Abstract
Within the context of the heterogeneous phenotypic stratification of asthmatic population, many patients are characterized by moderate-to-severe eosinophilic asthma, not adequately controlled by relatively high dosages of inhaled and even oral corticosteroids. Therefore, these subjects can obtain significant therapeutic benefits by additional biologic treatments targeting interleukin-5 (IL-5), given the key pathogenic role played by this cytokine in maturation, activation, proliferation, and survival of eosinophils. In particular, reslizumab is a humanized anti-IL-5 monoclonal antibody that has been found to be an effective and safe add-on therapy, capable of decreasing asthma exacerbations and significantly improving disease control and lung function in patients experiencing persistent allergic or nonallergic eosinophilic asthma, despite the regular use of moderate-to-high doses of inhaled corticosteroids. These important therapeutic effects of reslizumab, demonstrated by several controlled clinical trials, have led to the recent approval by US Food and Drug Administration of its use, together with other antiasthma medications, for the maintenance treatment of patients suffering from severe uncontrolled asthma.
Introduction
Asthma is a chronic respiratory disease, clinically manifesting as wheezing, cough, shortness of breath, and chest tightness, which is characterized by bronchial obstruction mainly due to inflammatory and structural changes leading to airway hyperresponsiveness and acute bronchoconstriction.Citation1,Citation2 This widespread airway disorder affects over 300 million people worldwide, which may increase to more than 400 million by 2020.Citation3,Citation4 Rather than a single disease entity, asthma is currently believed to be a heterogeneous complex of multiple clinical and pathobiologic phenotypes characterized by different responses to pharmacological therapies.Citation5,Citation6 The majority of asthmatic patients can achieve a good control of their symptoms using standard treatments including inhaled corticosteroids and bronchodilators such as β2-adrenergic agonists, eventually integrated with oral leukotriene inhibitors and/or tiotropium.Citation7–Citation9
However, despite an optimized inhaled therapy, a minority of subjects with severe disease are not adequately controlled and experience frequent exacerbations. Moreover, asthma severity in these difficult-to-treat patients is often further worsened by the coexistence of one or more comorbidities, including chronic rhinitis and sinusitis, gastroesophageal reflux, obesity, obstructive sleep apnea, and even chronic obstructive pulmonary disease.Citation10 Although when considering the overall population of asthmatic subjects, patients suffering from severe disease constitute a relatively small percentage, ranging from 5% to 10%; however, they consume a huge share of economic resources, amounting to about 50% of the global asthma budget.Citation11–Citation13 This very high cost of severe asthma is caused by a higher utilization of health care services including unscheduled consultations and emergency visits, and also additional consumption of drugs as well as frequent hospitalizations for recurrent exacerbations. Furthermore, severe asthma is associated with significant losses of school- and work-days, and asthmatic subjects with uncontrolled disease also often experience anxiety and depression.Citation14 Therefore, patients expressing asthma phenotypes refractory to conventional treatments are characterized by the most urgent unmet medical needs, which thus require a closer attention to the assessment, monitoring, and therapeutic management of their disease. Hence, particularly in severe asthma, an accurate phenotypic characterization should be pursued to identify the relevant cellular and molecular targets involved in disease pathobiology. Through such a personalized strategy, it would be possible to tailor individual therapies aimed to achieve an adequate and persistent control of symptoms, as well as to decrease the risk of future exacerbations and to slow down the progression of lung function decline.Citation15 Within this context, the present and future cornerstone of patient-centered treatment of severe asthma is based on the use of biological drugs.Citation16–Citation19 Biologic therapies usually include monoclonal antibodies, soluble receptors, and genetically altered cytokines. Because biologic treatments for asthma point to specific molecular and cellular targets, eligible patients should be identified through a search for reliable and easily assessable biomarkers. Among these, the most commonly measured in asthmatic patients are serum IgE, sputum or blood eosinophils (the latter being used more often), fractional exhaled nitric oxide, and periostin, a matricellular protein produced by both inflammatory and airway structural cells.Citation20,Citation21
