Abstract
Background
The aim of this study was to evaluate the clinical and microbiological effects of local application minocycline HCl 2% gel, used as an adjunct to scaling and root planing (SRP) for treatment of chronic periodontitis (CP). CP is an inflammation of periodontal tissue that is caused mainly by bacterial infection, where periodontal destruction such as loss of attachment and bone destruction occurred.
Methods
A total of 81 subjects with moderate to severe periodontitis whose baseline clinical attachment loss (CAL) was ≥4 mm were randomly assigned to receive SRP alone (control group, N=39) or SRP followed by four times of local application of minocycline HCl gel (Periocline) once a week (test group, N=42). Pocket depth, CAL, and papilla bleeding index were examined at baseline, 21 days, 2, 3, and 6 months. Subgingival plaque samples were collected with sterile curettes and were analyzed by real-time polymerase chain reaction for the presence of three periodontal pathogens (Porphyromonas gingivalis [P.g.], Tannerella forsythia [T.f.], and Treponema denticola [T.d.]) at baseline, 2, 3, and 6 months.
Results
The number of bacteria was reduced in both groups at 2 months after baseline (SRP treatment). The changes (2–6 months) in T.d. and T.f. counts in the test group were significantly lower than those in the control group. In the control group, a significant regrowth of P.g., T.f., and T.d. was observed from 2 to 6 months and of P.g. and T.f. from 3 to 6 months. On the other hand, in the test group, the number of the three bacteria did not significantly increase during the 6-month period.
Conclusion
The results showed that local application of minocycline, used as an adjunct to SRP, was effective for suppressing regrowth of periodontal pathogens, suggesting its risk reduction of recurrent periodontal pathogens in CP.
Introduction
Chronic periodontitis (CP), the most common periodontal disease, is an inflammatory disease leading to the destruction of connective tissue and loss of the adjacent supporting bone. The initiation and progression of CP is a consequence of interaction between oral bacteria and the host immune responses. It is well known that the immune response to bacterial products such as lipopolysaccharide, proteolytic enzymes, and subsequent production of proinflammatory cytokines could also evoke an inflammatory reaction in periodontal tissue.
Periodontal treatment involves mechanical cleaning of tooth surfaces to remove calculus and dental biofilm, and strict control of biofilm prevents recolonization of the subgingival area. In some cases, scaling and root planing (SRP) is insufficient to solve periodontal infection. CP is caused by pathogens called the red complex, which includes Porphyromonas gingivalis [P.g.], Tannerella forsythia [T.f.], and Treponema denticola [T.d.]. These pathogens dominate the subgingival layers and are recognized as the most important pathogens in adult periodontal disease, currently thought to be closely associated with CP.Citation1,Citation2
The last 20 years have seen the emergence of a range of adjunctive antimicrobial regimens designed to aid the mechanical methods of dealing with subgingival plaque. Local application of antibiotic directly at the subgingival area (into the periodontal pocket) has become an alternative. SRP combined with local antibiotics has been shown to yield better results than SRP only.Citation3–Citation6 The local application of minocycline in the treatment of periodontal disease has promising results compared with other nonsurgical therapies.Citation7–Citation10 Local administration can avoid many of the side effects associated with systemic antibiotic therapy by limiting the agent to the periodontal pocket, minimizing systemic absorption. A stable, sustained-action formulation of minocycline gel has been developed for subgingival use. This formulation, extensively evaluated in clinical trials conducted in Japan, contains 2% minocycline, a concentration selected on clinical, bacteriological, and gingival crevicular criteria. It also demonstrated that repeated subgingival administration of minocycline ointment in the treatment of CP leads to significant adjunctive improvement after subgingival instrumentation. It possesses widespread bacteriostatic properties and acts slowly by binding to the tooth surface, inhibiting the activity of collagenase at low concentrations and thus preventing periodontal tissue destruction.Citation11,Citation12 Application of minocycline ointment as an adjunct to periodontal flap surgery in generalized CP demonstrated that there was significant reduction in the clinical parameters with improvement in the periodontal status.Citation13 Thus, a direct approach using antibacterial agents by topical administration has become an important part of periodontal disease management.Citation3,Citation14,Citation15 However, it has not been reported that local application of minocycline HCl 2% gel, used as an adjunct to SRP, has effectively suppressed the regrowth of periodontal pathogens.
