Abstract
Objectives
The aim of this study was to perform an updated systematic review and meta-analysis to assess the clinical efficacy, safety, and cost-effectiveness of negative-pressure wound therapy (NPWT) in the treatment of diabetic foot ulcers (DFUs).
Methods
We searched the Cochrane Library, MEDLINE, EMBASE, Ovid, and Chinese Biological Medicine databases up to June 30, 2016. We also manually searched the articles from reference lists of the retrieved articles, which used the NPWT system in studies of vacuum-assisted closure therapy. Studies were identified and selected, and two independent reviewers extracted data from the studies.
Results
A total of eleven randomized controlled trials, which included a total of 1,044 patients, were selected from 691 identified studies. Compared with standard dressing changes, NPWT had a higher rate of complete healing of ulcers (relative risk, 1.48; 95% confidence interval [CI]: 1.24–1.76; P<0.001), shorter healing time (mean difference, −8.07; 95% CI: −13.70– −2.45; P=0.005), greater reduction in ulcer area (mean difference, 12.18; 95% CI: 8.50–15.86; P<0.00001), greater reduction in ulcer depth (mean difference, 40.82; 95% CI: 35.97–45.67; P<0.00001), fewer amputations (relative risk, 0.31; 95% CI: 0.15–0.62; P=0.001), and no effect on the incidence of treatment-related adverse effects (relative risk, 1.12; 95% CI: 0.66–1.89; P=0.68). Meanwhile, many analyses showed that the NPWT was more cost-effective than standard dressing changes.
Conclusion
These results indicate that NPWT is efficacious, safe, and cost-effective in treating DFUs.
Introduction
Diabetes mellitus (DM) is a syndrome characterized by hyperglycemia that results from absolute or relative impairment in insulin secretion and/or insulin action.Citation1 With the development of people’s living standards and lifestyle changes, the incidence of diabetes has been rising. An estimated 382 million people had DM in 2013; this number will increase to 592 million by 2035.Citation2 Hazards of DM usually present as complications; diabetic foot ulcers (DFUs) are considered one of the most common and devastating chronic complications of diabetes because they contribute to high morbidity, high hospitalization rates, and high mortality, all of which seriously threaten the quality of life of DM patients. The expected lifetime risk of a DM patient developing a foot ulcer is 12%–25%,Citation3 with a 50%–70% recurrence rate over the ensuing 5 years. As a consequence of DFUs, a lower limb is lost every 30 seconds somewhere in the world, and the probability of losing the other leg is 50% after 3 years. DFUs contribute to 85% of non-traumatic, lower-extremity amputations and lead to a 13%–17% mortality rate in patients with DM.Citation4,Citation5 In comparison to non-DFU patients, DFU patients have more days of hospitalization and more days requiring home health care, emergency department visits, and outpatient/physician office visits.Citation6 Meanwhile, the cost of treating DFUs for complete healing and transtibial amputation ranges from US$3,959 to US$188,645 in the US.Citation7 These numbers indicate that DFUs also impose a substantial burden on public and private payers.
The standard of care for DFUs involves debridement, local wound care, infection control, and off-loading of pressure. Various treatments advocated in recent years include advanced wound dressings, growth factors, hyperbaric oxygen therapy, cultured skin substitutes, and other wound therapies. Negative-pressure wound therapy (NPWT) is a newer, noninvasive adjunctive therapy system. A vacuum-assisted closure (VAC) device to control sub-atmospheric pressure helps promote wound healing by removing fluid from open wounds, preparing the wound bed for closure, reducing edema, and promoting formation and perfusion of granulation tissue.Citation8 Some clinical evidence has suggested that NPWT is an effective and safe method for promoting diabetic foot wounds’ healing,Citation9,Citation10 but some serious complications related to NPWT have been reported in recent years.Citation11 It is also worth noting that NPWT appears to be more expensive than conventional methods in the treatment of DFUs. Some of the previous literature focused on one or a few of the several factors of NWPT for DFUs such as evaluating efficacy, safety, and cost-effectiveness, but almost never evaluating all of them at the same time.
The aim of this study was to perform an updated systematic review and meta-analysis to assess the clinical efficacy, safety, and cost-effectiveness of NPWT in the treatment of DFUs, and to strengthen the evidence to support recommendations regarding the use of NPWT in DFU patients.
Methods
We conducted a systematic review, using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist.
