Abstract
Diabetes is a global health emergency projected to affect 642 million people by 2040. Type 2 diabetes (T2D) represents 90% of diabetes cases and is associated with a range of cardiovascular (CV) risk factors that are more than double the incidence of CV disease and significantly increase mortality rates. Diabetes treatments have typically focused on improving glycemic control but their effect on CV outcomes has remained uncertain. In 2008, the US Food and Drug Administration (FDA) looked to address this knowledge gap and mandated CV outcome trials (CVOTs) for all new antidiabetic therapies. In 2015, EMPA-REG OUTCOME® became the first CVOT to present results for a sodium/glucose cotransporter 2 (SGLT2; also known as SLC5A2) inhibitor, empagliflozin. Subsequently, a regional meeting of the Academy for Cardiovascular Risk, Outcomes and Safety Studies in Type 2 Diabetes (ACROSS T2D) brought together a respected faculty of international experts and 150 physicians from 14 countries to discuss the current unmet medical needs of patients with T2D, the results from the EMPA-REG OUTCOME study and the implications of these results for clinical practice. This article summarizes the current scientific evidence and the discussions that took place at the ACROSS T2D regional meeting, which was held in Vienna, Austria, on May 30, 2016.
Introduction
Diabetes accounts for 12% of worldwide health expenditure; one in 11 adults, or 412 million people, has diabetes (projected to increase to 642 million by 2040),Citation1 and of these patients, 90% have type 2 diabetes (T2D).Citation1 Diabetes affects multiple organs, including the eyes, kidneys, nerves, and heart, which results in a range of comorbidities, as well as increased risks of cancer, serious psychiatric illness, cognitive decline, chronic liver disease, and accelerated arthritis.Citation2
T2D is also associated with cardiovascular (CV) risk factors, such as dyslipidemia, hypertension, hyperglycemia, obesity, and increased oxidative stress.Citation3 CV disease (CVD) is the leading cause of mortality in patients with diabetes, accounting for ~50% of deaths and reducing the life expectancy of a 60-year-old patient with T2D by an average of 12 years compared with the general population.Citation1,Citation4 Although microvascular complications have been reduced by improved glycemic control with glucose-lowering drugs,Citation5–Citation8 the effect of these drugs on macrovascular outcomes is less certain.Citation9,Citation10 Consequently, in 2008, the US Food and Drug Administration (FDA) mandated CV outcome trials (CVOTs) for all new glucose-lowering therapies.
The impact of CVD on patients with T2D and the recent data from the EMPA-REG OUTCOME® trial (the CVOT for empagliflozin) were discussed by an international faculty of experts and 150 physicians from 14 countries at the regional meeting of the Academy for Cardiovascular Risk, Outcomes and Safety Studies in Type 2 Diabetes (ACROSS T2D) that was held in Vienna, Austria, on May 30, 2016. This article summarizes the data presented at the meeting and the thoughts and opinions put forward by the expert faculty and meeting delegates.
Managing CV risk in patients with T2D: how are we doing?
Professor Chaim Lotan, Hadassah-Hebrew University Medical Center, Jerusalem, Israel
CV risk factors have a significant and cumulative effect on patient prognosis and the risk of CVD death.Citation11 Indeed, patients with T2D are two to four times more likely to have CVD and twice as likely to have a stroke than the general population.Citation12,Citation13 The proportion of patients with diabetes who reach CV risk factor goals has improved in the past 20 years, but a considerable number of patients remain at unnecessarily high risk. A cross-sectional analysis of US National Health and Nutrition Examination Survey (NHANES) data found that only 18.8% of patients with diabetes met the American Diabetes Association (ADA) 2011 targets of HbA1c <7%, blood pressure <130/80 mmHg and LDL cholesterol <100 mg/dL (2.6 mmol/L) in 2007–2010. Although this percentage represents a substantial improvement from 1988 to 1994 (1.7%) and 1999 to 2002 (7.1%),Citation14 there is a clear need to further reduce CV risk factors in people with T2D.
Targeting CV risk factors
A range of therapies have been shown to reduce CV risks in patients with T2D and are now recommended by the ADA and the European Society of Cardiology (ESC).Citation15,Citation16 For those patients with T2D who have elevated lipid levels, statins form an important part of treatment. A meta-analysis of data from 18,686 patients with diabetes who were prescribed statins revealed, over a mean follow-up of 4.3 years, a 9% reduction in all-cause mortality, a 13% reduction in CV deaths and a 21% reduction in major vascular events compared with those not prescribed statins.Citation17
Professor Lotan also emphasized the importance of addressing hypertension, highlighting findings that show that a small decrease in blood pressure can have a large effect on the incidence of CV events. He pointed to data that showed a small reduction in systolic blood pressure (SBP; 4 mmHg) in high-risk patients (>21% 5-year risk of CVD) avoided as many CV events as a large reduction in SBP (16 mmHg) in low-risk patients (<11% 5-year risk of CVD),Citation18 although the benefits of a small decrease in blood pressure remain less clear in patients with a baseline SBP of <140 mmHg.Citation19
Glucose-lowering benefits?