The objective of the present study was to establish the efficacy of suppressing regrowth of periodontal pathogens and risk of recurrent periodontal pathogens of locally applied minocycline HCl 2% gel when used as an adjunct to SRP without repeated administration for 6 months after initial treatment.
Materials and methods
Type of study
A prospective randomized open blinded study was conducted in a single center (Periodontic Clinic, Faculty of Dentistry, Universitas Indonesia) from November 2013 to November 2014. Ethical approval was given by the Research Ethical Committee, Faculty of Dentistry, Universitas Indonesia (KEPKG 2013). Written informed consent was given and signed by subjects prior to the study. Subjects aged <16 years were not allowed and not included in this study.
Subjects
Patients were aged 30–55 years with localized CP, who had 4–6 mm proximal pocket depth (PD), clinical attachment loss (CAL) equal to or greater than 4 mm, and gingival bleeding on probing (BOP). Patients were included if they did not take any antibiotics in the last 3 months and had no periodontal treatment in the last 6 months. Patients were excluded if they were suffering from a systemic disease, allergic to minocycline hydrochloride, had proximal tooth restorations, or had proximal and cervical caries; pregnant or breast-feeding women and smokers were also excluded, as were also those with poor oral hygiene or malocclusion and patients on continuous medication.
Treatment regimen
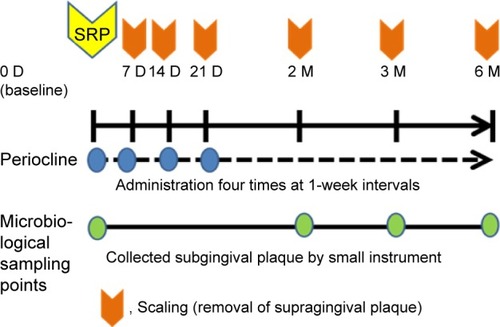
The drugs were applied locally into the gingival pocket by inserting the gel on the periodontal pocket base and then slowly pulling the ends of the syringe while continuing the injection. The microcapsule gel containing 2% minocycline HCl (Periocline, Sunstar, Osaka, Japan) was commercially packed (0.5 g) to achieve a dose of 20 mg of minocycline HCl per subject. Subjects that fulfilled inclusion and exclusion criteria were randomized and examined. Periodontal examinations included oral hygiene, bleeding scores, PD, attachment loss, and doing the SRP at day 0 of this study (baseline). The subjects were evaluated 1 month before receiving treatment; if the PD was still 4 mm, they were recruited into the study. A total of 84 subjects with CP and with baseline CAL above 4 mm were randomly assigned to receive SRP alone (control group) or SRP followed by four times of local application of minocycline HCl 2% gel (Periocline) once a week (test group). Subgingival plaque samples were collected using a sterile curette at days 1 (baseline), 2, 3, and 6 months before SRP (baseline) or scaling, for bacteriological testing. The outline of this study design is described in .
Clinical measurements
In this study, supra gingival SRP were performed in all subjects at baseline or day 1. In the minocycline group (test group), subgingival minocycline HCl gel 2% was added. The same procedures were repeated at days 7, 14, and 21. Oral hygiene instruction was given to the patients after each procedure. At days 14 and 21, the amount of plaque was recorded with Loe and Sillness index.Citation16 Papilla bleeding index (PBI) by Muhlemann and SonCitation17 modification index was scored. PD and CAL were examined using bite registration for probing. At month 2, we measured the bleeding scores, PD, and attachment loss in both groups. The subjects were followed up for 6 months. At months 3 and 6, subgingival plaque samples were taken for microbiologic testing before clinical examinations. The plaque index (PLI), PBI, PD, and loss of attachment were scored during the follow-up.