Search strategy
We searched the Cochrane Library, MEDLINE, EMBASE, Ovid, and Chinese Biological Medicine databases (up to June 30, 2016) to identify relevant reports of randomized controlled trials (RCTs) and manually searched articles from reference lists of retrieved articles to assemble a comprehensive collection of RCTs about NPWT in the treatment of DFUs. The search terms used were “diabetic foot”, “diabetic feet”, “foot ulcer, diabetic”, “foot, diabetic”, “feet, diabetic”, “negative pressure wound therapy”, “negative-pressure wound therapies”, “vacuum assisted closure”, “vacuum-assisted closure”, “topical negative pressure therapy”, “negative pressure dressings”, “VAC”, and “NPWT” (Supplementary material).
Selection criteria
Inclusion criteria were as follows: 1) RCTs comparing NPWT (VAC) with standard dressing changes in diabetic patients; 2) diabetic patients with chronic foot ulcers and surgical foot wounds; 3) English and Chinese publication languages only; 4) diabetic patients with chronic foot ulcers and surgical foot wounds regardless of pathogenesis; 5) NPWT, whether modified or commercial negative pressure devices, compared with standard dressing changes such as various advanced wound dressings and conventional moist gauze; 6) final indicators, in which the primary outcome is the rate of complete ulcer healing and complete wound closure defined as 100% re-epithelialization without drainage or dressing requirements, and the secondary outcomes included ulcer healing time, change in ulcer size, granulation tissue formation, quality of life, patient satisfaction, resource use, amputation rate, and treatment-related adverse effects (edema, infection, pain, bleeding). Exclusion criteria were as follows: 1) no RCT was performed; 2) NPWT (VAC) was not compared with standard dressing changes; 3) the study did not show corresponding outcomes.
Quality assessment and data collection
Two reviewers (Si Liu, Chao-zhu He) independently assessed the quality of each included study and extracted relevant data; differing opinions were resolved through discussion or a third reviewer’s judgment. The reviewers extracted the following information from every included RCT: first author; publication year; study design and size; demographic characteristics of participants; ulcer size, location, and severity; specific implementation of intervention measures (intervention settings, intervention time, the feature of VAC, and details of treatment received by each group); and final indicator measures. We assessed the quality of each included study using the Cochrane Collaboration tool for assessing risk of bias.Citation12 This tool addressed six domains including selection bias, performance bias, detection bias, attrition bias, reporting bias, and other bias.
Statistical analysis
We assessed all data using Revman 5.3 software. First, we conducted the chi-square test to determine whether there was heterogeneity among the studies. A result of P>0.1, I2,50% indicated no significant heterogeneity between studies; in this case, we used the fixed-effects model for analysis. However, if P<0.1, I2≥50% and in the absence of clinical heterogeneity, we chose the random-effects model. If P<0.1 and we were unable to judge the source of heterogeneity, we used descriptive analysis. We calculated a weighted mean difference (WMD) and 95% confidence intervals (CIs) for continuous variables and calculated the relative risk (RR) and 95% CI for dichotomous variables. We considered a two-sided P whose value is less than 0.05 to indicate statistical significance. Sensitivity analysis was performed for reduction of DFU area based on the leave-one-out approach.
Results
Characteristics of studies and assessment
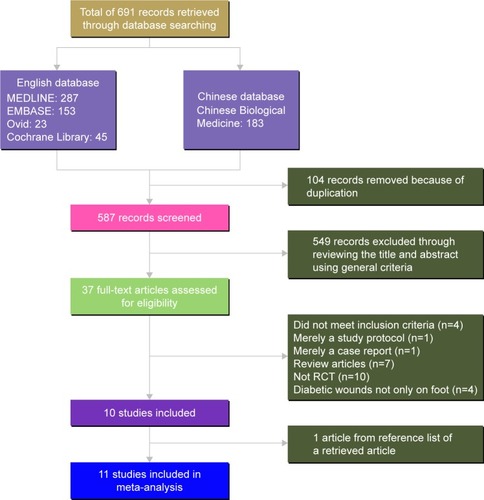
We retrieved 691 records through database searches. After removing duplicates, we found 587 articles, 549 of which we excluded by reviewing the title and abstract using general criteria, and assessed 37 full-text articles for eligibility. We then excluded 27 studies for the following reasons: did not meet inclusion criteria (n=4); merely a study protocol (n=1); merely a case report (n=1); they were review articles (n=7); they were not an RCT (n=10); they did not describe diabetic wounds on the foot only (n=4). One article was obtained from a reference list of a retrieved record. We subjected the resulting eleven articles to meta-analysis.Citation13–Citation23 shows the specific flow chart. For reasons for final exclusion of 27 studies, see Supplementary material.
