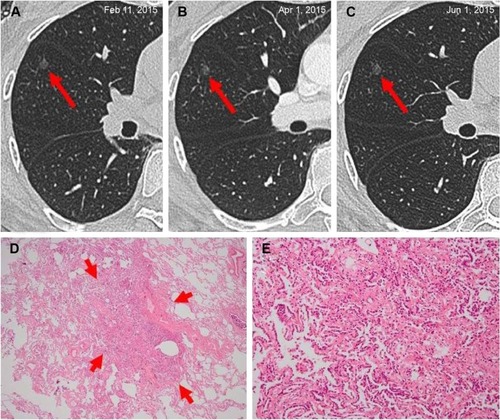
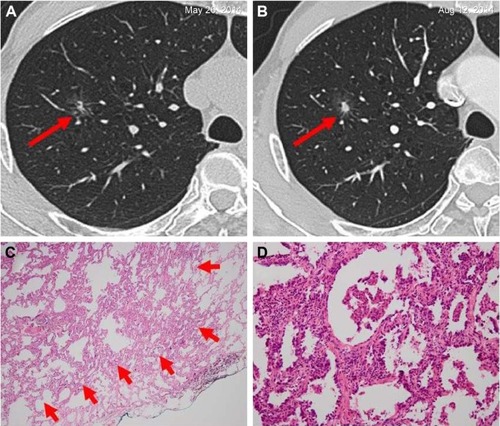
Figure 3 Invasive adenocarcinoma in a 66-year-old man.
Abbreviations: ALK, anaplastic lymphoma kinase; HRCT, high-resolution computed tomography.
Furthermore, HRCT was performed and the results showed that there were 35 (43.2%) pSGGNs and 46 (56.8%) mSGGNs. SGGNs were primarily found to be located in the superior lobe of right lung (n=35, 43.2%) and then in the superior lobe of left lung (n=26, 32.1%). Preoperative positron emission tomography (PET)/CT scanning was carried out in 10 of the 81 patients (12.3%), and only two patients’ standard uptake value (SUV) was above 2.5. The median follow-up period was 3.06 months. No recurrence or metastasis has been found until now.
Comparison of imaging features
Imaging features of the 81 SGGNs of different pathologic types are shown in . The size was larger and the shape was much more irregular along with an increased malignant degree of lesions (from AIS to IA) (P=0.016 and P=0.027, respectively). The density of IA nodules were more showed as mGGNs, however, AIS and MIA were more often showed as pGGNs on the contrary (P<0.001). Pleural tag was observed more frequently in IA than in AIS and MIA (P=0.018), but no difference was observed between AIS and MIA.
Table 2 Imaging features of GGNs classified into different histologic types
Furthermore, we compared the imaging features of pGGNs and mGGNs (). The results showed that mGGNs was significantly larger in size compared to pGGNs (P<0.001). Besides the presence of spiculated, lobular, pleural tag, vascular cluster, bubble lucency, and air bronchogram were observed more frequently in mGGNs than in pGGNs (all P<0.05), the shape of mGGNs were more irregular (P<0.001).
Table 3 Imaging features of GGNs classified into pGGNs and mGGNs
Relationship between immunohistochemistry and pathologic subtypes
Comparison of immunohistochemistry and pathologic subtypes showed that the expression of ALK-V and ROS-1 in different pathologic classifications had no statistical significance ().
Table 4 Comparison of immunohistochemistry and pathologic subtypes
In summary, the main results of this research are as follows: 1) among all the patients, with an average age of 55.9 years, the number of women who had lung adenocarcinoma with SGGNs images on HRCT was more than men, and most of them were nonsmokers and had no history of other cancers; 2) for the purpose of diagnosis and treatment, most of the patients underwent lobectomy, and blood tumor biomarkers such as CEA, CA19-9, and NSE were often found to be higher than normal; 3) most of the nodules were IA, and all the patients were at stage I and well differentiated; 4) none of the patients with SGGNs has tumor recurrence or metastasis until now; 5) the rate of positive expression of ALK-V and ROS-1 was very low, with 2.5% and 8.6%, respectively; and 6) the specificity and accuracy of HRCT of invasive lung adenocarcinoma were 85.2% and 87.4%. Based on the findings of imaging the following were concluded: 1) SGGNs was often found to be located in the superior lobe of right lung and then in the superior lobe of left lung; 2) results of PET/CT were often negative; and 3) nodule size was significantly larger in IA than in AIS and MIA, and the presence of pleural tag favored the diagnosis of IA.
Discussion
Our study explored the relationship between clinical pathological features and radiological characteristics of SGGNs on HRCT. Our results (malignant composition ratio was 82.7% [81/98] with 12 [14.8%] AIS, 14 [17.3%] MIA, and 55 [67.9%] IA) are in accordance with a previous study,Citation8 which showed that of the 330 surgically removed GGNs, malignant composition ratio was 95.2% (314/330), including 38 (12.1%) AIS, 63 (20.1%) MIA, and 213 (67.8%) IA. But this is in contrast to the results of Lim et al,Citation5 who showed that the main histologic type of GGNs in their research was AIS. Moreover, Lim et alCitation5 showed that the size (cutoff =16.4 mm, P=0.032), mass (cutoff =0.472 g, P=0.040), and bronchogram (P=0.012) of a nodule are determinants of IA in persistent pGGNs with a diameter ≥10 mm. However, our study showed that the size, shape, density, and the presence of pleural tag were statistically significant for SGGNs compared to other histological types. In addition, Lee et alCitation9 studied 80 GGNs and found that lobular marginal appearance of the nodules was an independent risk factor for malignancy (OR =13.769, P=0.016). But another study by Kim et alCitation10 carried out on 53 GGNs suggested that there was no relationship between marginal features of GGNs and degree of malignancy. Furthermore, some studies indicated that nodules with a maximum diameter of >8 mm and presence of solid element in the nodules, respectively, were independent risk factors for malignancyCitation11 and lymph node metastasis (OR =4.87, 95% confidence interval 1.51–15.77).Citation12
PET/CT scanning did not show any differentiation between malignancy and benign GGNs. In 53 patients with GGNs, Tsushima et alCitation13 found that the average and maximum SUV values obtained for benign lesions were significantly higher than that obtained for malignant lesions. Sensitivity, specificity, and accuracy of the diagnosis of benign lesions were 100.0%, 96.4%, and 100.0%, respectively, when SUV value was >1.5. Our results are found to be consistent with prior studies. Besides, it may reduce the sensitivity of PET/CT in diagnosis of malignant lesions because of lymph node metastasis and distant metastasis is not occurred in GGNs.