PLI, using an index of Loe and Silness, was measured on teeth 16, 12, 24, 36, 32, and 44.Citation16 The labial or buccal surface (divided into facial, mesio-facial, and disto-facial) was examined, as was palatal or lingual, considered as single surface. A probe was passed along the cervical portion of the tooth surface. Score 0 was given if no plaque adhered to the tip of the probe. Score 1 was given if a film of plaque adhered to the tip of the explorer. Score 2 was given for thin to moderate accumulation of plaque seen on the tooth surface at the cervical portion of the crown. Score 3 was given when an abundance of plaque was seen on the tooth surface at the cervical portion of the crown. The individual scores of all the teeth were then added and divided by the total number of teeth examined.
PBI by Muhlemann and SonCitation17 modification was measured by a Hu-Fredy periodontal probe. PBI of mesial surface was measured from the labial/buccal site, while PBI of distal surface was measured from the palatal/lingual site by carefully inserting the probe into the margin of the gingival sulcus. The PBI was scored as follows: 0, equals to no bleeding; 1, equals to bleeding in the form of a point; 2, equals to bleeding in the form of a line; 3, equals to bleeding in the form of a triangle; and 4, equals to widespread bleeding.
Periodontal PD was measured from the direction of the labial/buccal and the palatal/lingual. CAL was measured from the boundary between the cementum-enamel junction to the basic pocket. Increased tissue attachment loss is defined as the difference between the distance before and after treatment. We categorized the CAL, based on the cutoff point of 4 mm, as mild attachment loss and moderate to severe attachment loss.
The subjects were followed up for 6 months. At 2, 3, and 6 months, subgingival plaque samples for microbiologic testing were taken before clinical examinations. The PLI, PBI, PD, and loss of attachment were scored during the follow-up. In a prospective clinical study, threshold levels of P.g. and T.d. at a periodontal site as measured by reverse transcription–polymerase chain reaction (PCR) could predict CAL at that site over the following 3 months, suggesting that P.g. and T.d. were important pathogens in the progression of CP.Citation18
Treatments
In this study, supragingival SRP was performed in all subjects at baseline or day 1. In the minocycline group, subgingival minocycline HCl gel 2% was added. The same procedures were repeated at days 7, 14, and 21. Oral hygiene instruction was given to the patients after each procedure. Chemotherapeutic agents is reported to effectively eliminate the bacteria harbored at the bottom of deeper pockets or in dentine tubules that can not be removed with mechanical treatment only.Citation19
Quantification of periodontal pathogens by real-time PCR
Bacterial quantification was carried out using real-time PCR (7500 Fast RT-PCR System, Applied Biosystems, Tokyo, Japan). The bacterial genomic DNA was extracted separately from each sample using the QIAamp DNA Mini Kit (QIAGEN). The extracted DNA was diluted with distilled water to a concentration of 100 ng/μL. Quantification of target species from unknown plaques was achieved by projecting them to standard curves of targeted bacteria based on counts of pure bacterial cultures with serial tenfold dilution from 102 to 108 cell copies.
Bacteria-specific primer pairs (), according to the literature,Citation20–Citation22 were used to qualify each target bacterium. All samples were run in duplicate in 96-well plates in 7500 Fast RT-PCR System (Applied Biosystems). RT-PCR amplification was performed in a 20 μL reaction mixture containing 2 μL template DNA, 10 μL ds DNA-binding dye SYBR® Green MIX (Invitrogen, Tokyo, Japan), 2 μL, 2.5 μM bacterium-specific primer pair, and 4 μL distilled water. The amplification cycling conditions were 95°C for 10 minutes and then 40 cycles of 15 seconds at 95°C and 60 seconds at 60°C. Melting curve analysis was performed for each run to evaluate the specificity of the PCR products. The detection limits of all three bacteria were less than log 102.
Table 1 PCR primer sequences for red complex periodontal pathogens
Real-time PCR was performed with the ds DNA-binding dye SYBR® Green MIX (Invitrogen) using species-specific primers ().
Statistical procedure
In this study, the sample size was calculated based on power of 80% and alpha level of 5% using standard deviation of 0.8 mm and precision of 0.3736.Citation23 We got the minimal sample size of 36 subjects for each group.