Figure 1 Flow diagram for identification of studies for inclusion in meta-analysis.
and summarize the details of the eleven studies. The eleven RCTs included 1,044 patients. The number of patients in each included article ranged from ten to 342, the mean ages ranged from 50.2 to 66.5, and the intervention time ranged from 14 to 112 days. We evaluated the quality of the included RCTs according to the Cochrane reviewers’ handbook.Citation12 For the included studies, seven of the eleven published articlesCitation13–Citation15,Citation17,Citation18,Citation22,Citation23 (63.6%) described specific randomized methods and processes; we judged one reportCitation21 to be at high risk of bias for this domain because of randomization based on the date of admission. Three articlesCitation13,Citation14,Citation18 (27%) reported allocation concealment methods. Two articlesCitation17,Citation21 employed different treatments according to the odevity of case number and date of admission, so we judged them as being at high risk of bias for the allocation concealment domain. It was difficult to achieve a blinded study of participants and personnel in NPWT, but un-blinded health professionals were able to make decisions about closure surgery that could then have resulted in more wound closure or amputation in one group than in the other,Citation24 so we classified the risk of bias in this part as unclear. Six articlesCitation13,Citation14,Citation16,Citation17,Citation21,Citation22 explained the specific tools used for image processing and analysis and had the corresponding data; thus, we may conclude that the outcome assessment was based on the blinded method. Other studies did not contain enough details for us to make a judgment for this domain, so we also judged their risk as unclear. We classified only one studyCitation18 as having a low risk of bias, because a group independent from the research team, masked the assigned treatment and evaluated the percentage of granulation tissue formation. Five articlesCitation13,Citation17–Citation19,Citation23 provided information on the loss of cases and the reasons why participants withdrew; another article also provided that information, but the number of cases lost from the experimental and control groups was not clear. Two articlesCitation19,Citation20 showed some results that had not previously been mentioned, so it was thought to have a risk of publication bias. All studies showed that the baseline data for the experimental group and the control group were comparable. and show the risk of bias in the included studies (details in Supplementary material).
Figure 2 Risk of bias graph.
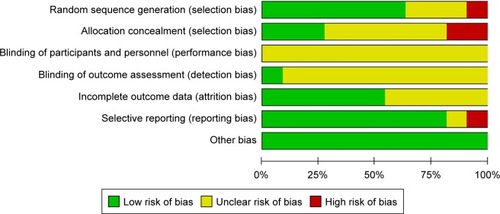
Figure 3 Risk of bias summary.
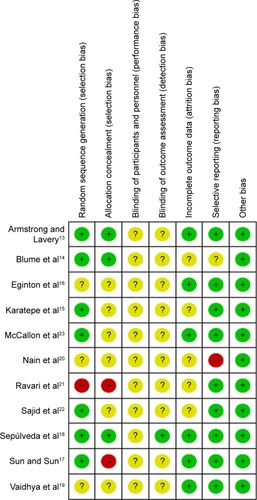
Table 1 Characteristics of participants in included studies
Table 2 Specific implementation of intervention measures
The DFUs’ complete healing rate
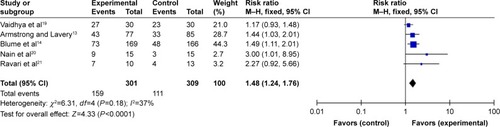
Five articlesCitation13,Citation14,Citation19–Citation21 reported the complete ulcer healing rate. In pooling the data, we found no significant heterogeneity among the five studies (Q=6.31, degrees of freedom [df] =4, P=0.18; I2=37%) (); therefore, we used a fixed-effects model for the analysis. All reports showed the same results, and the combined RR of 1.48 indicated that the complete ulcer healing rate in the NPWT group was significantly higher than that of the control group (95% CI: 1.24–1.76, P<0.0001).