Glycemic control is central to diabetes management; however, its role in managing CV risk factors has remained unclear. Several studies (ACCORD, ADVANCE, UKPDS, and VADT) have looked at the effect of more intensive glycemic control on macrovascular outcomes in T2D.Citation5–Citation8 Although these studies showed no beneficial effect of glycemic control per se on major CV events (CV death, nonfatal myocardial infarction [MI], and nonfatal stroke), a meta-analysis found that more intensive therapy offered a 9% reduction in the risk of major CV events compared with less intensive therapy.Citation20 This finding was driven by a hazard ratio of 0.85 for MI, with no significant reductions for stroke and CV death. There was also no evidence that more intensive glycemic control reduced the risk of heart failure.Citation20 Long-term follow-up of these studies showed mixed results; a 6-year follow-up of ADVANCE showed no effect of intensive glycemic control on CVD or mortality, whereas a 10-year follow-up of VADT showed a reduction in CVD and a 20-year follow-up of UKPDS showed reductions in both CVD and mortality.Citation21
Of the large number of glucose-lowering drug classes available, none, as yet, are recognized unequivocally to reduce CV events over and above any modest effects of glucose lowering itself,Citation22 and some may actually exacerbate CV risk.Citation23 This uncertainty over the effects of glucose-lowering agents on CV risk resulted in the FDA requesting CVOTs for all new diabetes treatments.Citation24 CVOT results, which are now becoming available, have provided the first evidence showing that some of the new glucose-lowering agents, such as empagliflozin, are capable of significantly reducing CV risks, which demonstrates that these drugs have benefits beyond lowering blood glucose levels.Citation25
A complete approach to treatment
Tackling CVD in patients with T2D evidently requires a multifactorial approach. The Steno-2 trial randomized 160 patients at a single center to receive conventional treatment or intensive multifactorial control of CV risk factors with goals of HbA1c <6.5%, total cholesterol <175 mg/dL (4.5 mmol/L), triglycerides <150 mg/dL (1.7 mmol/L) and blood pressure <130/80 mmHg.Citation26 Over a mean treatment period of ~8 years, and an average subsequent follow-up of 5.5 years, intensive treatment was associated with a significantly reduced risk of all-cause mortality, CV mortality, and CV events.Citation26
Panel discussion: audience insights and faculty perspectives
Professor Lotan urged doctors to look beyond glycemic control when managing patients with T2D – focusing colleagues on the need to pay equal attention to the control of lipids and blood pressure and to start treating the whole patient, not just the patient’s diabetes. In response, a delegate asked whether the EMPA-REG OUTCOME study heralded a shift in the approach to T2D management, from a glucose-centric approach to a vascular-centric approach. Professor Wascher agreed that the EMPA-REG OUTCOME study could indeed help refocus T2D management strategies toward a more vascular approach, and he added that clinicans’ focus on glycemic control in T2D was a consequence of the approach used for type 1 diabetes treatment. The discussion concluded with Professor Lotan reiterating his belief that T2D treatment should focus on the patient as a whole rather than on an individual aspect of the disease. He elaborated on this point by highlighting the need to treat comorbidities, such as depression, that can affect adherence and treatment outcomes.
CVOTs: expectations and reality
Professor Dr Thomas Wascher, Hanuschkrankenhaus, Vienna, Austria
Most of the major trials of antidiabetic drugs have focused on establishing glucose-lowering properties, whereas assessment of the effect of these drugs on CV outcomes has been limited.Citation27 For example, the effects of sulfonylureas and metformin on CV risk have not been evaluated in long-term trials,Citation15 and class effects for other agents cannot be assumed – illustrated by the differences between the thiazolidinediones rosiglitazone, and pioglitazone.Citation28,Citation29 In light of the uncertainty surrounding the effect of glucose-lowering agents on CV risk, the FDA issued guidance in 2008 mandating a more thorough CV assessment of new drugs for T2D.Citation24 In 2012, the European Medicines Agency (EMA) published a guideline requiring CVOTs for new antidiabetic drugs for which specific CV claims are made or that are suspected of having detrimental CV effects.Citation27,Citation30
CVOTs for T2D drugs are designed to assess drug-specific CV effects and CV safety beyond the modest effects of blood glucose lowering on CV risk factors. The FDA requires CVOTs to enroll patients at high CV risk and to compare the drug in question with placebo. CVOTs are also encouraged to have study designs that offer adjustment of background antihyperglycemic therapy and that drive composite primary endpoints by events rather than fixed time durations.Citation31 summarizes the timings of completed and ongoing CVOTs for the newer T2D agents.Citation32
Figure 1 Completed and ongoing cardiovascular outcome trials in type 2 diabetes.
Abbreviations: CVOT, cardiovascular outcome trial; SGLT2, sodium/glucose cotransporter 2.
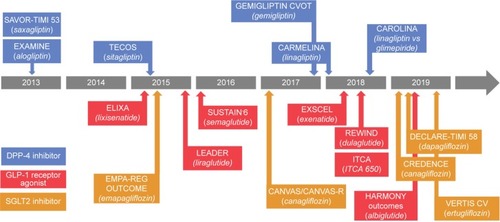
CVOTs: mixed results
The three completed large prospective CVOTs of the DPP-4 inhibitors saxagliptin, alogliptin, and sitagliptin generally indicate a neutral effect on CV events.Citation33–Citation35 However, in the SAVOR-TIMI 53 study, saxagliptin was observed to increase hospitalization for heart failure (HHF).Citation33 On the basis of this finding, the FDA issued a warning regarding the risk of heart failure for saxagliptin.Citation36 The warning also included alogliptin, for which heart failure risk is unclear because it was not assessed in the alogliptin CVOT (EXAMINE).Citation34,Citation36
GLP-1 receptor agonists seem to have mixed effects on CV outcomes. In patients with acute coronary syndrome, lixisenatide did not significantly reduce the rate of major CV events, having a similar effect to placebo.Citation37 By comparison, liraglutide was shown to significantly reduce CV risk (CV death, nonfatal MI, and nonfatal stroke) when compared with placebo.Citation38,Citation39
EMPA-REG OUTCOME, which assessed empagliflozin, was the first CVOT to publish data for a sodium/glucose cotransporter 2 (SGLT2; also known as SLC5A2) inhibitor and was the first study to provide evidence that glucose-lowering agents can reduce mortality and CV risks in patients with T2D, independently of glycemic control.Citation25
Panel discussion: audience insights and faculty perspectives
Professor Prázný commented on the importance of the CVOT trials for patients with T2D – 95% of delegates agreed that CVOTs are valuable or extremely valuable for informing clinical practice.