Several inflammatory phenotypes of asthma have been characterized, which include eosinophilic, neutrophilic, mixed, and paucigranulocytic patterns.Citation2,Citation22 Eosinophils are the inflammatory cells most frequently infiltrating the airways of asthmatic patients, and they are crucially implicated in the development of both allergic and nonallergic asthma.Citation23,Citation24 Eosinophilic asthma originates from the activation of immunopathologic and proinflammatory pathways, mainly coordinated by T-helper (Th)2 lymphocytes, which release interleukins (IL)-5, 4, and 13. In addition to being driven by adaptive immune responses, airway eosinophilia can also arise from innate immune mechanisms, which are mediated by intercellular communications involving bronchial epithelial cells and innate lymphoid cells.Citation25,Citation26
Because of the central role played by IL-5 in maturation, activation, proliferation, and survival of eosinophils, this cytokine is a key target for the treatment of eosinophilic asthma.Citation27–Citation30 In this regard, it is noteworthy that, among the pleiotropic effects of corticosteroids, inhibition of IL-5 synthesis is one of the most important mechanisms underlying the very effective antiasthma action of these drugs.Citation31 Corticosteroids are indeed powerful inducers of eosinophil apoptosis;Citation32,Citation33 nevertheless, despite a regular or almost continuous use of inhaled and even systemic corticosteroids, some subgroups of asthmatic subjects display persistent bronchial and/or blood eosinophilia, associated with an inadequate control of asthma.Citation34 Therefore, these patients can potentially benefit from additional therapies based on the use of biological drugs targeting IL-5. Moreover, previous studies showed that neutrophilic asthma may be related to corticosteroid resistance. Indeed, it is known that corticosteroids inhibit neutrophil apoptosis and contribute to activation of these cells, suggesting that corticosteroid treatment itself could have some role in the development of neutrophilia, thus contributing to worsening of severe asthma.Citation35
Role of IL-5 in eosinophilic asthma
IL-5 plays a pivotal pathogenic role in eosinophilic asthma. In asthmatic airways, the main cellular sources of IL-5 include Th2 lymphocytes, CD4+ invariant natural killer T-cells, Type 2 innate lymphoid cells, mast cells, and eosinophils themselves.Citation26,Citation36–Citation39 Production of Th2 cytokines, also including IL-5, is markedly enhanced by IL-25.Citation40 In patients with allergic asthma, the bone marrow is able to respond to allergen challenge with an enhanced capacity of producing eosinophils, and this effect is associated with higher concentrations of IL-5 mRNA in subjects experiencing dual early and late asthmatic responses, when compared to patients showing only early bronchoconstrictive reactions.Citation41 The stimulatory action of IL-5 on eosinophil differentiation extends from the bone marrow to the airways; indeed, increased levels of IL-5, eosinophil progenitors, and mature eosinophils have been detected in the induced sputum of dual-responder asthmatics.Citation42 IL-5 synergizes with powerful eosinophil-chemotactic chemokines, namely, eotaxins 1, 2, and 3, in eliciting airway eosinophilia and bronchial hyperresponsiveness.Citation30 Furthermore, significantly increased sputum levels of IL-5 and eotaxins have been found in patients experiencing acute asthma exacerbations when compared with both healthy controls and subjects with mild persistent disease.Citation43 IL-5 and eotaxins cooperate in favoring eosinophil accumulation into the airways, especially during asthma exacerbations, and this effect is at least in part dependent on IL-5-induced inhibition of eosinophil apoptosis.Citation44,Citation45 Sputum levels of IL-5 were indeed found to be inversely correlated with the numbers of apoptotic eosinophils in both stable patients and asthmatic subjects experiencing exacerbations. Also in nonallergic, late-onset eosinophilic asthma, IL-5 exerts a key pathogenic role.Citation24 In this particular asthmatic phenotype, large amounts of IL-5 are produced by Type 2 innate lymphoid cells,Citation46 in the absence of active allergic pathways triggered by Th2 lymphocytes.
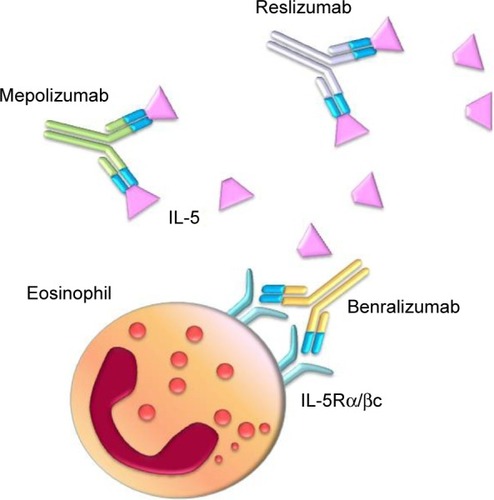
On eosinophils, the cellular effects of IL-5 are mediated by its binding to a membrane receptor including a ligand-specific α subunit (IL-5Rα) and a nonspecific signaling βc subunit (), which also interacts via a shared extracellular domain with two other hematopoietic cytokines IL-3 and granulocyte-macrophage colony stimulating factor.Citation47,Citation48 High-affinity binding of IL-5 to IL-5Rα is followed by ligation of this activated IL-5/IL-5Rα complex to the βc subunit, which probably triggers signal transduction via dimerization of its cytoplasmic domain.Citation49,Citation50 The signaling pathways activated by the interaction of IL-5 with its receptor involve several transducing enzymes, mainly including intracellular kinases such as Janus kinases (JAK), mitogen-activated protein kinases, Lyn tyrosine kinase, Raf-1 kinase, and phosphoinositide 3-kinase (PI3K) ().Citation29
Figure 1 Molecular mechanisms and signaling pathways activated by IL-5 in eosinophils.
