Abstract
Incontinentia pigmenti (IP; Bloch–Sulzberger syndrome) is a rare, genetic syndrome inherited as an X-linked dominant trait. It primarily affects female infants and is lethal in the majority of males during fetal life. The clinical findings include skin lesions, developmental defects, and defects of the eyes, teeth, skeletal system, and central nervous system. Cardiovascular complications of this disease in general, and pulmonary hypertension in particular, are extremely rare. This report describes the case of a 3-year-old girl with IP complicated by pulmonary arterial hypertension. Extensive cardiology workup done to the patient indicates underlying vasculopathy. This report sheds light on the relationship between IP and pulmonary hypertension, reviews the previously reported cases, and compares them with the reported case.
Introduction
Incontinentia pigmenti (IP; Bloch–Sulzberger syndrome; Online Mendelian Inheritance in Man [OMIM# 308300]) is a rare hereditary disease inherited as an X-linked dominant trait. It primarily affects female infants and is lethal in the majority of males during fetal life.Citation1 Approximately 1,000 cases have been reported in the literature and the female: male ratio ranges from ~20–37:1.Citation2
IP occurs due to a genetic mutation in the IKBKG gene (inhibitor of the kappa light polypeptide gene enhancer in B-cells, kinase gamma; NM_003639.4), which is located on the X chromosome at position q28.Citation3 This gene is also known as nuclear factor-kappa B (NF-κB) essential modulator (NEMO). It encodes for regulatory NEMO/IKKγ (I-kappa B kinase), which causes phosphorylation and degradation of the inhibitor bound to NF-κB in the cytosol. The dissociated, active, NF-κB enters the nucleus and then assumes the role of a transcription factor.Citation4,Citation5 The NF-κB pathway plays an important role in the immune system and regulates the expression of many genes outside the immune system, including that involved in embryonic development and the development of bone, mammary glands, skin, and central nervous system (CNS).Citation6,Citation7
IP has a broad spectrum of clinical features reflecting the involvement of different organs of the body, including the eyes, skeletal system, skin, and CNS. Landy and Donnai initially proposed the diagnostic criteria for IP.Citation8 They divided patients into two groups based on the family history of IP in first-degree female relatives and set the major criteria as typical skin manifestations, which are usually the first sign to appear. In addition, they subdivided the skin manifestations into four stages comprising blisters preceded by erythema, hyperkeratotic verrucous lesions, hyperpigmentation, and finally, hypopigmentation and loss of hair stage. Minor criteria comprised dental manifestations and hair, nail, and retinal involvement. In the absence of familial history, the presence of at least one major criterion is required, while the presence of minor criteria further supports a diagnosis of IP. Complete absence of minor criteria leads to an uncertain diagnosis.
In 2014, Minic et al proposed the addition of CNS, palate, breast, and nipple anomalies, multiple male miscarriages, and pathohistological manifestations to the minor criteria for the disease.Citation9 Skin manifestation stages appear sequentially over time, but patients might harbor variable stages simultaneously.Citation8 In more uncommon cases, cardiac malformations may present as ventricular endomyocardial fibrosis, tricuspid insufficiency, and pulmonary hypertension.Citation10
This report describes a case of a 3-year-old girl diagnosed with IP complicated by pulmonary arterial hypertension.
Methods
Clinical evaluation
A retrospective chart review was undertaken for the patient, and all data were collected and summarized.
Analysis of gene mutation
Genomic DNA was screened by single-tier polymerase chain reaction (PCR) amplification for the common deletion of exons 4–10 in the IKBKG (NEMO) gene. The patient’s specimen, along with control samples, was amplified by long-range PCR amplification with primers designed to produce DNA fragments of specific sizes in the presence of the concurrent deletion. Normal control samples analyzed concurrently on an agarose gel along with the patient’s DNA were not expected to produce a visible band when amplified under the used PCR conditions. The result was confirmed in a new PCR amplicon/preparation of DNA by repeat analysis.Citation11
Case report
This report describes a 3-year-old girl who was a product of nonconsanguineous marriage, and who was born at full term by cesarean section owing to non-reassuring cardiotocography. The family history was negative for a similar condition, recurrent miscarriages, or neonatal deaths.
A few hours after birth, the newborn was taken to the neonatal intensive care unit (NICU) because of respiratory distress which necessitated intubation and mechanical ventilation.
Investigations including cardiac echo showed persistent pulmonary hypertension (PHTN) of the newborn. Throughout her stay in the NICU, she developed skin rash with vesicles and pustules, which were initially suspected to be herpes simplex or impetigo, but over time, the lesions became hyperpigmented.
At 30 days of age, her condition improved; so she was extubated and discharged home without a definitive diagnosis. Her pulmonary hypertension persisted, and she started to develop episodes of cyanotic spells and exhibited decreased level of consciousness mainly with crying.
At 18 months of age, she had a viral illness, after which the cyanotic spells became more frequent. The patient was admitted and an echocardiogram was performed revealing persistent pulmonary hypertension; so she was started on diuretics (furosemide). Later, at the age of 2 years, phosphodiesterase-5 inhibitor (sildenafil) was added to the existing regimen. Thereafter, the patient was referred to the tertiary center at King Abdulaziz Medical City – because of complicated pulmonary hypertension – for further investigations and management.