For the clinical analysis, statisticians were blinded on the treatment arms. Differences between means of both groups at each time point were tested using Student’s t-test for numerical data (PD, CAL) and Wilcoxon rank-sum test for nonparametric, categorical data (PLI, bleeding index, calculus index). Changes between different time frames (baseline vs month 3 vs month 6) were tested using Wilcoxon signed-rank test. Differences between means of both groups were tested using analysis of variance (ANOVA) and Friedmann’s two-way ANOVA. Data were analyzed using Statistical Package for the Social Sciences (SPSS) 20 (StataCorp LP, College Station, TX, USA).
For the microbiological analysis, the results were analyzed using IBM SPSS software.23 (StataCorp LP); nonparametric tests were selected. In order to analyze the microbiological counts within the group from baseline to 6 months after treatment, a Friedman test followed by a post hoc test (signed test with Bonferroni correction) was adopted. The changes in bacterial counts were compared between the two groups, on a patient basis, using Mann–Whitney U-test. The results are presented as the mean difference with a 95% confidence interval and are regarded as statistically significant when P<0.05, unless otherwise stated.
Results
We recruited 84 patients into the clinical study. We analyzed 42 patients for the test group (age =43.67±6.88, men/women =9/33, PLI score =1.04±0.54, calculus index score =1.26±0.64, oral hygiene index score =2.31±1.10) and 39 patients for the control group (age =44.00±6.93, men/women =9/30, PLI score =1.08±0.49, calculus index score =1.44±0.84, oral hygiene index score =2.53±1.13), who were eligible for microbiological assay.
The P.g. bacterial counts were significantly reduced in both groups at 2 months when compared with the baseline (). In the control group, a significant regrowth of P.g., T.d., and T.f. was observed from 2 to 6 months and of P.g. and T.f. from 3 to 6 months (). P.g. in the control group had reduced significantly (P<0.05) after therapy observed in 2 months (0.00±0.17 log10 copies/mL); later on, increased slightly in 3 months (0.17±0.72 log10 copies/mL); in 6 months the P.g. is back (1.11±1.81 log10 copies/mL) similar to baseline. P.g. in test group (minocycline applied) had reduced significantly (P<0.05) after therapy observed in 2 months (0.40±1.28 log10 copies/mL); later on increased slightly in 3 months (0.51±1.47 log10 copies/mL); in 6 months the P.g. returned (0.83±1.68 log10 copies/mL) similar to baseline. T.f. in the control group tended to be reduced after therapy observed in 2 months (0.09±0.55 log10 copies/mL); it increased significantly later on (P<0.05) in 3 months (0.55±1.13 log10 copies/mL); in 6 months, the T.f. returned higher than baseline (1.41±1.81 log10 copies/mL). T.f. in the test group (minocycline applied) tended to be reduced after therapy observed in 2 months (0.88±1.71 log10 copies/mL); later on stable in 3 months (0.81±1.75 log10 copies/mL); in 6 months, even though the T.f. is increased (1.26±1.94 log10 copies/mL), it is still lower than baseline. T.d. in the control group had reduced significantly (P<0.05) after therapy observed in 2 months (0.63±1.40 log10 copies/mL); later it increased slightly in 3 months (1.16±1.72 log10 copies/mL); in 6 months the T.d. returned (2.08±2.02 log10 copies/mL) twice significantly higher than baseline. T.d. in the test group (minocycline applied) had tended to be reduced after therapy observed in 2 months (1.30±1.93 log10 copies/mL); later it reduced slightly in 3 months (1.10±1.97 log10 copies/mL); in 6 months the T.d. is back (1.70±2.13 log10 copies/mL) similar to baseline. On the other hand, in the test group, regrowth of P.g., T.d., and T.f. was not significantly observed from 2 to 6 months ().