Abbreviations: IL-5, interleukin-5; JAK, Janus kinases; STAT, signal transducers and activators of transcription; PI3K, phosphoinositide 3-kinase; MAPK, mitogen-activated protein kinases.
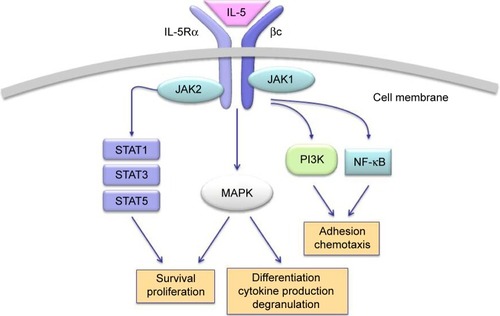
In the absence of IL-5, JAK2 and JAK1 are constitutively associated with IL-5Rα and the βc chain, respectively.Citation51 Upon IL-5 binding, the receptor construct undergoes a dynamic conformational change, leading to the association of JAK1 with IL-5Rα.Citation51 Therefore, IL-5 activates both JAK1 and JAK2, thus triggering the assembly of a functional IL-5Rα-βc complex. As a result of this IL-5-dependent stimulatory process, JAK2 induces the activation of signal transducers and activators of transcription (STAT)1, 3, and 5, which enhance the expression of pim-1, cyclin D3, and other IL-5-inducible genes involved in cell cycle progression and eosinophil proliferation.Citation52,Citation53 JAK2 is also implicated, via a cooperative action with Lyn and Raf-1 kinases, in IL-5-mediated inhibition of eosinophil apoptosis, thereby contributing to cell survival.Citation54 Moreover, Raf-1 plays a key role in eosinophil activation and degranulation.Citation54
Within the context of the intricate IL-5-stimulated signaling network activated by the βc receptor subunit, a central role is played by both extracellular-signal-regulated kinase (ERK) and p38 subgroups of mitogen-activated protein kinases (MAPK). In particular, Ras/Raf-1/MEK-mediated activation of ERK is crucially responsible for induction of c-fos gene expression and eosinophil differentiation, proliferation and survival, and also for the release of leukotriene C4.Citation55–Citation58 Furthermore, p38 MAPK mainly induces, also acting through activation of the transcription factor NF-κB, cytokine production by eosinophils, as well as eosinophil adhesion and chemotaxis occurring during allergic inflammation.Citation58–Citation60 IL-5-induced interaction of eosinophils with intercellular adhesion molecule-1 is also promoted by phosphoinositide 3-kinase, and this effect is mediated by downstream stimulation of protein kinase C and phosphorylation-dependent activation of ERK1/2.Citation61
Given the pivotal role played by IL-5 in eosinophil functions and asthma pathobiology, this cytokine and its receptor are suitable targets of biological therapies and are being evaluated for treatment of eosinophilic asthma.Citation62 In this regard, several preclinical studies have been carried out in experimental animal models of asthma. Indeed, the anti-IL-5 antibody TRFK-5 suppressed airway eosinophilia in allergen-sensitized mice.Citation63 Moreover, in nonhuman primate models of asthma, TRFK-5 inhibited the influx of eosinophils into bronchi and the associated airway hyperre-sponsiveness.Citation64 Later, other monoclonal antibodies directed against IL-5 (mepolizumab and reslizumab) or IL-5Rα (benralizumab) have been developed and evaluated in clinical trials ().Citation16,Citation65–Citation67
Reslizumab: mechanism of action, efficacy, and safety
Reslizumab is an IgG4/κ monoclonal antibody, also known as SCH-55700, which was humanized from the rat monoclonal IgG2a antibody JES1-39D10 via a synthetic process based on recombinant technology using complementarity-determining region grafting, aimed to incorporate rat antigen recognition sites for human IL-5 onto a human IgG4 structure.Citation68–Citation70 Reslizumab has a molecular weight of 146 kDa and binds with high affinity to an epitope region corresponding to amino acids 89–92 of human IL-5, thus preventing this cytokine from binding to IL-5Rα.Citation71–Citation73
The first clinical study aimed to assess the efficacy of reslizumab in asthma treatment was carried out by Kips et alCitation74 in a small group of asthmatic subjects. This Phase II, double-blind, randomized, and dose-ranging pilot trial evaluated the biological, clinical, and functional effects, as well as the safety and pharmacokinetic profiles of reslizumab. Enrolled patients were recruited on the basis of their severe persistent asthma, treated with oral glucocorticoids or high doses of inhaled corticosteroids, regardless of the underlying inflammatory phenotypes. Reslizumab was compared with placebo (n=8) and administered as a single intravenous infusion at four rising doses of 0.03 mg/kg (n=2), 0.1 mg/kg (n=4), 0.3 mg/kg (n=6), or 1.0 mg/kg (n=12), respectively. When compared with placebo, reslizumab doses ≥0.3 mg/kg significantly reduced eosinophil counts in peripheral blood with respect to baseline values, thus inducing mean decreases in circulating eosinophils ranging from 52.5% at 48 hours to 18.9% at day 30. Moreover, reslizumab lowered sputum eosinophil numbers in three of four patients with documented bronchial eosinophilia. However, no significant changes were detected in both symptom control and physician evaluation of overall clinical status. With regard to lung function, in comparison with placebo reslizumab elicited a transiently significant increase in forced expiratory volume in one second (FEV1), recorded 24 hours after administration of the 0.3 mg/kg dosage. Although a trend toward FEV1 improvement also persisted at subsequent time points, no dose of reslizumab was able to induce further significant FEV1 changes. On day 30, FEV1 increases with respect to baseline values were 11.2% in the 0.3 mg/kg group, 8.6% in the 1.0 mg/kg arm, and 4.0% in patients assigned to