Abstract
Purpose
Evaluation of the efficacy of single-shot, low-dose urokinase administration for the treatment of port catheter-associated fibrin sheaths.
Methods
Forty-six patients were retrospectively evaluated for 54 episodes of port catheter dysfunction. The presence of a fibrin sheath was detected by angiographic contrast examinations. On an outpatient basis, patients subsequently received thrombolysis consisting of a single injection of urokinase (15.000 IU in 1.5 mL normal saline) through the port system. A second attempt was made in cases of treatment failure. Patients were followed up for technical success, complications and long-term outcome.
Results
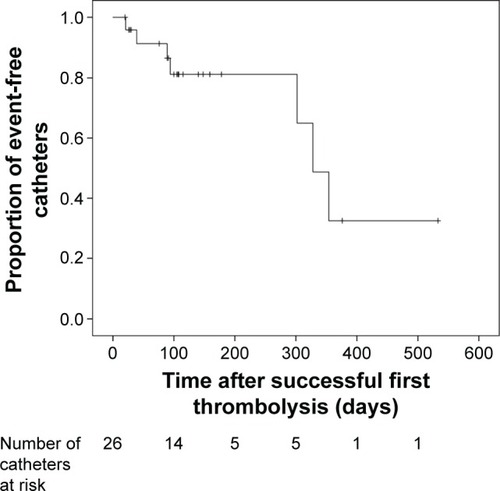
Port dysfunction occurred at a median of 117 days after implantation (range: 7–825 days). The technical success after first port dysfunction by thrombolysis was 87% (40/46); thereof, initial thrombolysis was effective in 78% (36/46). Nine patients (20%) received a second dose of urokinase after previous treatment failure. Follow-up was available for 26 of 40 patients after successful thrombolysis. In 8 of these, rethrombosis occurred after a median of 98 days (range: 21–354 days), whereby rethrombolysis was effective in 5 of 7 (63%) patients. The overall success of all thrombolyses performed was 70% (45/64). No procedure-related technical or clinical complications occurred. After first favorable thrombolysis, a Kaplan–Meier analysis yielded a 30-, 90- and 180-day probability of patency of 96%, 87% and 81%.
Conclusion
Thrombolytic therapy on an outpatient basis appears to be a safe and efficient. Three-month patency rates are comparable to more invasive treatment options, including catheter exchange over a guide wire and percutaneous fibrin sheath stripping.
Introduction
Central venous port catheters are widely used for the intravenous administration of chemotherapy and parenteral nutrition in patients with oncologic diseases. During the course of usage, catheter-related vein thrombosis, infection and fibrin sheath formation are the most frequent complications.Citation1,Citation2 Fibrin sheaths encase the outer wall and the endhole of the catheter, leading to port dysfunction in terms of difficult aspiration and/or high resistance to the injection of fluids. Different approaches to resolve the fibrin sheath have been described, including exchange of the impaired catheter,Citation3 the use of a loop snare from a transfemoral venous approach to pull off the sheath from the catheterCitation4,Citation5 and thrombolysis.Citation6–Citation9 In the latter case, the reported application regimens (single injections vs continuous infusions, local lysis through the catheter vs intravenous systemic application) as well as the thrombolytic agents (streptokinase, urokinase, recombinant tissue-type plasminogen activator [rt-PA]) and the dosages administered vary significantly. Overall, all therapies appear to be comparable regarding primary and secondary technical success and complication rates.Citation10,Citation11 Therefore, the method of choice should aim to be minimally invasive and cost effective. The purpose of the present study was the evaluation of a noninvasive and uncomplex approach for the treatment of port catheter-associated fibrin sheaths.
Methods
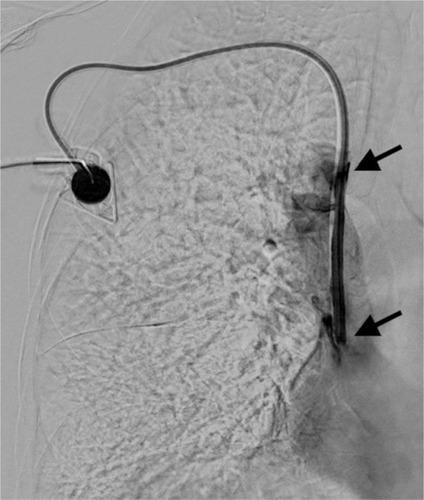
Our study was approved by the local ethics committee of the medical faculty of the University of Cologne (Registration Number 16-212), which waived the requirement for written informed consent because of the retrospective, observational character of the study. Written informed consent was obtained for publication of the accompanying images. From January 2015 to May 2016, 46 patients (31 female, 15 male; age 56.8, mean ±10.5; median 59, range: 24–76 years) with underlying malignant diseases and port catheter dysfunction due to fibrin sheath formation were referred to our radiology department for evaluation and treatment. Dysfunction of the port was defined as difficulty in blood aspiration and/or high resistance to injection of fluids over the course of ambulatory chemotherapy. Fluoroscopy was used in each case to confirm correct positioning of the catheter tip in the vena cava superior. Subsequently, 5 mL normal saline solution was slowly injected through the pectoral port chamber. If the flow was preserved, 5 mL of contrast agent (Accupaque 300 mg/mL; GE Health-care, Fairfield, CT, USA) was injected in the next step and the run off was depicted using digital subtraction angiography at 2 frames/s. A fibrin sheath was confirmed by presentation of retrograde tracking of a contrast agent along the catheter wall. The length of the fibrin sheath was determined by measuring the distance from the tip to the most proximal exit point of the contrast medium (). Patients with associated venous thrombosis, depicted by angiography or ultrasound examination, or a complete intraluminal occluded port system with unfeasible injection were not included in the study. Furthermore, patients with contraindication to thrombolysis, for example, recent gastrointestinal bleeding or stroke, were also excluded from the study.