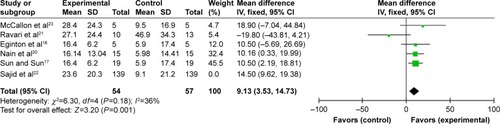
Time to complete DFU healing
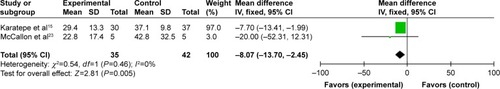
Four reportsCitation13–Citation15,Citation23 provided the time to complete DFU healing, but Armstrong et alCitation13 and Blume et alCitation14 offered the estimated time to complete ulcer healing, so we took the other two results into meta-analysis. The two studies showed some homogeneity after we pooled the data (P=0.46; I2=0%) (). Our meta-analysis result showed that the NPWT group had a shorter time to complete healing of DFUs (mean difference: −8.07, 95% CI: −13.70– −2.45, P=0.005) compared with that of the standard dressing changes group.
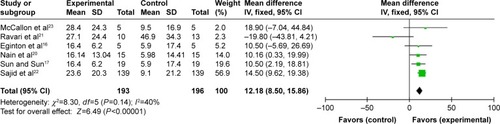
Change in DFUs’ size
Six articlesCitation16,Citation17,Citation20–Citation23 described a reduction of the DFU area. We found no significant heterogeneity among the six reports after pooling the data (Q=8.30, df=5, P=0.14; I2=40%) () and therefore used a fixed-effects model for the analysis. The combined WMD of 12.18 indicated that NPWT more effectively reduced DFUs’ area than standard dressing changes (95% CI: 8.50–15.86, P<0.00001).
Figure 6 NPWT compared with standard dressing changes, outcome 3: reduction of DFU area.
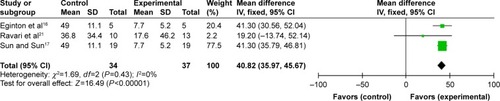
Three articlesCitation16,Citation17,Citation21 described reduction of DFUs’ depth. The three studies showed some homogeneity after we pooled the data (P=0.43; I2=0%) (). The combined WMD of 40.82 indicated that NPWT significantly reduced DFUs’ depth in comparison to standard dressing changes (95% CI: 35.97–45.67, P<0.00001).
Granulation tissue formation
Four articlesCitation13,Citation14,Citation18,Citation19 assessed the granulation tissue formation, but the evaluation results were not unified; therefore, we used descriptive analysis. Armstrong et alCitation13 showed that the time during which 76%–100% of granulation tissue formed in the NPWT group, was shorter than that in the moist dressings change group. Sepúlveda et alCitation18 and Vaidhya et alCitation19 provided the average time to reach 90% or over 90% of wound granulation tissue formation (18.8±6 days and 17.2±3.55 days, respectively) in the NPWT group; both time periods were shorter than corresponding times in the control group.
Quality of life
Karatepe et alCitation15 had patients fill out the 36-item short form health survey (SF-36) questionnaire at the beginning of treatment and in the follow-up month, to ascertain whether the patients’ quality of life improved after treatment. The SF-36 questionnaire included two sections regarding the patient’s physical and mental state. The results showed that the effect of the NPWT treatment was significantly positive for both mental (P=0.0287) and physical (P=0.004) health in comparison to treatment using conventional wound dressing.
Resource use
Armstrong et alCitation13 reported an average total cost per participant of US$26,972 in the NPWT group, compared to US$36,887 in the moist dressing group, with no other information provided. Vaidhya et alCitation19 reported that the mean number of dressings needed to achieve satisfactory healing in the NPWT group was 7.46±2.25, compared to 69.8±11.93 (P<0.001) for the conventional treatment group. Irrespective of the cost of daily treatment or hospital stay, the average cost of NPWT and of conventional dressing was US$55 and US$103 respectively.
Amputation
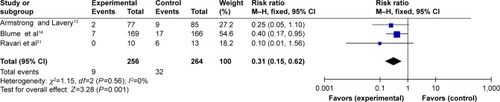
Three reportsCitation13,Citation14,Citation21 provided amputation information. Armstrong et alCitation13 and Blume et alCitation14 analyzed the incidence of re-amputation, Ravari et alCitation21 analyzed the number of patients requiring major and minor amputations. We found no heterogeneity among the three studies after pooling the data (Q=1.15, df=2, P=0.56; I2=0%) (). The combined RR of 0.31 indicated that the incidence of amputation in the NPWT group was lower than in the standard dressing changes group (95% CI: 0.15–0.62, P=0.001).