placebo treatment. Furthermore, no meaningful variations were observed in terms of either FEV1/FVC (forced vital capacity) ratio or peak expiratory flow. With regard to safety, all single doses of reslizumab were well tolerated, and no significant alterations of vital signs or laboratory parameters were found. The most common adverse events included headache and fatigue, which were reported with the same frequency also in the placebo group. Similarly, no significant differences were detected with regard to asthma worsening, which occurred in three of 12 patients treated with 1.0 mg/kg of reslizumab, and in one of eight subjects of the placebo arm. In one patient treated with the dosage of 1.0 mg/kg, nonneutralizing serum antibodies to reslizumab were found. The plasma levels of reslizumab were dose proportional. At 6.9 hours after administration of the 1.0 mg/kg dose, the pharmacokinetic profile of reslizumab was characterized by a mean maximal concentration of 30.3 μg/mL. After the same drug dosage, mean concentrations of 0.87 and 0.43 μg/mL were detected on days 90 and 120, respectively. The elimination half-life ranged between 24.5 and 30.1 days.
Later, in a Phase II multicenter, double-blind study performed in Northern America (United States and Canada) and specifically targeted to eosinophilic asthma, Castro et alCitation75 evaluated the effects of reslizumab in 106 patients with inadequately controlled disease despite the use of high doses of inhaled corticosteroids. Patient enrollment was made on the basis of an eosinophil percentage of at least 3% in induced sputum. In particular, 53 subjects were randomly assigned to treatment with placebo, and the other 53 patients were treated with four intravenous infusions of 3.0 mg/kg of reslizumab, administered every 4 weeks for 12 weeks, respectively. With respect to baseline counts, at the end of treatment, reslizumab induced a significant, median 95.4% reduction of sputum eosinophils. In clinical terms, this effect was associated with a positive trend toward better asthma control, which however did not reach the threshold of statistical significance; greater improvements in asthma control were achieved by a subgroup of patients characterized by the highest levels of blood and sputum eosinophils, associated with nasal polyposis. In comparison with placebo, a positive but not significant difference in favor of the reslizumab arm was also observed in the rate of asthma exacerbations. With regard to lung function, when compared to placebo reslizumab elicited significant improvements in both FEV1 (mean FEV1 increase: 180 mL) and FVC. This trial confirmed the good tolerability profile of reslizumab. Indeed, in reslizumab and placebo arms, the proportions of patients who experienced adverse events were very similar. The most frequently reported adverse event was nasopharyngitis.
More recently, two large, multicenter, double-blind, randomized, and placebo-controlled, Phase III trials have been carried out by Castro et alCitation76 with the primary end point of evaluating the effects of reslizumab on asthma exacerbations in patients with poorly controlled disease and blood eosinophilia. Inclusion criteria were based on the occurrence of one or more exacerbations treated with systemic corticosteroids during the previous year, associated with blood eosinophil counts ≥400 cells/μL, and with an inadequate asthma control despite the use of medium-to-high doses of inhaled corticosteroids, with the eventual addition of other drugs including long-acting β2 adrenergic agonists, leukotriene modifiers, cromolyn sodium, and even oral glucocorticoids. Recruited patients continued their usual asthma therapies at constant doses throughout both studies. Among 2,597 patients screened, 953 were enrolled and randomly assigned to receive intravenous infusions of either placebo or reslizumab, administered at a dosage of 3.0 mg/kg every 4 weeks for 52 weeks. When compared with placebo, reslizumab significantly lowered the annual rate of clinical asthma exacerbations by 50%–59%. Reslizumab also prolonged the time to first exacerbation. Moreover, in both trials reslizumab significantly decreased blood eosinophil numbers, improved asthma symptom control, and increased FEV1 values. These two parallel trials confirmed the good safety profile of reslizumab, which was found to be similar to that of placebo.Citation76 In both studies, the most commonly reported adverse events, occurring in more than 5% of patients receiving reslizumab, were worsening of asthma symptoms, nasopharyngitis, upper respiratory tract infections, sinusitis, influenza, and headache. Overall, when compared with subjects treated with placebo, serious adverse events were less frequent in patients receiving reslizumab. Local reactions at injection sites were uncommon and not different between placebo and reslizumab arms. In study 2, two patients receiving reslizumab experienced anaphylactic reactions judged to be treatment related, which responded well to standard therapy at clinic site, but led to withdrawal from the trial;Citation76 antidrug antibodies (ADA) were not detected in either subject. Transient, low-titer antireslizumab antibodies were found in eight patients (3%) in study 1, and in seven patients (2%) in study 2, respectively. However, in these subjects, the overall safety pattern of reslizumab was not different from that globally recorded in both study populations.