Figure 1 Digital subtraction angiography of clinically dysfunctional venous port system correctly placed into the cavoatrial junction.
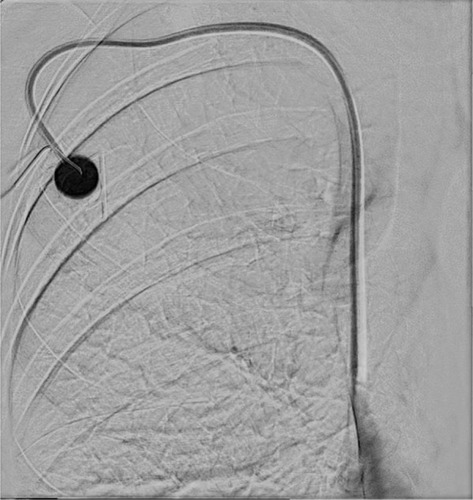
Thrombolysis was performed by administering a single injection of 15.000 IU urokinase (medac, Wedel, Germany) dissolved in 1.5 mL normal saline solution through the port chamber. No other treatment-related medication was given. A control angiography was scheduled either 6 hours later or in the morning hours of the next day if patients were referred in the afternoon. The port was flushed with saline and blocked with heparin (500 IU) if the catheter function was restored effortlessly and the patient was discharged from the hospital (). In cases of persistent port dysfunction, a second thrombolytic injection was given right after the control angiography with 20.000 IU urokinase dissolved in 2 mL normal saline solution. The follow-up of these patients was the same as described for the initial attempt.
Figure 2 Effective local thrombolysis resulted in complete dissolution of the fibrin sheath.
Patients were retrospectively evaluated for primary (success rate after the first attempt), secondary (success rate after the second attempt) and overall (all attempts including rethrombolysis in long-term follow-up) technical success, which was defined as restitution of aspiration and infusion through the port chamber. Procedure-related complications were classified according to the guidelines of the Society of Interventional Radiology.Citation12 Catheter patency after initial thrombolysis was extracted from the patients’ charts using records from computed tomography staging examinations and ambulatory chemotherapy treatment.
Baseline statistics and survival analysis using Kaplan–Meier Curves were calculated using Statistical Package for the Social Sciences software (Version 22; IBM Corporation, Armonk, NY, USA). Statistical differences were calculated using the Wilcoxon test. Statistical significance was set at P<0.05.
Results
In all 46 patients, the port was implanted for intravenous chemotherapy of underlying malignant diseases, which were lung cancer (8/46; 17%), gastrointestinal malignancies (7/46; 15%), breast cancer (6/46; 13%), colorectal cancer (6/46; 13%), lymphoma (6/46; 13%), gynecologic cancer (4/46; 9%), malignant melanoma (4/46; 9%) and other reasons (5/46; 11%). Port catheters were inserted on the left side in 27 cases (59%) and on the other side in 19 cases (41%), each through the subclavian vein.
Patients were referred with either an indication of difficulties in blood aspirating through the port system (22/46 cases [48%]) or a combination of the former plus resistance to injection of fluids (24/46 cases [52%]).
Port dysfunction occurred in a range of 7–825 days after implantation (median 117 days). All 46 patients were initially treated with a single injection of 15.000 IU urokinase. Nine of 46 (20%) patients received a second dose due to initial treatment failure. One patient refused a second urokinase injection and underwent port explantation. Clinical and angiographic control after thrombolysis was performed within 5–140 hours after urokinase therapy (median 24 hours). The primary and secondary technical success rates of thrombolysis were 78% (36/46) and 87% (40/46), respectively. Repeated thrombolysis after a failed attempt restored aspiration and infusion capabilities in 4/9 patients (44%). The length of fibrin sheaths (mean ± standard deviation [SD] 4±2 cm; range: 1−10 cm) was not significantly associated with treatment failure (P=0.31). There were no major or minor complications related to the procedure.