EMPA-REG OUTCOME: getting to the heart of the matter
Professor Guntram Schernthaner, chairperson, Rudolfstiftung Hospital, Vienna, Austria
As an SGLT2 inhibitor, empagliflozin reduces hyperglycemia by inhibiting reabsorption of glucose within the kidney.Citation30 Pooled results from pivotal Phase III trials have shown that empagliflozin, either as a monotherapy or as an add-on therapy, significantly reduces HbA1c levels, weight, and SBP compared to placebo.Citation40
Furthermore, SGLT2 inhibitors can also modulate other CV risk factors, including visceral adiposity, hyperinsulinemia, arterial stiffness, albuminuria, circulating uric acid levels, oxidative stress, and lipids.Citation22 EMPA-REG OUTCOME was a multicenter, randomized, double-blind, placebo-controlled trial to assess the effect of empagliflozin (10 mg or 25 mg doses, once daily) compared to placebo on CV events in adults with T2D and high CV risk (N=7,020) against a background of stable glucose-lowering therapy.Citation25 Selected baseline patient characteristics are listed in .
Table 1 Selected baseline patient characteristics from the EMPA-REG OUTCOME® trial
Reducing CV risk with empagliflozin
summarizes the outcomes from the EMPA-REG OUTCOME study; study results were similar for the 10 mg and 25 mg doses of empagliflozin and were pooled for analysis.Citation24 Empagliflozin met the primary outcome of three-point major adverse CV event (3P-MACE; time to first occurrence of CV death or nonfatal MI or stroke), with 10.9% of patients in the empagliflozin group experiencing a CV event compared to 12.1% of those in the placebo group (HR: 0.86, 95% CI: 0.74–0.99; P<0.001 for noninferiority and P=0.04 for superiority). In the key secondary outcome, 4P-MACE (3P-MACE plus hospitalization for unstable angina), 12.8% of patients in the empagliflozin group experienced a CV event compared to 14.3% of those in the placebo group (HR: 0.89, 95% CI: 0.78–1.01; P<0.001 for noninferiority and P=0.08 for superiority).Citation25 Importantly, compared with placebo, empagliflozin was associated with significantly lower rates of CV death, all-cause mortality, and HHF; these benefits were consistent across patient subgroups, including age, HbA1c levels, body mass index (BMI), estimated glomerular filtration rate (eGFR), and use of CV medications.Citation25 The EMPA-REG OUTCOME study is the first CVOT to demonstrate a significant improvement in CV outcomes in high-risk patients with T2D.Citation25
Table 2 Outcomes of EMPA-REG OUTCOME® trialTable Footnotea
The incidence of adverse events, serious adverse events, and adverse events leading to the discontinuation of a study drug was similar between the empagliflozin and placebo groups, except for genital infections, which were reported in 1.8% of patients in the placebo group and 6.4% of patients in the empagliflozin group ().Citation25
Empagliflozin and heart failure
The reductions in CV death, HHF, and all-cause mortality in the empagliflozin cohort in the EMPA-REG OUTCOME study occurred regardless of the presence or absence of preexisting heart failure at baseline compared to those in the placebo cohort ().Citation41 Again, the effect of empagliflozin was consistent across subgroups.Citation41 Interestingly, empagliflozin also reduced CV death in patients receiving baseline loop diuretics, indicating that even patients with significant CV comorbidities can benefit from empagliflozin.Citation42
Panel discussion: audience insights and faculty perspectives
As patients in the EMPA-REG OUTCOME study had high CV risk, one delegate was interested to know whether the faculty felt the data could be generalized outside of this patient population. The chairperson stated that, from his perspective as a scientist, he was compelled to say that the data only provided evidence for the benefits of empagliflozin to a high CV risk patient population; however, despite this caveat, he believed that many other patients could also benefit from this treatment. Dr Jarvis added that patients in the EMPA-REG OUTCOME study were already well controlled and that perhaps an even greater improvement in CV outcomes would have been observed if the patients had instead been more representative of those seen in the clinic.
One physician did have concerns with the study data and asked what explanation the faculty could provide for the very early separation in the empagliflozin and placebo curves for many of the primary and secondary outcomes in EMPA-REG OUTCOME.Citation25 Professor Lotan agreed that the early separation was different to what is usually seen in clinical trials but expressed confidence in the data. He suggested that the early benefits were a consequence of empagliflozin effects beyond glucose lowering, such as electrolyte/volume changes and reduced arterial stiffness. When pressed for what might be the most important physiological effect of empagliflozin, the chairperson suggested that hemodynamic effects, specifically volume reduction through reduced renal blood flow and vascular resistance, were key benefits of the drug.Citation43,Citation44 Professor Lotan agreed, stating that empagliflozin reduces volume without affecting the sympathetic nervous system, which probably benefits the heart.