The positive effects on lung function, mainly referring to enlargement of proximal airways, can be integrated by further improvements also occurring at level of peripheral airways, as shown by another Phase III study conducted by Bjermer et al.Citation77 In particular, the results of this trial (aimed to evaluate the effects of two different doses [0.3 and 3.0 mg/kg] of intravenous reslizumab) refer to 311 patients with persistent asthma, reversible airflow limitation, and high levels of blood eosinophils (≥400 cells/μL), not adequately controlled by inhaled corticosteroids. At the end of the first part of this investigation, 271 patients chosen among those receiving either drug (n=179) or placebo (n=92) were enrolled in an open-label extension study and received the 3.0 mg/kg dose of reslizumab. When compared to placebo after 16 weeks of treatment, both doses of reslizumab induced significant increases in mean FEV1 values (115 and 160 mL with 0.3 and 3 mg/kg, respectively). Furthermore, only when administered at the 3.0 mg/kg dose, reslizumab also elicited significant increases in mean values of both FVC (130 mL) and forced expiratory flow at 25%–75% of FVC (233 mL/s). At both doses (0.3 mg/kg and 3.0 mg/kg), reslizumab improved symptom control evaluated through asthma control questionnaire score, as well as decreased the use of inhaled rescue medications. Additionally, although both reslizumab doses significantly lowered blood eosinophil counts, a greater effect was observed when the 3 mg/kg dose was used. Treatment with reslizumab at both dosages was well tolerated, and only mild-to-moderate and self-limiting adverse events related to the study drug, including headache, nasopharyngitis, upper respiratory tract infections, and sinusitis, were recorded. Low ADA titers were detected in 12% and 11% of patients treated with reslizumab 0.3 and 3 mg/kg, respectively. However, the majority of these patients were found to be ADA-positive only once during the 16-week treatment period. Moreover, the safety profile of ADA-positive patients was similar to that observed in the global study population, and ADA positivity did not have any impact on blood eosinophil suppression caused by reslizumab, thus suggesting that ADAs were not neutralizing.Citation77
The aforementioned data were indirectly confirmed by a further Phase III study performed by Corren et al,Citation78 who completed their analysis in 492 patients with poorly controlled asthma, not selected on the basis of their blood eosinophil counts, who over a period of 16 weeks received every 4 weeks placebo (n=97) or 3.0 mg/kg of intravenous reslizumab (n=395). The authors did not report significant changes in FEV1 in the overall study population and in the subgroup of patients with less than 400 blood eosinophils/μL. However, in the subgroup of patients with more than 400 blood eosinophils/μL, with respect to placebo, reslizumab produced a significant mean FEV1 increase (270 mL). Reslizumab was well tolerated, and patients receiving this drug experienced fewer overall adverse events when compared with subjects assigned to the placebo arm (55% versus 73%). Low and transient ADA titers were detected in 5% of patients treated with reslizumab, but ADA positivity did not affect either safety profile or eosinophil depletion pattern, thereby indicating a lack of neutralizing activity.Citation78
Conclusion
The well-established awareness of the role of IL-5 as a key player in the pathobiology of eosinophilic asthma has promoted the development of effective therapeutic strategies aimed to neutralize this cytokine. Indeed, as a result of several randomized controlled trials, mepolizumab and reslizumab have been recently approved for biological treatment of asthma. In particular, the pharmacologic profile of reslizumab is very interesting because this drug is characterized by a high affinity for an IL-5 epitope including residues 89–92 of the amino acid sequence.Citation73 This small region of IL-5 molecule is critically involved in cytokine binding to its receptor α subunit. Therefore, reslizumab strongly prevents IL-5/IL-5Rα interaction, thereby very effectively blocking IL-5 bioactivity.