Follow-up after treatment was available for 30/46 patients (65%) for a period of 104 days (median) with the range being 7−533 days. After successful treatment, recurrence of fibrin sheath formation and consecutive port dysfunction occurred in 8/26 patients (31%) after a median of 98 days (range: 21−354 days). Port catheter patency after successful treatment was 96%, 87% and 81% at 30, 90 and 180 days, respectively (). The technical success rate of rethrombolysis was 71% (5/7): 57% (4/7) in the first and 50% (1/2) in the second attempt. The overall success rate of thrombolyses was 70% (45/64).
Figure 3 Kaplan–Meier curve showing the distribution of events over time after previous successful first thrombolysis (n=26 patients); ticks denote censored patients.
Among 8 patients with persistent port dysfunction, 3 patients (37.5%) underwent fibrin sheath stripping and 5 patients decided on explantation of the port system. Fibrin sheath stripping was successful in 1 out of 3 patients.
Discussion
Port catheter dysfunction due to a fibrin sheath formation is a common complication that can occur at any time after implantation. The inability to withdraw blood or to inject fluids is a serious problem that leads to unintended interruption of therapy or ultimately to removal and reinsertion of the catheter if the therapeutic approaches to restore the port function fail. Clinical practice for the treatment of fibrin sheath-associated port dysfunction varies greatly among institutions. These include pulling off the fibrin sheath using a snare, catheter exchange over the wire or thrombolytic therapy with different agents and dosages.
Exchanging the catheter and percutaneous snaring of the fibrin sheath are both invasive procedures with additional costs and potentially severe complications such as arrhythmia and pulmonary embolism.Citation13,Citation14 In comparison, local thrombolysis through direct injection into the port appears to be atraumatic and very safe.Citation6,Citation15–Citation17 There were no minor or major bleeding assigned to local thrombolytic therapy, either in our or in any of the other studies. In a double-blind, placebo-controlled trial, Haire et alCitation18 showed that a low-dose injection of recombinant urokinase (15.000 IU) was more effective than placebo in restoring the patency of occluded central venous access devices and may obviate the need for catheter replacement. Furthermore, long-term follow-up shows no benefit of the invasive procedures compared with thrombolysis, regarding the 3-month patency rates ranging from 37% to 85%.Citation3,Citation6,Citation13,Citation19
Various thrombolytic protocols with different dosages and agents have been implemented for the treatment of fibrin sheath formation. Among these, urokinase and alteplase (tissue plasminogen activator) are most frequently used either as single application into the port catheter or through a peripheral venous access in terms of a prolonged systemic thrombolysis.Citation6 The purpose of the present study was to analyze a simple and cost-effective treatment scheme on an outpatient basis. Urokinase was selected as it is well controllable due to quick elimination (half-life period about 10−20 min); also, adverse events such as allergic reactions are less compared to streptokinase. In addition, the current cost of urokinase (50.000 IU) is approximately 5 times less than that of alteplase (10 mg) in our institution.
Furthermore, we decided on a single injection of urokinase instead of a systemic thrombolysis. With this protocol, patients were able to leave the hospital right after the examination. There was no need for close control in the ward or even at an intermediate care unit. For example, Gray et alCitation11 used a urokinase infusion of 60.000 IU/hour, with a total amount of 250.000 IU for the treatment of malfunctioning dialysis catheters. Despite the low dosage and short time of administration used in our study, a comparably high rate of initial technical success was obtained in relation to the more time-consuming infusion protocols with rates ranging from 79.5% to 97%.Citation9,Citation11,Citation20 There are no definitive guidelines or recommendations of the manufacturer regarding the dosage or volume of urokinase in the treatment of fibrin sheath formation. Deitcher et alCitation21 showed that lower doses of urokinase (5000 IU/mL) is as effective as higher doses (15.000 or 25.000 IU/mL) with less hemorrhagic risks. However, it must be pointed out that, although there was a significant difference among the treatment groups in the rates of patients experiencing any bleeding complication (P=0.024), only 1 bleeding complication (minor bleeding at a vascular access site) was considered by the investigator to be possibly related to urokinase (25,000 IU/mL). The dose of urokinase adopted in this study (10.000 IU/mL) was based on a literature review favoring those at the lower end of the dose range and the empirical experience from our institution. The volume of urokinase used in this study (15.000 IU urokinase in 1.5 mL) was just sufficient to ensure infiltration of the catheter-adherent thrombus (port system volume: port chamber [0.6 mL] + catheter length × 0.02 mL/cm; 8F PowerPort isp MRI; Bard Access Systems Inc., Salt Lake City, UT, USA). A limitation of this study is that the analysis was not randomized and had no reference group. Also, the retrospective study design may have led to a bias in the long-term follow-up evaluation due to incomplete records in the patients’ charts.