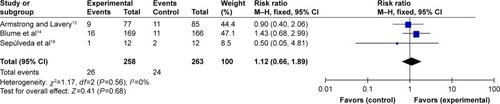
Treatment-related adverse events
Treatment-related adverse DFU events include edema, infection, pain, and bleeding. Infection was the most common adverse event assessed in three RCTs.Citation13,Citation14,Citation16 Sepúlveda et alCitation18 included data for bleeding and pain in addition to infection. The result of the meta-analysis indicated that treatment-related adverse events related to DFU showed no significant difference between the NPWT group and the standard dressing changes group (95% CI: 0.66–1.89, P=0.68) ().
Sensitivity analysis
Regarding reduction of the DFU area, when we removed a report that contributed to the final result, the direction and magnitude of the pooled RRs did not vary substantially. This indicated a good reliability of this meta-analysis (95% CI: 3.53–14.73, P=0.001) ().
Discussion
Evaluation of NPWT efficacy
In this systematic review and meta-analysis, we found that NPWT facilitated wound granulation formation and complete DFU closure, reduced DFU healing time, and decreased DFU size in comparison with standard dressing changes. Those results were similar to results of prior system reviews.Citation24,Citation25 However, another systematic reviewCitation26 concluded that the method of measuring and evaluating ulcer size reduction and complete wound closure may affect the reliability of the results. Therefore, for outcome measures, it is important to focus on the use of blinded measures. Wound bed preparation and granulation tissue formation are also important prerequisites for wound healing. The Patient Outcome Group suggested that the appropriate primary endpoint may not be DFU healing but, rather, percentage granulation tissue formation.Citation27 Four articlesCitation13,Citation14,Citation18,Citation19 assessed granulation tissue formation, and two of them used 90% or more than 90% of granulation tissue formation, preparation of re-epithelialization, and skin grafting as endpoints. The evaluation results showed that NPWT could accelerate granulation formation in comparison to standard dressing changes. It is known that foot wounds secondary to amputation are deeper, with exposed bone and tendons and pre-existing infection, and would lead to delayed wound healing. Armstrong et alCitation13 enrolled 162 diabetic patients with post-operative wounds to receive NPWT treatment or moist dressing treatment. The rate of complete wound healing for patients receiving NPWT (56%) was higher than for the moist dressings group (39%); the median time to reach 76%–100% granulation tissue for patients receiving NPWT (42 days) was less than for the control group (84 days), which suggested that NPWT had the potential to promote more complex and severe wound healing and prepare an adequately granulated wound bed.
Evaluation of NWPT safety
Treatment-related adverse DFU events include edema, infection, pain, and bleeding. The meta-analysis results showed that NPWT neither increased nor decreased the incidence of treatment-related side effects as compared with the standard dressing change group; which suggested that adverse events related to NPWT were not serious. However, in 2011 the US Food and Drug Administration updated a report on serious complications associated with NPWT and cited 12 deaths and 174 injuries since 2007.Citation11 Ren and Li reported sepsis in a burns patient treated with NPWT.Citation28 It should be noted that acute hemorrhages caused all of the deaths because large, exposed blood vessels and bleeding were ignored. Meanwhile, some of these serious adverse events occurred at home or in a long-term care facility, where the patients, nurses, and home care providers might not have received adequate training to do NPWT properly. In the eleven RCTs that we included in our meta-analysis, the intervention settings were hospitals or wound centers where professionals are familiar with NPWT indications and adhere to treatment guidelines.Citation29 This may be why serious complications did not occur in the studies we reviewed in our meta-analysis. DFUs are a leading cause of non-traumatic foot amputation; Armstrong et alCitation13 reported that the number of patients who received NPWT treatment were a quarter less likely to need re-amputation compared to controls. The result of our meta-analysis also indicated that NPWT could effectively reduce the occurrence of amputation. The rate of amputation decreased in the NPWT treatment group and is attributed to faster removal of infectious material, better preparation of granulated wound bed, and more rapid healing.
Evaluation of NPWT cost-effectiveness
A post hoc retrospective analysis indicated that for patients with DFUs who achieved complete wound closure, the median cost per 1 cm2 of closure was US$1,227 with NPWT and US$1,695 with advanced moist wound therapy, which showed greater cost-effectiveness in the NPWT group for treating recalcitrant wounds.Citation30 Two analysesCitation31,Citation32 based on economic models also concluded that, compared to patients treated with advanced wound care, patients treated with VAC therapy had increased quality-adjusted life years and a higher healing rate at a lower cost. Vaidhya et alCitation19 concluded that the mean dressings and total cost of dressings needed to achieve satisfactory healing in the NPWT group, were less than for the conventional dressing changes group. However, in this RCT, the VAC was modified to the standard KCI VAC therapy kit, so subsequent RCTs are needed to evaluate the cost of commercial VAC NPWT for treating DFUs. Moreover, considering the actual situation of medical resources available in developing countries, a modified NPWT device may be a future research direction for NPWT experiments in resource-poor settings.