Of course, a focused selection of eligible asthmatic patients for anti-IL-5 biotherapies requires a careful phenotypic stratification based on reliable clinical, functional, and biologic features. In particular, subjects who can take the best advantages from the use of biologic drugs targeting IL-5 are likely those suffering from uncontrolled eosinophilic, allergic or nonallergic asthma, also experiencing recurrent disease exacerbations despite the use of inhaled corticosteroids at relatively high doses. Of course, the most important biomarkers of eosinophilic airway inflammation are sputum eosinophils. However, because of the practical unfeasibility of induced sputum in many clinical settings of real-life routine medical activity, peripheral blood eosinophils represent a very useful and easily measurable parameter to characterize these patients. Indeed, the levels of circulating eosinophils approximately reflect, even better than fractioned exhaled nitric oxide, the state of ongoing bronchial inflammation.Citation79–Citation81 Therefore, it seems very reasonable that anti-IL-5 add-on treatments will soon contribute to satisfy the unmet needs of many patients with moderate-to-severe eosinophilic asthma, not adequately controlled by current standard therapies.
Disclosure
No external help was received in writing this manuscript, and no author has a financial interest related to reslizumab or its manufacturer. The authors report no conflicts of interest in this work.
References
- HolgateSTArshadHSRobertsGCHowarthPHThurnerPDaviesDEA new look at the pathogenesis of asthmaClin Sci2013118743945020025610
- PelaiaGVatrellaABuscetiMTCellular mechanisms underlying eosinophilic and neutrophilic airway inflammation in asthmaMediators Inflamm201587978325878402
- MasoliMFabianDHoltSBeasleyRGlobal Initiative for Asthma (GINA) ProgramThe global burden of asthma: executive summary of the GINA dissemination committee reportAllergy200459546947815080825
- ChanezPHumbertMAsthma: still a promising future?Eur Respir Rev20142313440540725445937
- RayAOrissTBWenzelSEEmerging molecular phenotypes of asthmaAm J Physiol Lung Cell Mol Physiol20153082L130L14025326577
- GauthierMRayAWenzelSEEvolving concepts of asthmaAm J Respir Crit Care Med2015192666066826161792
- BatemanEDBousheyHABousquetJCan guideline-defined asthma control be achieved? The Gaining Optimal Asthma ControL (GOAL) studyAm J Respir Crit Care Med2004170883684415256389
- FantaCHDrug therapy: asthmaN Engl J Med2009360101002101419264689
- Global Initiative for Asthma (GINA) [homepage on the Internet]Global strategy for asthma management and prevention Available from: http://www.ginasthma.orgAccessed June 27, 2016
- BouletLPInfluence of comorbid conditions on asthmaEur Respir J200933489790619336592
- Serra-BatllesJPlazaVMorejonEComellaABruguésJCosts of asthma according to the degree of severityEur Respir J1998126132213269877485
- AntonicelliLBuccaCNeriMAsthma severity and medical resource utilisationEur Respir J200423572372915176687
- AccordiniSCorsicoAGBraggionMThe cost of persistent asthma in Europe: an international population-based study in adultsInt Arch Allergy Immunol201316019310122948386
- HeaneyLGConwayEKellyCPredictors of therapy resistant asthma: outcome of a systematic evaluation protocolThorax200358756156612832665
- ReddelHKBatemanEDBeckerAA summary of the new GINA strategy: a roadmap to asthma controlEur Respir J201546362263926206872
- PelaiaGVatrellaAMaselliRThe potential of biologics for the treatment of asthmaNat Rev Drug Discov2012111295897223197041
- FajtMLWenzelSEBiologic therapy in asthma: entering the new age of personalized medicineJ Asthma201451766967624712500
- DarveauxJBusseWWBiologics in asthma – the next step toward personalized medicineJ Allergy Clin Immunol Pract20153215216025754716
- MitchellPDEl-GammalAIO’ByrnePMEmerging monoclonal antibodies as targeted innovative therapeutic approaches to asthmaClin Pharmacol Ther2016991384826502193
- SzeflerSJWenzelSBrownRAsthma outcomes: biomarkersJ Allergy Clin Immunol20121293 SupplS9S2322386512
- IzuharaKConwaySJMooreBBRoles of periostin in respiratory disordersAm J Respir Crit Care Med2016193994995626756066
- WenzelSEComplex phenotypes in asthma: current definitionsPulm Pharmacol Ther201326671071523880027
- LambrechtBNHammadHThe immunology of asthmaNat Immunol2015161455425521684