EMPA-REG OUTCOME: renal subanalysis
Professor Christoph Wanner, University Hospital, Würzburg, Germany
Chronic kidney disease (CKD) manifests as a progressive decline in kidney function and is associated with an increased risk of death, mainly from CV causes, in patients with T2D.Citation45 CKD affects as many as 18% of prediabetics and 40% of patients with T2D;Citation45 however, no CKD-specific treatments have been launched since the renin–angiotensin system (RAS)-blocking drugs that were introduced in the 2000s.Citation46,Citation47
Empagliflozin and kidney disease
Renal outcomes were a prespecified objective of EMPA-REG OUTCOME, and >1,800 of the 7,020 enrolled patients at baseline had stage 3 or worse kidney disease (eGFR <60 mL/min/1.73 m2).Citation25 Empagliflozin slowed kidney disease progression in patients with T2D, with new onset or worsening of kidney disease reduced, compared with placebo both in the overall patient population (HR: 0.61, 95% CI: 0.53–0.70; P<0.001) and in patients with prevalent kidney disease (HR: 0.58, 95% CI: 0.47–0.71; P<0.001), as well as across various patient subgroups, including baseline eGFR, urine albumin-to-creatine ratio (UACR), HbA1c levels, and BMI.Citation48 These improved renal outcomes in the empagliflozin cohort were also consistent between the 10 mg and 25 mg dose study arms.Citation48 Interestingly, the reduction in new onset or worsening of kidney disease was also consistent regardless of kidney function, with patients with an eGFR of <45 mL/min/1.73 m2 experiencing the same effects as patients with normal kidney function.Citation48 However, empagliflozin is currently not recommended for patients whose eGFR is persistently <45 mL/min/1.73 m2.Citation49
Empagliflozin also significantly reduced a series of other prespecified renal endpoints compared with placebo in the EMPA-REG OUTCOME study (). Furthermore, post hoc analyses indicated that, compared with placebo, empagliflozin delayed patient progression toward end-stage renal disease and also reduced acute renal failure and acute kidney injury.Citation42,Citation48
Table 3 Secondary endpoints from the EMPA-REG OUTCOME® renal subanalysis
SGLT2 inhibitors, kidney disease, and the FDA
Acute kidney injury and renal failure may be a concern for drugs of the SGLT2 inhibitor class. Accordingly, the FDA has added warnings to the labels of canagliflozin and dapagliflozin regarding acute kidney injury; these warnings include recommendations to minimize patient risk, requiring physicians to consider renal risk factors (such as decreased blood volume, congestive heart failure, diuretics, and blood pressure medications) before initiating treatment.Citation50
By contrast, in addition to slowing kidney disease progression, the EMPA-REG OUTCOME indicated that empagliflozin may reduce the incidence of acute kidney injury and acute renal failure.Citation48 In patients with a baseline eGFR of <59 mL/min/1.73 m2, 3.6% of patients receiving placebo experienced acute kidney injury compared to 2.1% of patients receiving empagliflozin.Citation48 A similar effect was seen for acute renal failure: 14.3% of patients receiving placebo experienced renal failure compared to 11.2% of patients receiving empagliflozin.Citation48 Finally, in patients with a baseline eGFR of ≥60 mL/min/1.73 m2, acute kidney injury was observed in 0.9% and 0.5% – and acute renal failure in 3.9% and 3.2% – of patients receiving placebo and empagliflozin, respectively.Citation48
Panel discussion: audience insights and faculty perspectives
Professor Wanner thought that the 44% reduction in the combined endpoint of doubling of serum creatinine, initiation of renal replacement or death due to renal disease compared with placebo was a particularly notable finding of the EMPA-REG OUTCOME study, as this reduction was greater than that seen in studies assessing CKD therapies, such as angiotensin-converting enzyme (ACE) inhibitors.Citation48
Importantly, reported adverse events with empagliflozin were consistent between patients with an eGFR of <60 mL/min/1.73 m2, patients with an eGFR of <45 mL/min/1.73 m2 and patients in the overall EMPA-REG OUTCOME study population.Citation48 According to the EMA, empagliflozin should not be initiated in patients with an eGFR of <60 mL/min/1.73 m2, and should be discontinued in patients whose eGFR falls persistently <45 mL/min/1.73 m2, even when these patients tolerate the drug.Citation49 Many of the panel felt that these restrictions in empagliflozin treatement were unwarranted and that more evidence-based decision making was needed. Dr Jarvis suggested that national guidance bodies, such as NICE in the UK, can help circumvent restrictions that limit use.
One member of the audience asked whether empagliflozin should be contraindicated in nephrectomy patients. Professor Wanner responded that the filtration rate is the important factor for evaluating empagliflozin treatment and not whether patients have one or two kidneys.
CV outcomes and SGLT2 inhibitors – a class effect?
Professor Martin Prázný, Charles University, Prague, Czech Republic
The EMPA-REG OUTCOME study provides the first evidence that treatment with an SGLT2 inhibitor, empagliflozin, can achieve significant improvements in CVD, heart failure, and renal and mortality endpoints in high CV risk patients with T2D.Citation25 Based on these findings, can we now assume an SGLT2 inhibitor class effect? Professor Prázný suggested not, as we need to wait for the results from the ongoing canagliflozin (CANVAS) and dapagliflozin (DECLARE-TIMI 58) CVOTs before we can consider a class effect.Citation51,Citation52 Indeed, there are precedents for differences in efficacy and safety between members of the same drug class, including between members of the SGLT2 inhibitor class. For example, canagliflozin is associated with increased osteoporotic bone fractures, whereas empagliflozin is not,Citation50,Citation53 as confirmed in the EMPA-REG OUTCOME study, which found that bone fractures occurred in 3.9% of patients receiving placebo group and 3.8% of patients receiving empagliflozin.Citation25
Beyond empagliflozin – what do we know so far?