Evaluation of other aspects of NPWT
One RCTCitation17 evaluated quality of life using the SF-36 questionnaire, which suggested that NPWT remarkably improved the quality of life of DFU patients. Another RCT, in which no amputation was performed,Citation21 evaluated patient satisfaction by that measure, indicating that patients in the NPWT group were more satisfied. However, we would prefer to survey patients rather than relying on a secondary outcome to assess patient satisfaction.
Limitations
From the details of included studies, important information was not fully available. Only four articlesCitation13,Citation14,Citation17,Citation18 offered data related to the ankle brachial index (ABI), even though ABI measurement is a simple and effective method of judging lower limb vascular disease to determine whether amputation is necessary.Citation33 Two studiesCitation15,Citation22 provided the duration of DM, which could influence the peripheral neuropathy leading to the formation of DFUs.Citation34 It was reported that body mass was significantly associated with pressure in the mid-foot models.Citation35 Two articles calculated average weight, and another two calculated body mass index, whereas no relevant details about local pressure on the foot were provided in the remaining seven studies. Stratified randomization was not performed for the severity of DFUs, thus, the patient characteristics in each group were not balanced. Meanwhile, there were many other influencing factors, including the relatively small sample sizes, insufficient description of methodologic details, inadequate follow-up time, and so on, which can result in clinical heterogeneity. Finally, because we retrieved only published literature, the document collection may be incomplete.
Conclusion
This meta-analysis of eleven RCTs extends support for the use of NPWT in the treatment of DFUs and post-operative wounds in diabetic patients. Additional robust RCT research is necessary to solidify support for the treatment.
Acknowledgments
The authors would like to thank Ming Huang for guidance in terms of writing and submission of the paper.
Disclosure
The authors report no conflicts of interest in this work.
References
- HeubleinHBaderAGiriSPreclinical and clinical evidence for stem cell therapies as treatment for diabetic woundsDrug Discov Today201520670371725603421
- ToosizadehNMohlerJArmstrongDGTalalTKNajafiBThe influence of diabetic peripheral neuropathy on local postural muscle and central sensory feedback balance controlPLoS One2015108e013525526258497
- BoultonAJThe diabetic footMedicine20103812644648
- SetacciFSirignanoPDe DonatoGPrimary amputation: is there still a place for itJ Cardiovasc Surg (Torino)20125315359
- KvitkinaTNarresMClaessenHIncidence of lower extremity amputation in the diabetic compared to the non-diabetic population: a systematic review protocolSyst Rev201547426001384
- RiceJBDesaiUCummingsAKBirnbaumHGSkornickiMParsonsNBBurden of diabetic foot ulcers for medicare and private insurersDiabetes Care201437365165824186882
- CavanaghPAttingerCAbbasZBalARojasNXuZRCost of treating diabetic foot ulcers in five different countriesDiabetes Metab Res Rev201228Suppl 1S107S111
- SöylemezMSÖzkanKKılıçBErincSIntermittent negative pressure wound therapy with instillation for the treatment of persistent periprosthetic hip infections: a report of two casesTher Clin Risk Manag20161216116626929628
- VassalloIMFormosaCComparing calcium alginate dressings to vacuum-assisted closure: a clinical trialWounds201527718019026192736
- ModyGNNirmalIADuraisamySPerakathBA blinded, prospective, randomized controlled trial of topical negative pressure wound closure in IndiaOstomy Wound Manage200854123646
- Safety Communications-UPDATE on serious complications associated with negative pressure wound therapy systems: FDA safety communication2011 Available from: https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm244211.htmAccessed April 7, 2017
- HigginsJPGreenSCochrane Handbook for Systematic Reviews of Interventions 5.1.0The Cochrane Collaboration2011
- ArmstrongDGLaveryLADiabetic Foot Study ConsortiumNegative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trialLancet200536694981704171016291063