However, Naidich et alCitation14 in their study reported the following: 1) no CT follow-up is required for solitary pGGNs when the diameter of GGNs ≤5 mm; 2) the patient has to be initially followed up for CT at 3 months to confirm persistence and then followed up annually for CT for a minimum of 3 years; PET/CT is of limited value; and 3) the patient has to be initially followed up for CT at 3 months to confirm persistence. If persistent and solid component <5 mm is observed, then the patient should be annually followed up for CT for a minimum of 3 years. If persistent and solid component ≥5 mm is found, then biopsy or surgical resection is required. PET/CT should be considered for part-solid nodules >5 mm. The results of our study are consistent with that of Naidich et al.
We also analyzed the relationship between the expression of ALK-V and ROS-1 among different histologic types. The results showed that ALK-V and ROS-1 were expressed in only IA, and positive expression rates were only 2.5% and 8.6%, respectively.
Our study had several limitations. First, it was a retrospective research, and the number of patients was relatively small as we analyzed only patients with SGGNs. Second, the median follow-up period was too short, only 3.06 months, as most of the patients did not want a long follow-up period and decided on surgical resection after no more than two reexaminations or even without any reexamination. Third, we included nodules with a diameter ≤30 mm, which may have contributed to a selection bias. Finally, variations in nodule measurement and characterization of lesions might have been possible due to different observers.
Conclusion
The size, density, shape, and pleural tag of a nodule are determinants of IA in SGGNs with diameter ≤30 mm on HRCT, and detection of ALK-V expression and performance of PET/CT scan are not preferred as routine examinations.
Author contributions
ZXQ and WML conceived and designed the experiments. ZXQ and YC performed the experiments. ZXQ, YC, WYW, XW, and WLW analyzed the data. WYW, XW, and WLW contributed reagents/materials/analysis tools. ZXQ and DL wrote the paper. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No 81372504), the Science and Technology Support Program of Science and Technology Department of Sichuan Province (2014SZ-0148), and the International Cooperation Program of Science and Technology Department of Sichuan Province (2014AA022202-2).
Supplementary material
Table S1 Comparison of features between patients with benign GGNs and adenocarcinomas
Disclosure
All authors declare no conflicts of interest in this work.
References
- AustinJHMullerNLFriedmanPJGlossary of terms for CT of the lungs: recommendations of the Nomenclature Committee of the Fleischner SocietyRadiology199620023273318685321
- CollinsJSternEJGround-glass opacity at CT: the ABCsAJR Am J Roentgenol199716923553679242736
- LeeHYLeeKSGround-glass opacity nodules: histopathology, imaging evaluation, and clinical implicationsJ Thorac Imaging201126210611821508733
- HenschkeCIYankelevitzDFMirtchevaRCT screening for lung cancer: frequency and significance of part-solid and nonsolid nodulesAJR Am J Roentgenol200217851053105711959700
- LimHJAhnSLeeKSPersistent pure ground-glass opacity lung nodules ≥10 mm in diameter at CT scan: histopathologic comparisons and prognostic implicationsChest201314441291129923722583
- ChangBHwangJHChoiYHNatural history of pure ground-glass opacity lung nodules detected by low-dose CT scanChest2013143117217822797081
- TravisWDBrambillaENoguchiMInternational association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinomaJ Thorac Oncol20116224428521252716
- ChoJKoSJKimSJSurgical resection of nodular ground-glass opacities without percutaneous needle aspiration or biopsyBMC Cancer20141483825406492
- LeeHJGooJMLeeCHPredictive CT findings of malignancy in ground-glass nodules on thin-section chest CT: the effects on radiologist performanceEur Radiol200919355256018925404
- KimHYShimYMLeeKSHanJYiCAKimYKPersistent pulmonary nodular ground-glass opacity at thin-section CT: histopathologic comparisonsRadiology2007245126727517885195
- HeoEYLeeKWJheonSLeeJHLeeCTYoonHISurgical resection of highly suspicious pulmonary nodules without a tissue diagnosisJpn J Clin Oncol20114181017102221697137
- MatsugumaHYokoiKAnrakuMProportion of ground-glass opacity on high-resolution computed tomography in clinical T1 N0 M0 adenocarcinoma of the lung: a predictor of lymph node metastasisJ Thorac Cardiovasc Surg2002124227828412167787
- TsushimaYTateishiUUnoHDiagnostic performance of PET/CT in differentiation of malignant and benign non-solid solitary pulmonary nodulesAnn Nucl Med200822757157718756359
- NaidichDPBankierAAMacMahonHRecommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner SocietyRadiology2013266130431723070270