- BrusselleGGMaesTBrackeKREosinophils in the spotlight: eosinophilic airway inflammation in nonallergic asthmaNat Med201319897797923921745
- ErleDJSheppardDThe cell biology of asthmaJ Cell Biol2014205562163124914235
- YuSKimHYChangYJDeKruyffRHUmetsuDTInnate lymphoid cells and asthmaJ Allergy Clin Immunol2014133494395024679467
- WeltmanJKKarimASIL-5: biology and potential therapeutic applicationsExpert Opin Investig Drugs200093491496
- StirlingRGvan RensenEIBarnesPJChungKFInterleukin-5 induces CD34+ eosinophil progenitor mobilization and eosinophil CCR3 expression in asthmaAm J Respir Crit Care Med20011648 Pt 11403140911704586
- MolfinoNAGossageDKolbeckRParkerJMGebaGPMolecular and clinical rationale for therapeutic targeting of interleukin-5 and its receptorClin Exp Allergy201242571273722092535
- FulkersonPCRothenbergMETargeting eosinophils in allergy, inflammation and beyondNat Rev Drug Discov201312211712923334207
- BarnesPJAdcockIMHow do corticosteroids work in asthma?Ann Intern Med20031395 Pt 135937012965945
- ZhangXMoilanenEKankaanrantaHEnhancement of human eosinophil apoptosis by fluticasone propionate, budesonide, and beclomethasoneEur J Pharmacol2000406332533211040338
- ZhangXMoilanenEAdcockIMLindsayMAKankaanrantaHDivergent effect of mometasone on human eosinophil and neutrophil apoptosisLife Sci200271131523153412127907
- BarnesPJCorticosteroid resistance in patients with asthma and chronic obstructive pulmonary diseaseJ Allergy Clin Immunol2013131363664523360759
- KatoTTakedaYNakadaTSendoFInhibition by dexamethasone of human neutrophil apoptosis in vitroNat Immun19951441982088696009
- WoodruffPGModrekBChoyDFT-helper type 2-driven inflammation defines major sub-phenotypes of asthmaAm J Respir Crit Care Med2009180538839519483109
- SakuishiKOkiSArakiMPorcelliSAMiyakeSYamamuraTInvariant NKT cells biased for IL-5 production act as crucial regulators of inflammationJ Immunol200717963452346217785779
- ShakooryBFitzgeraldSMLeeSAChiDSKrishnaswamyGThe role of human mast cell-derived cytokines in eosinophil biologyJ Interferon Cytokine Res200424527128115153310
- HoganSPRosenbergHFMoqbelREosinophils: biological properties and role in health and diseaseClin Exp Allergy200838570975018384431
- WangYHLiuYJThymic stromal lymphopoietin, OX40-ligand, and interleukin-25 in allergic responsesClin Exp Allergy200939679880619400908
- WoodLJSehmiRDormanSAllergen-induced increases in bone marrow T lymphocytes and interleukin-5 expression in subjects with asthmaAm J Respir Crit Care Med2002166688388912231502
- DormanSCEfthimiadisABabiradISputum CD34+ IL-5Rα+ cells increase after allergen: evidence for in situ eosinophilopoiesisAm J Respir Crit Care Med2004169557357714630618
- ParkSWKimDJChangHSAssociation of interleukin-5 and eotaxin with acute exacerbation of asthmaInt Arch Allergy Immunol2003131428329012915771
- XuJJiangFNayeriFZetterstromOApoptotic eosinophils in sputum from asthmatic patients correlate negatively with levels of IL-5 and eotaxinRespir Med200710171447145417379492
- IlmarinenPMoilanenEKankaanrantaHRegulation of spontaneous eosinophil apoptosis – a neglected area of importanceJ Cell Death201471925278781
- WalkerJABarlowJLMcKenzieAMInnate lymphoid cells: how did we miss them?Nat Rev Immunol2013132758723292121
- RossjohnJMcKinstryWJWoodcockJMStructure of the activation domain of the GM-CSF/IL-3/IL-5 receptor common β-chain bound to an antagonistBlood20009582491249810753826
- MurphyJMYoungIGIL-3, IL-5, and GM-CSF signaling: crystal structure of the human β-common receptorVitam Horm20067413017027509
- JohansonKAppelbaumEDoyleMBinding interactions of human interleukin 5 with its receptor α subunit. Large scale production, structural, and functional studies of Drosophila-expressed recombinant proteinsJ Biol Chem199527016945994717721873
- IshinoTHarringtonAEGopiHChaikenIStructure-based rationale for interleukin 5 receptor antagonismCurr Pharm Des200814121231123918473871
- KouroTTakatsuKIL-5- and eosinophil-mediated inflammation: from discovery to therapyInt Immunol200921121303130919819937
- PazdrakKStaffordSAlamRThe activation of the JAK-STAT 1 signalling pathway by IL-5 in eosinophilsJ Immunol199515513974027602114
- StoutBABatesMELiuLYIL-5 and granulocyte-macrophage colony-stimulating factor activate STAT3 and STAT5 and promote Pim-1 and cyclin D3 protein expression in human eosinophilsJ Immunol2004173106409641715528381