- BlumePAWaltersJPayneWAyalaJLantisJComparison of negative pressure wound therapy using vacuum-assisted closure with advanced moist wound therapy in the treatment of diabetic foot ulcers: a multicenter randomized controlled trialDiabetes Care200831463163618162494
- KaratepeOEkenIAcetEVacuum assisted closure improves the quality of life in patients with diabetic footActa Chir Belg2011111529830222191131
- EgintonMTBrownKRSeabrookGRTowneJBCambriaRAA prospective randomized evaluation of negative-pressure wound dressings for diabetic foot woundsAnn Vasc Surg200317664564914534844
- SunJWSunJHVacuum assisted closure technique for repairing diabetic foot ulcers: analysis of variance by using a randomized and double-stage crossover designJ Clin Rehab Tissue Eng Res2007114489088911
- SepúlvedaGEspíndolaMMaureiraMCuración asistida por presión negativa comparada con curación convencional en el tratamiento del pie diabético amputado. Ensayo clínico aleatorio. [Negative-pressure wound therapy versus standard wound dressing in the treatment of diabetic foot amputation. A randomised controlled trial]Cir Esp2009863171177 Spanish19616774
- VaidhyaNPanchalAAnchaliaMMA new cost-effective method of NPWT in diabetic foot woundIndian J Surg201577Suppl 2525529
- NainPSUppalSKGargRBajajKGarqSRole of negative pressure wound therapy in healing of diabetic foot ulcersJ Surg Tech Case Rep201131172222022649
- RavariHModagheghMHKazemzadehGHComparison of vacuum-assisted closure and moist wound dressing in the treatment of diabetic foot ulcersJ Cutan Aesthet Surg201361172023723599
- SajidMTMustafaQuShaheenNHussainSMShukrIAhmedMComparison of negative pressure wound therapy using vacuum-assisted closure with advanced moist wound therapy in the treatment of diabetic foot ulcersJ Coll Physicians Surg Pak2015251178979326577962
- McCallonSKKnightCAValiulusJPCunninghamMWMcCullochJMFarinasLPVacuum-assisted closure versus saline-moistened gauze in the healing of postoperative diabetic foot woundsOstomy Wound Manage2000468283234
- DumvilleJCHinchliffeRJCullumNNegative pressure wound therapy for treating foot wounds in people with diabetes mellitusCochrane Database Syst Rev20131010CD010318
- ZhangJHuZCChenDGuoDZhuJYTangBEffectiveness and safety of negative-pressure wound therapy for diabetic foot ulcers: a meta-analysisPlast Reconstr Surg2014134114115124622569
- PeinemannFSauerlandSNegative-pressure wound therapy: systematic review of randomized controlled trialsDtsch Arztebl Int20111082238138921712971
- GottrupFApelqvistJPricePEuropean Wound Management Association Patient Outcome GroupOutcomes in controlled and comparative studies on non-healing wounds: recommendations to improve the quality of evidence in wound managementJ Wound Care201019623726820551864
- RenHLiYSevere complications after negative pressure wound therapy in burned wounds: two case reportsTher Clin Risk Manag20141051351625061310
- SchintlerMVNegative pressure therapy: theory and practiceDiabetes Metab Res Rev201228Suppl 1727722271727
- DriverVRBlumePAEvaluation of wound care and health-care use costs in patients with diabetic foot ulcers treated with negative pressure wound therapy versus advanced moist wound therapyJ Am Podiatr Med Assoc2014104214715324725034
- FlackSApelqvistJKeithMTruemanPWilliamsDAn economic evaluation of VAC therapy compared with wound dressings in the treatment of diabetic foot ulcersJ Wound Care2008172717818389832
- WhiteheadSJForestbendienVLRichardJLHalimiSVanGHTruemanPEconomic evaluation of Vacuum Assisted Closure® therapy for the treatment of diabetic foot ulcers in FranceInt Wound J201181223220875048
- Al-RubeaanKAl DerwishMOuiziSDiabetic foot complications and their risk factors from a large retrospective cohort studyPLoS One2015105e012444625946144
- AlaviASibbaldRGMayerDDiabetic foot ulcers: Part I. Pathophysiology and preventionJ Am Acad Dermatol20147011.e1e1824355275
- BarnRWaaijmanRNolletFWoodburnJBusSAPredictors of barefoot plantar pressure during walking in patients with diabetes, peripheral neuropathy and a history of ulcerationPLoS One2015102e011744325647421