Table 2 Mean bacterial counts (log10, copies/mL)
The changes in T.d. and T.f. counts from 2 to 6 months were found to be significantly higher in the control group than in the test group (). The comparison of P.g. in the control group and in the test group in a time-dependent manner had no significant differences (P<0.05) after therapy observed in 2, 3, and 6 months. A comparison of T.f. in the control group and in the test group in a time-dependent manner showed a significant difference (P<0.05) after therapy observed in 2 and 6 months, 1.32±1.71 log10 copies/mL and 0.37±2.16 log10 copies/mL, respectively (P=0.033). Also, a comparison of T.d. in the control group and in the test group in a time-dependent manner showed a significant difference (P<0.05) after therapy observed in 2 and 6 months, 1.45±1.99 log10 copies/mL and 0.39±2.15 log10 copies/mL, respectively (P=0.034).
Table 3 Mean changes in bacterial counts (log10, copies/mL)
In clinical parameters, the change in mean CAL at 2 months from baseline was significantly (P<0.001) reduced in the test group (−1.58±0.57 mm) when compared with the control group (−0.76±0.99 mm), although the changes in PD and PBI were not significantly different between the two groups. The changes in PD from 2 to 3 months were significantly improved (P<0.05) in the control group (−0.33±0.76 mm) when compared with the test group (−0.01±0.58 mm), (P=0.036). Moreover, the PBI was also observed to be significantly reduced (P<0.05) in the control group (−0.06±0.14 mm) compared with the test group (−0.01±0.06 mm), (P=0.03) ().
Table 4 Mean changes in clinical parameters
Discussion
SRP is recognized as the gold standard of periodontal therapy. Conventional mechanical debridement sometimes cannot reach the site with the deep periodontal pocket or difficult positions of the tooth, resulting in the recurrence of periodontal pathogen. The local application of minocycline in the treatment of periodontal disease as an adjunct to SRP has promising results compared with other nonsurgical therapies.Citation7–Citation10 Local administration of antibiotic can limit the agent to the periodontal pocket, minimizing systemic absorption and avoiding the side effects associated with systemic antibiotic therapy. The choice of antibacterial agents is based on the bacterial etiology of the infection. As we know, the subgingival pocket contains many pathogens rather than a single pathogenic species, and it is resistant to many antibacterial therapies.Citation24
Minocycline gel, a semisynthetic derivative of tetracycline, has a wide spectrum of action against anaerobic as well as aerobic bacteria. Local delivery of antimicrobial agents can be used systemically or topically as an adjunct to SRP in an attempt to further reduce the number of periodontal pathogens, thereby improving the periodontal condition. In past years, these agents have been extensively researched.
As shows, the clinical parameters analyzed in this study show that clinical attachment levels increased rapidly in the minocycline group and are significantly different compared with those in the control group. The improvement in the clinical attachment levels in the minocycline group was consistent with the decrease in inflammation in the deep pocket. This condition showed that the differences were related to local administration of antibiotic gel minocycline rather than to clinical procedures or oral hygiene. The PBI in the minocycline group was decreased and significantly different compared with that in the control group and remained stable until 6 months of follow-up. These results are similar to two short-term, double-blind, parallel studies by Nakagawa et alCitation6 and Steenberghe et alCitation5 that evaluated the effect of subgingivally administered 2% minocycline in addition to mechanical debridement.Citation5,Citation6 Their studies showed that the treatment group had a better response than patients in the placebo group.
In this study, the pocket reduction in both groups was not significant; however, the mean reduction in probing depth from the 21st day to the 6th month was more in the test group compared with that in the control group. This is in accordance with the studies done by Timmerman et alCitation4 and Cortelli et alCitation25 and could be due to supragingival SRP on both groups every 1 month following mechanical debridement, and causing the formation of the fully epithelialized gingival crevice in both groups. The result of this study are similar to those of Timmerman et al,Citation4 showing no statistically significant differences between the test and the control groups in probing depth and attachment level.
In the control group, the number of three bacteria was significantly increased at 6 months. Although SRP was effective in bringing about a temporary reduction of bacteria, it alone may not suppress the regrowth of three periodontopathic bacteria without additional periodontal treatment for 6 months. This study result is consistent with that pertaining to other groups in that the bacterial counts including P.g. were higher than 6 months after SRP.Citation26,Citation27 The efficacy of SRP can be limited in cases with less access to deep pockets and furcations. In addition, there are well-documented secondary effects, such as gingival recession, loss of tooth substance, and dentin hypersensitivity. SRP alone suggested that the risk of recurrent CP was higher than in the case where local application of minocycline HCl 2% gel was used as an adjunct to SRP. On the other hand, in the test group, the number of three bacteria was not significantly higher during the 6-month period. SRP adjunctive local minocycline ointment may suppress the regrowth of three periodontopathic bacteria for 6 months without retreatment.