- PazdrakKOlszewska-PazdrakBStaffordSLyn, Jak2, and Raf-1 kinases are critical for the antiapoptotic effect of interleukin-5, whereas only Raf-1 kinase is essential for eosinophil activation and degranulationJ Exp Med199818834214299687520
- AdachiTAlamRThe mechanism of IL-5 signal transductionAm J Physiol19982753 Pt 1C623C6339730944
- TakatsuKNakajimaHIL-5 and eosinophiliaCurr Opin Immunol200820328829418511250
- BatesMEGreenVLBerticsPJERK1 and ERK2 activation by chemotactic factors in human eosinophils is interleukin 5-dependent and contributes to leukotriene C4 biosynthesisJ Biol Chem200027515109681097510753897
- PelaiaGCudaGVatrellaAMitogen-activated protein kinases and asthmaJ Cell Physiol2005202364265315316926
- AdachiTChoudhuriBKStaffordSThe differential role of extracellular signal-regulated kinases and p38 mitogen-activated protein kinase in eosinophil functionsJ Immunol200016542198220410925307
- IpWKWongCKWangCBInterleukin-3, -5, and granulocyte macrophage colony-stimulating factor induce adhesion and chemotaxis of human eosinophils via p38 mitogen-activated protein kinase and nuclear factor-κBImmunopharmacol Immunotoxicol200527337139316237950
- SanoMLeffARMyouSRegulation of interleukin-5-induced β2-integrin adhesion of human eosinophils by phosphoinositide 3-kinaseAm J Respir Cell Mol Biol2005331657015802551
- GallelliLBuscetiMTVatrellaAUpdate on anticytokine treatment for asthmaBioMed Res Int201310431523853765
- GarlisiCGKungTTWangPEffects of chronic anti-interleukin-5 monoclonal antibody treatment in a murine model of pulmonary inflammationAm J Respir Cell Mol Biol19992022482559922215
- MauserPJPitmanAMFernandezXEffects of an antibody to interleukin-5 in a monkey model of asthmaAm J Respir Crit Care Med199515224674727633694
- WalshGMTherapeutic potential of targeting interleukin-5 in asthmaBioDrugs201327655956323728885
- PattersonMFBorishLKennedyJLThe past, present, and future of monoclonal antibodies to IL-5 and eosinophilic asthmaJ Asthma Allergy2015812513426604804
- VarricchiGBagnascoDBorrielloFInterleukin-5 pathway inhibition in the treatment of eosinophilic respiratory disorders: evidence and unmet needsCurr Opin Allergy Clin Immunol201616218620026859368
- ChungKFTargeting the interleukin pathway in the treatment of asthmaLancet201538699981086109626383000
- WalshGMProfile of reslizumab in eosinophilic disease and its potential in the treatment of poorly controlled eosinophilic asthmaBiologics2013771123326187
- EganRWAthwalDBodmerMWEffect of Sch 55700, a humanized monoclonal antibody to human interleukin-5, on eosinophilic responses and bronchial hyperreactivityArzneimittelforschung199949977979010514907
- CardetJCIsraelEUpdate on reslizumab for eosinophilic asthmaExpert Opin Biol Ther201515101531153926372797
- LimHNairPEfficacy and safety of reslizumab in patients with moderate to severe eosinophilic asthmaExpert Rev Respir Med20159213514225578680
- ZhangJKuvelkarRMurgoloNJMapping and characterization of the epitope(s) of Sch 55700, a humanized mAb, that inhibits human IL-5Int Immunol199911121935194410590259
- KipsJCO’ConnorBJLangleySJEffects of SCH55700, a humanized anti-human interleukin-5 antibody, in severe persistent asthma: a pilot studyAm J Respir Crit Care Med2003167121655165912649124
- CastroMMathurSHargreaveFReslizumab for poorly controlled, eosinophilic asthma: a randomized, placebo-controlled studyAm J Respir Crit Care Med2011184101125113221852542
- CastroMZangrilliJWechslerMEReslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trialsLancet Respir Med20153535536625736990
- BjermerLLemiereCMasperoJWeissSZangrilliJGerminaroMReslizumab for inadequately controlled asthma with elevated blood eosinophil levels: a randomized phase 3 studyChest2016S0012-3692164755147553
- CorrenJWeinsteinSJankaLZangrilliJGarinMPhase 3 study of reslizumab in patients with poorly controlled asthma: effects across a broad range of eosinophil countsChest2016S0012-3692164571545716
- WagenerAHde NijsSBLutterRExternal validation of blood eosinophils, FE(NO) and serum periostin as surrogates for sputum eosinophils in asthmaThorax201570211512025422384
- PavordIDAgustiABlood eosinophil count: a biomarker of an important treatable trait in patients with airway diseaseEur Respir J20164751299130327132257
- GeorgeLBrightlingCEEosinophilic airway inflammation: role in asthma and chronic obstructive pulmonary diseaseTher Adv Chronic Dis201671345126770668