Although it is too soon to assume an SGLT2 inhibitor class effect on CV outcomes, initial data for other members of the class seem promising. Pooled analyses of canagliflozin and dapagliflozin trial data indicate a generally favorable effect on 4P-MACE,Citation24,Citation54 and Archimedes modeling estimated that, over a 20-year period, patients with T2D receiving dapagliflozin plus standard of care would experience relative reductions in the incidence of MI (13.8%), stroke (9.1%), CV death (9.6%), and all-cause mortality (5%).Citation55 In addition, a prespecified meta-analysis of CV events from 21 Phase IIb/III clinical trials of dapagliflozin in different patient populations found no suggestion of increased risk of MACE in any population and the potential for a beneficial effect in the overall population and patients with a history of CVD.Citation11 However, as Professor Prázný explained, these models are based on heterogeneous, short-term studies, and the results of the ongoing CVOTs once completed will be required to determine whether the CV benefits of empagliflozin extend to the other SGLT2 inhibitors.
Panel discussion: audience insights and faculty perspectives
The question of class effects prompted much discussion within the ACROSS T2D regional faculty. Although some felt that a class effect was likely, all were agreed that it was essential to wait for the outcomes of other SGLT2 inhibitor CVOTs before drawing any conclusions. However, the chairperson urged caution, as significant differences in adverse events exist between the SGLT2 inhibitors. The differences between empagliflozin and other SGLT2 inhibitors, such as bone fracture risk and use with loop diuretics, suggest that similar behavior between the drugs cannot be assumed. Professor Prázný supported this point and drew upon the example of the differences in heart failure risk observed between members of the DPP-4 inhibitor class to emphasize the need for more data before making any conclusions about a class effect.Citation35,Citation56
Our patients at heart: how can we collectively design better treatment solutions?
The ACROSS T2D regional faculty
An increasing number of glucose-lowering agents are now available, and deciding which treatment is most appropriate for a patient is an increasingly complex choice. The results from the EMPA-REG OUTCOME study have shown that add-on empagliflozin provides CV and mortality benefits in T2D patients with high CV risk – but how can this inform clinical practice decisions regarding which real-world patients should receive empagliflozin?Citation25
To understand the audience’s current clinical practice, Professor Wanner presented an interactive patient case involving an elderly male patient with T2D. With glycemic control being the key treatment aim for the patient, ~50% of the audience chose to add a DPP-4 inhibitor to his metformin, as DPP-4 inhibitors offer glycemic control with a low risk of hypoglycemia.Citation57 Six months later, the patient had signs of reduced renal function, and ~63% of the audience agreed with Professor Wanner’s suggestion that empagliflozin may be a more suitable alternative to the previous treatment, owing to its ability to slow kidney disease progression while also lowering glucose levels.Citation40,Citation48
In small working groups, delegates explored a series of complex patient cases that examined best practice with various antidiabetic therapies and a range of comorbidities, including heart failure, atrial fibrillation, kidney disease, hyperlipidemia, and obesity. In a series of engaging discussions, delegates advocated a range of treatment options, among which a theme emerged of the general suitability of empagliflozin for patients with moderate-to-high CV risk or renal function concerns.
Guiding best practice?
T2D can be a complex condition with associated comorbidities that significantly reduce the quality of life and increase mortality. The presence of multiple comorbidities can complicate the patient treatment pathway and result in care that does not always meet best practice. Approximately 80% of delegates believed that the EMPA-REG OUTCOME study should lead to changes in clinical practice, but how should this be achieved? Over 75% of delegates felt that local diabetes guidelines should be updated to reflect the latest data, which was a position that was supported by Dr Jarvis because, with the rationale that many primary care physicians (PCPs) find it difficult to challenge guidelines. She encouraged the specialists in the room to assist in updating their local guidelines and to ensure that the guidelines reflect the latest data and best practice. Some in the audience felt that increased support and engagement with local medical societies could provide the necessary impetus and expertise to revise T2D guidelines and increase patient access to recent therapeutic advances.
In response to Professor Lotan’s earlier call to treat the whole patient, not just the diabetes, many physicians advocated for the establishment of multidisciplinary treatment teams. Although the patient’s physician would retain overall responsibility, a team of specialists – such as a nephrologist and a cardiologist – would be available to review patient progress and offer advice. Concerns around time and costs were raised, but these were not considered insurmountable, with email and virtual clinics offered as cost-saving alternatives to face-to-face meetings.
Continuing education
The suggestion that was most consistently voiced by the delegates was the need for more education. With an increasing number of treatments available for T2D, it was felt that local peer-to-peer educational meetings and practical workshops were essential to increase understanding and ensure best practice. However, education should not be limited to PCPs or diabetologists; similar to the need for multidisciplinary treatment teams, all physicians and health care practitioners involved in the treatment of patients with T2D should be involved in education.