Sugi et al reported that in recurrent periodontitis patients, IgG titer to Gram-negative obligative anaerobe (Prevotella intermedia, T.d., and C. rectus) was significantly higher than in the control group.Citation28 The study assumed that these bacteria were one of the causes of recurrence in periodontitis. Matesanz-Pérez et alCitation29 in his meta-analysis concluded that the effect of the subgingival application of antimicrobials was statistically significant (P=0.000) for changes in both periodontal pocket depth (0.407 mm) and CAL (0.310 mm).Citation29 The goal to reduce the incidence of recurrence of periodontitis caused by the return of bacteria was achieved. Previous studies have demonstrated that repeated subgingival administration of minocycline ointment in the treatment of CP leads to a significant adjunctive effect for suppression of regrowth of red complex in combination with SRP. The changes (2–6 months) in T.d. and T.f. counts in the test group were significantly lower than those in the control group. It is suggested that local application of minocycline changes the composition of subgingival microbiota and controls regrowth on the subgingival plaque. An adjunctive therapy of local minocycline ointment may reduce the risk of recurrent periodontal pathogens compared with SRP only.
Conclusion
Red complex periodontal bacteria are one of the important etiologies contributing to the activity of periodontitis. Although SRP is recognized as the gold standard of periodontal therapy, occasionally mechanical debridement cannot effectively reach the site with deep periodontal pocket or difficult position of the tooth and resulting residual periodontal pathogen. The results showed that local application of minocycline HCl 2% gel as an adjunct to SRP was effective for suppressing regrowth of periodontal pathogens, suggesting risk reduction of recurrent periodontal pathogens in CP compared with SRP only. With a view to obtaining clearer insights, the author is planning, in future studies, to expand this experiment by investigating human cytokines responses, microbial alteration based on clinical and radiographic findings.
Author contributions
Y Soeroso, H Sunarto, Y Kemal, R Salim, M Octavia, A Viandita, J Setiawan, and BM Bachtiar contributed as researchers in this study. The coauthor T Akase (Sunstar Company) reviewed the paper. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
A financial award from Sunstar and a grant from The International Collaboration of Research by Directorate of Research and Community Services, Universitas Indonesia (DRPM-UI) 2015, were used to conduct this study.
Disclosure
The authors report no conflicts of interest in this work.
References
- SocranskySHaffajeeADCuginiMASmithCKentRLJrMicrobial complexes in subgingival plaqueJ Clin Periodontol199825Suppl 21341449495612
- SlotsJSubgingival microflora and periodontal diseaseJ Clin Periodontol19796351356393729
- RadvarMPourtaghiNKinaneDFComparison of three periodontal local antibiotic therapies in resistant periodontal pocketsJ Periodontol1996678608658884642
- TimmermanMFWeijdenGASteenbergenTJMantelMSde GraaffJVeldenUEvaluation of the long-term efficacy and safety of locally-applied minocycline in adult periodontitis patientsJ Clin Periodontol1996237077168877655
- SteenbergheDVBercyPKohlJSubgingival minocycline hydrochloride ointment in moderate to severe chronic adult periodontitis: a randomized, double-blind, vehicle-controlled, multicenter studyJ Periodontol19936476376448396177
- NakagawaTYamadaSOosukaYClinical and microbiological study of local minocycline delivery (Periocline) following scaling and root planing in recurrent periodontal pocketsBull Tokyo Dent Coll199132263701819445
- JonesAAKornmanKSNewboldDAManwellMAClinical and microbiological effects of controlled-release locally delivered minocycline in periodontitisJ Periodontol199465105810667853130