Conclusion
In conclusion, low-dose local thrombolysis with urokinase on an outpatient basis proved to be safe and achieved high technical success rates in the treatment of port catheter dysfunction due to fibrin sheath formation. Long-term catheter patency is comparable to other more invasive treatment options, including percutaneous snare maneuver or definitive exchange of the catheter.
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sector. All data will be made available upon request in an anonymized manner.
Disclosure
The authors report no conflicts of interest in this work.
References
- ChangDHBoeckerJHellmichMKrugKBExperiences with ultrasound guided port impantations via the lateral subclavian vein: a retrospective analysis of 1532 patientsRofo2012184872673322618474
- ShettyPCModyMKKastanDJOutcome of 350 implanted chest ports placed by interventional radiologistsJ Vasc Interv Radiol1997869919959399468
- DuszakRJrHaskalZJThomas-HawkinsCReplacement of failing tunneled hemodialysis catheters through pre-existing subcutaneous tunnels: a comparison of catheter function and infection rates for de novo placements and over-the-wire exchangesJ Vasc Interv Radiol1998923213279540917
- JohnstoneRDStewartGAAkohJAFleetMAkyolMMossJGPercutaneous fibrin sleeve stripping of failing haemodialysis cathetersNephrol Dial Transplant199914368869110193820
- MerportMMurphyTPEgglinTKDubelGJFibrin sheath stripping versus catheter exchange for the treatment of failed tunneled hemodialysis catheters: randomized clinical trialJ Vasc Interv Radiol20001191115112011041466
- MassmannAJagodaPKranzhoeferNBueckerALocal low-dose thrombolysis for safe and effective treatment of venous port-catheter thrombosisAnn Surg Oncol20152251593159725287441
- MeersCToffelmireEBUrokinase efficacy in the restoration of hemodialysis catheter functionJ CANNT1998821719
- SavaderSJHaikalLCEhrmanKOPorterDJOtehamACHemodialysis catheter-associated fibrin sheaths: treatment with a low-dose rt-PA infusionJ Vasc Interv Radiol20001191131113611041468
- WhighamCJLindseyJIGoodmanCJFisherRGVenous port salvage utilizing low dose tPACardiovasc Intervent Radiol200225651351612391517
- Janne d’OthéeBThamJCSheimanRGRestoration of patency in failing tunneled hemodialysis catheters: a comparison of catheter exchange, exchange and balloon disruption of the fibrin sheath, and femoral strippingJ Vasc Interv Radiol20061761011101516778235
- GrayRJLevitinABuckDPercutaneous fibrin sheath stripping versus transcatheter urokinase infusion for malfunctioning well-positioned tunneled central venous dialysis catheters: a prospective, randomized trialJ Vasc Interv Radiol20001191121112911041467
- LewisCAAllenTEBurkeDRQuality improvement guidelines for central venous accessJ Vasc Interv Radiol2003149 Pt 2231235
- CrainMRMewissenMWOstrowskiGJPaz-FumagalliRBeresRAWertzRAFibrin sleeve stripping for salvage of failing hemodialysis catheters: technique and initial resultsRadiology1996198141448539402
- WinnMPMcDermottVGSchwabSJConlonPJDialysis catheter ‘fibrin-sheath stripping’: a cautionary tale!Nephrol Dial Transplant1997125104810509175070
- SembaCPDeitcherSRLiXResnanskyLTuTMcCluskeyERCardiovascular thrombolytic to Open Occluded Lines InvestigatorsTreatment of occluded central venous catheters with alteplase: results in 1,064 patientsJ Vasc Interv Radiol200213121199120512471182
- TrerotolaSOJohnsonMSHarrisVJOutcome of tunneled hemodialysis catheters placed via the right internal jugular vein by interventional radiologistsRadiology199720324894959114110
- UldallRBesleyMEThomasASalterSNuezcaLAVasMMaintaining the patency of doublelumen silastic jugular catheters for haemodialysisInt J Artif Organs199316137408458669
- HaireWDDeitcherSRMullaneKMRecombinant urokinase for restoration of patency in occluded central venous access devices. A double-blind, placebo-controlled trialThromb Haemost200492357558215351854
- BradyPSSpenceLDLevitinAMickolichCTDolmatchBLEfficacy of percutaneous fibrin sheath stripping in restoring patency of tunneled hemodialysis cathetersAJR Am J Roentgenol199917341023102710511171
- LundGBTrerotolaSOScheelPFJrOutcome of tunneled hemodialysis catheters placed by radiologistsRadiology199619824674728596851
- DeitcherSRFraschiniGHimmelfarbJDose-ranging trial with a recombinant urokinase (urokinase alfa) for occluded central venous catheters in oncology patientsJ Vasc Interv Radiol20041557558015178717