Conclusion
Although T2D is a condition that is characterized by reduced glycemic control,Citation15 the associated CV risk factors account for much of the increased morbidity and mortality observed in patients with the disease: CVD is responsible for ~50% of T2D deathsCitation1 and 68% of diabetes patients >65 years die from heart disease.Citation58 Metformin is the most widely used oral glucose-lowering medication for patients with T2D and remains the first-line treatment of choice.Citation15 Despite initial CV concerns, metformin was shown to not raise the incidence of heart failure in patients with T2D and to even reduce the risk of MI in these patients.Citation56,Citation59 However, with the incidence of CVD-associated mortality so high in patients with T2D, additional therapies are clearly needed.Citation58
Empagliflozin in patients with T2D and high CV risk
The EMPA-REG OUTCOME study was the first CVOT to identify a positive benefit on CV outcomes,Citation25 showing that adding empagliflozin to standard of care significantly reduced CV death, all-cause mortality, HHF, and kidney disease progression.Citation41,Citation48 Members of the ACROSS T2D faculty at the regional meeting agreed that the EMPA-REG OUTCOME study provided robust data on the benefits that empagliflozin offers patients with T2D. But what do these benefits mean for clinical practice?
EMPA-REG OUTCOME was performed in patients with high CV risk, and Professor Lotan remarked that the use of add-on therapies in these patients was a matter of risk assessment. He stated that the risks associated with empagliflozin are very low, and patients with high CV risk would therefore benefit from starting empagliflozin treatment early. Reflecting on the improvements in CV outcomes, many faculty and delegates felt that patients with more moderate CV risks could also benefit from add-on empagliflozin – ~48% of delegates believed that the EMPA-REG OUTCOME data could be generalized to all patients with T2D and another 45% felt that generalization might be possible.
An SGLT2 inhibitor class effect?
Similar to the generalization of the results of the EMPA-REG OUTCOME study to all patients with T2D, can the results also be generalized to all SGLT2 inhbitors? “Not yet” was the opinion of the panel. Despite belonging to the same class, there are pharmacological differences between the SGLT2 inhibitors – in particular selectivity for SGLT2 over SGLT1 – that may influence efficacy and safety. Indeed, differences in safety have already been identified in the incidence of osteoporotic bone fractures, which supports the notion that it is too soon to consider a class effect for the SGLT2 inhibitors.Citation49,Citation60,Citation61 However, the results from the dapagliflozin and canagliflozin CVOTs, which are expected between 2017 and 2019, should provide clarification on the question of a class effect.Citation51,Citation52
Improving CV outcomes
CV risks need to be addressed in patients with T2D; 93% of delegates at the regional ACROSS T2D meeting were very likely or quite likely to review their patients’ CV risks when assessing their treatment. Empagliflozin is the first SGLT2 inhibitor to provide significant benefits for CV outcomes in patients with T2D.Citation25 Despite this, a third of delegates had no local guidelines to support decision making or reported restricted access to SGLT2 inhibitors. The regional ACROSS T2D panel considered empagliflozin as an important addition to the T2D armamentarium that offers significant benefits to patients with T2D at moderate and high CV risk. Updates to local guidelines and working practices should be considered to ensure that patients with T2D and high CV risk receive optimal management of their condition.
Acknowledgments
This article is a report on the regional meeting of ACROSS T2D that took place in Vienna, Austria, on May 30, 2016. Faculty members were supported by Fortis Pharma Communications in the preparation of this article. The meeting was sponsored by Boehringer Ingelheim.
Disclosure
CL has previously received honoraria for speaking from Boehringer Ingelheim, MSD, Boston Scientific, Medtronic, and AstraZeneca and has previously received honoraria for consultancy from Medtronic, Bendit, Healthwatch and Arineta. CW has previously received honoraria for speaking from AbbVie, Amgen, Boehringer Ingelheim, Janssen, Actelion, and Sanofi-Genzyme and has served on advisory boards for Actelion, Boehringer Ingelheim, Celgene, GSK, and Novo Nordisk. GS has previously received research grants and honoraria for speaking from Abbot, Amgen, Andromeda, AstraZeneca, Bayer, Boehringer Ingelheim, BMS, DeveloGen, Eli Lilly, GSK, Janssen, Merck, MSD, Novartis, Novo Nordisk, Pfizer, Roche, Sanofi-Aventis, Serono, Servier, and Takeda and has served as principal investigator in >40 studies. GS and CW have received honoraria from Boehringer Ingelheim for the EMPA-REG OUTCOME® trial. MP has previously received honoraria for speaking and consultancy from Abbott, AstraZeneca, Boeh-ringer Ingelheim, Eli Lilly, Medtronic, Novo Nordisk, Roche, Sanofi, and Takeda. SJ has received honoraria for speaking and moderating meetings from AstraZeneca, Boehringer Ingelheim, Janssen, MSD, Novartis, and Takeda. TCW has received honoraria for educational lectures as well as consulting from Astra Zeneca, Boehringer Ingelheim, MSD, Takeda, Novo Nordisk, Eli Lilly, Sanofi, Novartis, Johnson & Johnson, Amgen, and Roche. The authors report no other conflicts of interest in this work.