- GracaMAWattsTLWilsonRFPalmerRMA randomized controlled trial of a 2% minocycline gel as an adjunct to non-surgical periodontal treatment, using a design with multiple matching criteriaJ Clin Periodontol1997242492539144047
- WilliamsRCPaquetteDWOffenbacherSTreatment of periodontitis by local administration of minocycline microspheres: a controlled trialJ Periodontol2001721535154411759865
- PaquetteDOringerRLessemJLocally delivered minocycline microspheres for the treatment of periodontitis in smokersJ Clin Periodontol20033078779412956654
- StabholzAKetteringJAprecioRZimmermanGBakerPJWikesjoUMAntimicrobial properties of human dentin impregnated with tetracycline HCl or chlorhexidine. An in vitro studyJ Clin Periodontol1993205575628408717
- ChoiDHMoonISChoiBKEffects of sub-antimicrobial dose doxycycline therapy on crevicular fluid MMP-8, and gingival tissue MMP-9, TIMP-1 and IL-6 levels in chronic periodontitisJ Periodontol Res2004392026
- AbbasSMahendraJAriGMinocycline ointment as a local drug delivery in the treatment of generalized chronic periodontitis – a clinical studyJ Clin Diagn Res2016106ZC15ZC19
- PetersilkaGJEhmkeBFlemmigTFAntimicrobial effects of mechanical debridementPeriodontol. 2000200228567112013348
- PeterEBettinaDTi-SunKAntibiotics in periodontal therapyPERIO200524235251
- LöeHThe gingival index, the plaque index and the retention index systemsJ Periodontol1967386 Suppl610616
- MuhlemannHRSonSGingival sulcus bleeding – a leading symptom in initial gingivitisHelv Odontol Acta19711521071135315729
- ByrneSJDashperSGDarbyIBAdamsGGHoffmannBReynoldsECProgression of chronic periodontitis can be predicted by the levels of Porphyromonas gingivalis and Treponema denticola in subgingival plaqueOral Microbiol Immunol20092446947719832799
- AdriaensPADe BoeverJALoescheWJBacterial invasion in cementum and radicular dentin of periodontally diseased teeth in humansJ Periodontol1988592222303164373
- MorilloJMLauLSanzMHerreraDSilvaAQuantitative real-time PCR based on single copy gene sequence for detection of Actinobacillus actinomycetemcomitans and Porphyromonas gingivalisJ Periodontal Res20033851852412941077
- ShelburneCEPrabhuAGleasonRMMullallyBHCoulterWAQuantitation of Bacteroides forsythus in subgingival plaque comparison of immunoassay and quantitative polymerase chain reactionJ Microbiol Methods2000399710710576699
- KurataHAwanoSYoshidaAAnsaiTTakeharaTThe prevalence of periodontopathogenic bacteria in saliva is linked to periodontal health status and oral malodourJ Med Microbiol20085763664218436598
- PiantadosiSSample size and powerPiantadosi Steven Clinical Trials: A Methodologic Perspective2nd edHoboken, NJJohn Wiley and Sons, Inc2005
- NakaoRTakigawaSSuganoNImpact of minocycline ointment for periodontal treatment of oral bacteriaJpn J Infect Dis201164215616021519133
- CortelliJRQueridoSMAquinoDRRicardoLHPallosDLongitudinal clinical evaluation of adjunct minocycline in the treatment of chronic periodontitisJ Periodontol20067716116616460239
- EickSRenatusAHeinickeMPfisterWStratulSIJentschHHyaluronic acid as an adjunct after scaling and root planing: a prospective randomized clinical trialJ Periodontol201384794194923088524
- SteenbergheDVRoslingBSoderPOA 15-month evaluation of the effects of repeated subgingival minocycline in chronic adult periodontitisJ Periodontol19997065766710397521
- SugiNNaruishiKKudoCPrognosis of periodontitis recurrence after intensive periodontal treatment using examination of serum IgG antibody titer against periodontal bacteriaJ Clin Lab Anal201125253221254239
- Matesanz-PérezPGarcía-GargalloMFigueroEBascones-MartínezASanzMHerreraDA systematic review on the effects of local anti-microbials as adjuncts to subgingival debridement, compared with subgingival debridement alone, in the treatment of chronic periodontitisJ Clin Periodontol20134022724123320860