References
- International Diabetes Federation [webpage on the Internet]IDF Diabetes Atlas7th edBrusselsThe Federation2015 Available from: http://www.Diabetesatlas.org/Resources/2015-Atlas.htmlAccessed October 17, 2016
- Emerging Risk Factors CollaborationSeshasaiSRKKaptogeSDiabetes mellitus, fasting glucose, and risk of cause-specific deathN Engl J Med2011364982984121366474
- LauDCWDhillonBYanHSzmitkoPEVermaSAdipokines: molecular links between obesity and atheroslcerosisAm J Physiol Heart Circ Physiol20052885H2031H204115653761
- Emerging Risk Factors CollaborationDi AngelantonioEKaptogeSAssociation of cardiometabolic multimorbidity with mortalityJAMA20153141526026151266
- Action to Control Cardiovascular Risk in Diabetes Study GroupGersteinHCMillerMEEffects of intensive glucose lowering in type 2 diabetesN Engl J Med2008358242545255918539917
- Advance Collaborative GroupPatelAMacMahonSIntensive blood glucose control and vascular outcomes in patients with type 2 diabetesN Engl J Med2008358242560257218539916
- UK Prospective Diabetes Study (UKPDS) GroupIntensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33)Lancet199835291318378539742976
- DuckworthWAbrairaCMoritzTGlucose control and vascular complications in veterans with type 2 diabetesN Engl J Med2009360212913919092145
- HolmanRRSourijHCaliffRMCardiovascular outcome trials of glucose-lowering drugs or strategies in type 2 diabetesLancet201438399332008201724910232
- SonessonCJohanssonPAJohnssonEGause-NilssonICardiovascular effects of dapagliflozin in patients with type 2 diabetes and different risk categories: a meta-analysisCardiovasc Diabetol20161513726895767
- StamlerJVaccaroONeatonJDWentworthDDiabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention TrialDiabetes Care19931624344448432214
- World Heart Federation [webpage on the Internet]Cardiovascular Disease Risk Factors – DiabetesWorld Heart Federation Available from: http://www.World-Heart-Federation.org/Cardiovascular-Health/Cardiovascular-Disease-Risk-Factors/Diabetes/Accessed October 17, 2016
- JeerakathilTJohnsonJASimpsonSHMajumdarSRShort-term risk for stroke is doubled in persons with newly treated type 2 diabetes compared with persons without diabetesStroke20073861739174317478738
- Stark CasagrandeSFradkinJESaydahSHRustKFCowieCCThe prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988–2010Diabetes Care20133682271227923418368
- American Diabetes AssociationStandards of medical care in diabetes – 2015Diabetes Care201538suppl 1S1S99
- PiepoliMFHoesAWAgewallS2016 European Guidelines on cardiovascular disease prevention in clinical practiceEur Heart J201637292315238127222591
- CollaboratorsCTTCEfficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysisLancet2008371960711712518191683
- Blood Pressure Lowering Treatment Trialists’ CollaborationSundströmJArimaHBlood pressure-lowering treatment based on cardiovascular risk: a meta-analysis of individual patient dataLancet2014384994359159825131978
- EmdinCARahimiKNealBCallenderTPerkovicVPatelABlood pressure lowering in type 2 diabetes: a systematic review and meta-analysisJAMA2015313660361525668264
- Control GroupTurnbullFMAbrairaCIntensive glucose control and macrovascular outcomes in type 2 diabetesDiabetologia200952112288229819655124
- BergenstalRMBaileyCJKendallDMType 2 diabetes: assessing the relative risks and benefits of glucose-lowering medicationsAm J Med20101234374.e9374.e18
- InzucchiSEZinmanBWannerCSGLT-2 inhibitors and cardiovascular risk: proposed pathways and review of ongoing outcome trialsDiab Vasc Dis Res20151229010025589482
- AACEACE [webpage on the Internet]Comprehensive Type 2 Diabetes Management AlgorithmAACEACE Available from: https://www.aace.com/publications/algorithmAccessed October 17, 2016
- Center for Drug Evaluation and Research (CDER)Diabetes Mellitus – Evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes [Guidance for Industry]Silver Spring, MDCenter for Drug Evaluation and Research (CDER)2008 Available from: http://www.Fda.Gov/Downloads/Drugs/Guidancecompli-anceregulatoryinformation/Guidances/Ucm071627.pdfAccessed October 17, 2016
- ZinmanBWannerCLachinJMEmpagliflozin, cardiovascular outcomes, and mortality in type 2 diabetesN Engl J Med2015373222117212826378978
- GaedePLund-AndersenHParvingH-HPedersenOEffect of a multifactorial intervention on mortality in type 2 diabetesN Engl J Med2008358658059118256393
- HirshbergBKatzACardiovascular outcome studies with novel antidiabetes agents: scientific and operational considerationsDiabetes Care201336suppl 2S253S25823882054
- LincoffAMWolskiKNichollsSJNissenSEPioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trialsJAMA2007298101180118817848652
- LokeYKKwokCSSinghSComparative cardiovascular effects of thiazolidinediones: systematic review and meta-analysis of observational studiesBMJ2011342d1309d130921415101
- European Medicines AgencyGuideline on Clinical Investigation of Medicinal Products in the Treatment or Prevention of Diabetes MellitusLondonCommittee for Medicinal Products for Human Use (CHMP)2012 CPMP/EWP/1080/00. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/06/WC500129256.pdfAccessed October 17, 2016
- GeigerMJMehtaCRick TurnerJClinical development approaches and statistical methodologies to prospectively assess the cardiovascular risk of new antidiabetic therapies for type 2 diabetesTher Innov Regul Sci20154915064
- JohansenOEInterpretation of cardiovascular outcome trials in type 2 diabetes needs a multiaxial approachWorld J Diab20156910921096
- SciricaBMBhattDLBraunwaldESaxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitusN Engl J Med2013369141317132623992601
- WhiteWBCannonCPHellerSRAlogliptin after acute coronary syndrome in patients with type 2 diabetesN Engl J Med2013369141327133523992602
- GreenJBBethelMAArmstrongPWEffect of sitagliptin on cardiovascular outcomes in type 2 diabetesN Engl J Med2015373323224226052984
- US Food and Drug AdministrationDiabetes Medications Containing Saxagliptin and Alogliptin: Drug Safety Communication – Risk of Heart FailureSilver Spring, MDSafety Information and Adverse Event Reporting Program2016 Available from: http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm494252.htmAccessed May 5, 2016
- PfefferMAClaggettBDiazRLixisenatide in patients with type 2 diabetes and acute coronary syndromeN Engl J Med2015373232247225726630143
- MarsoSPDanielsGHBrown-FrandsenKLiraglutide and cardiovascular outcomes in type 2 diabetesN Engl J Med2016375431132227295427
- TuckerME webpage on the InternetTop-Line Data Show CV Benefit for Liraglutide in Type 2 DiabetesMedscape2016 Available from: http://www.medscape.com/viewarticle/859905Accessed May 5, 2016
- HachTGerichJSalsaliAEmpagliflozin improves glycemic parameters and cardiovascular risk factors in patients with type 2 diabetes (T2DM): pooled data from four pivotal phase III trialsDiabetol Stoffwechsel20149suppl01
- FitchettDZinmanBWannerCHeart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME® trialEur Heart J201637191526153426819227
- WannerCThe American College of Cardiology Meeting 2016 (ACC.16) Oral PresentationChicago, IL2016
- CherneyDZIPerkinsBASoleymanlouNThe renal hemodynamic effect of SGLT2 inhibition in patients with type 1 diabetesCirculation2014129558759724334175
- Abdul-GhaniMDel PratoSChiltonRDeFronzoRASGLT2 inhibitors and cardiovascular risk: lessons learned from the EMPA-REG OUTCOME StudyDiabetes Care201639571772527208375
- GrossJLde AzevedoMJSilveiroSPCananiLHCaramoriMLZelmanovitzTDiabetic nephropathy: diagnosis, prevention, and treatmentDiabetes Care200528116417615616252
- BrennerBMCooperMEde ZeeuwDEffects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathyN Engl J Med20013451286186911565518
- LewisEJHunsickerLGClarkeWRRenoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetesN Engl J Med20013451285186011565517
- WannerCInzucchiSELachinJMEmpagliflozin and progression of kidney disease in type 2 diabetesN Engl J Med2016375432333427299675
- European Medicines AgencyJardiance Summary of Product CharacteristicsLondonEuropean Medicines Agency2016 EMEA/H/C/002677. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/SGLT2_inhibitors__20/European_Commission_final_decision/WC500206515.pdfAccessed June 14, 2016
- US Food and Drug Administration [webpage on the Internet]Canagliflozin (Invokana, Invokamet) and Dapagliflozin (Farxiga, Xigduo XR): Drug Safety Communication – Strengthened Kidney Warnings [Safety Alert]Silver Spring, MDSafety Information and Adverse Event Reporting Program2016 Available from: http://www.Fda.Gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm506554.htmAccessed June 15, 2016
- WattsNBBilezikianJPUsiskinKCANVAS – CANagliflozin cardiovascular Assessment Study. NCT01032629J Clin Endocrinol Metab2016101115716626580237
- AstraZenecaMulticentre Trial to Evaluate the Effect of Dapagliflozin on the Incidence of Cardiovascular Events (DECLARE-TIMI58) Available from: https://clinicaltrials.gov/ct2/show/NCT01730534?term=NCT01730534&rank=1. NLM identifier: NCT01730534Accessed October 17, 2016
- NauckMAUpdate on developments with SGLT2 inhibitors in the management of type 2 diabetesDrug Des Devel Ther2014813351380
- US Food and Drug AdministrationNDA 204042: Invokana (Canagliflozin) Tablets [Briefing Document]Silver Spring, MDEndocrinologic and Metabolic Drugs Advisory Committee2013 Available from: http://www.Fda.Gov/Downloads/%20AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/%20EndocrinologicandMetabolicDrugsAdvisory-Committee/UCM334550.pdfAccessed May 6, 2016
- DziubaJAlperinPRacketaJModeling effects of SGLT – 2 inhibitor dapagliflozin treatment versus standard diabetes therapy on cardiovascular and microvascular outcomesDiabetes Obes Metab201416762863524443793
- GilbertREKrumHHeart failure in diabetes: effects of antihyperglycaemic drug therapyLancet201538599822107211726009231
- DickerDDPP-4 inhibitorsDiabetes Care201134suppl 2S276S27821525468
- Centers for Disease Control and PreventionNational Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States, 2011 ChapAtlanta, GAU.S. Department of Health and Human Services, Centers for Disease Control and Prevention2011 Available from: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdfAccessed October 17, 2016
- HolmanRRPaulSKBethelMAMatthewsDRNeilHAW10-year follow-up of intensive glucose control in type 2 diabetesN Engl J Med2008359151577158918784090
- ShaSPolidoriDFarrellKPharmacodynamic differences between canagliflozin and dapagliflozin: results of a randomized, double-blind, crossover studyDiabetes Obes Metab201517218819725421015
- MunirKMDavisSNDifferential pharmacology and clinical utility of empagliflozin in type 2 diabetesClin Pharmacol20168193427186083
- US National Institutes of HealthClinical trials Available from. https://clinicaltrials.govAccessed October 17, 2016