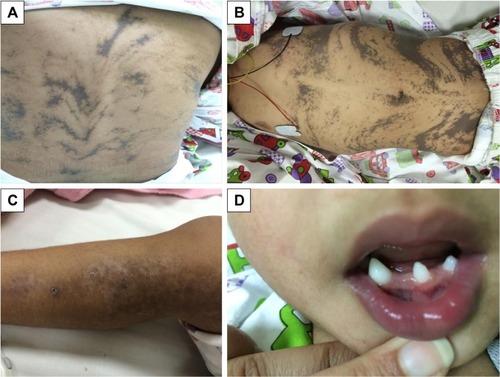
On examination, she was found to have hyperpigmented skin over her chest, abdomen, and back which followed the lines of Blaschko (). Hyperpigmented lesions were also found over her scalp. She had hypopigmentation over her lower limbs with atrophic scar () and had missing teeth, conoid teeth (), and wooly hair. There were no noticeable changes in her nails. Her neurological examination was unremarkable; she had normal power, tone, and reflexes.
Figure 1 Patient’s skin and teeth manifestations.
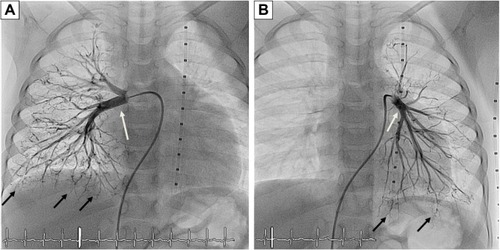
Laboratory investigations were normal with no eosinophilia. Her echocardiogram showed severe pulmonary, supra-systemic, hypertension; severe tricuspid regurgitation with peak gradient at 135 mmHg; D-shaped compressed left ventricle; severely dilated hypertrophied right ventricle; and atrial septal defect (5 mm) with mainly right-to-left shunt. The computed tomography (CT) angiogram of the chest showed that her pulmonary arteries were not dilated, which raised the concern of native pulmonary artery hypoplasia. Accordingly, cardiac catheterization was performed and showed distal bilateral pulmonary artery stenosis (type 5); left pulmonary artery, 7.9 mm; right pulmonary artery, 7.8 mm; and Nakata index, 203 (330±30 mm2/m2).Citation12 The cardiac catheterization revealed collaterals from plural arteries and complete obstruction of some arterioles bilaterally ().
Figure 2 Cardiac catheterization.
Based on clinical findings, IP was suspected. Definitive diagnosis was reached by molecular investigation, as described in the “Analysis of gene mutation” section, which showed deletion of exons 4–10 on the IKBKG gene.
Now, she is a 3-year-old girl, and her developmental milestones are appropriate for her age. All her growth parameters are below the fifth percentile (height 88.7 cm, weight 11 kg, head circumference 44 cm). Her cardiovascular condition is stable on furosemide 6 mg once daily and sildenafil 9 mg once daily.
Discussion
IP is a rare inherited neurocutaneous disorder, distinguished by classical skin lesions with frequent multisystem involvement.
It primarily affects females, as in this case, because in affected hemizygous male fetuses the pregnancy will terminate with spontaneous abortion owing to extensive apoptosis in fetal cells, although IP was reported previously in XXY male patients and males with somatic mosaicism.Citation1,Citation13,Citation14
In 80% of cases, there is a deletion of exons 4–10 on the IKBKG gene,Citation1,Citation15,Citation16 which was established in the present case by molecular testing. Most cases develop skin lesions on the trunk, extremities, and scalp, which were spotted in the patient observed in this study. Characteristic skin lesions evolve through the following four known stages.Citation17
Stage 1, known as the inflammatory or vesicular stage, is recognized by the development of papules, vesicles, and pustules on an erythematous base, spread linearly along the lines of Blaschko. In most patients (>90%), lesions present at birth or develop during the first 2 weeks of life and can be confused with herpes simplex or impetigo, which was the case with this study’s patient too.Citation18,Citation19
Stage 2, the verrucous stage, is characterized by plaques and warty papules. In most patients, these develop within 2–6 weeks and commonly vanish by 6 months of age.Citation17
Stage 3, the hyperpigmented stage, is determined by the development of linear lesions with a brownish pigmentation, which will also follow the lines of Blaschko – as seen in this report ().
Stage 4 is the atrophic or hypopigmented stage, which is characterized by areas of hypopigmentation, atrophy, and absence or loss of hair, most frequently observed on the lower extremities – as seen in the patient studied here also ().Citation20,Citation21
According to the diagnostic criteria described by Landy and Donnai,Citation8 the patient in this study had three major and two minor criteria. She matched the trends in presentation of the IP patient registry described by Fusco et alCitation22 in most of the domains, except for CNS and ophthalmological defects.
One extremely rare complication of IP is PHTN, which has been reported in only five cases worldwide (), and was not included in the clinical domain of Fusco et al’s registry.Citation22
Table 1 Summary of IP cases with PHTN
The first case that reported the association between primary PHTN and IP was published in 1992, where the meticulously described baby died at 2 months of age. In addition, he had convulsions on the fifth day of life and a brain CT scan showed diffuse hypodensities in the left hemisphere.Citation23
The second case was reported by Miteva and Nikolova.Citation24 The patient was a female, who presented after birth with typical skin manifestations with neurological and ophthalmological symptoms. The patient’s cardiac anomalies included massive tricuspid insufficiency and abnormal shunt from the right pulmonary vein to the superior vena cava in addition to PHTN.
A third case reported by Hayes et alCitation25 was familial with a positive family history of IP. This case was characterized by unilateral acheiria and was associated with CNS manifestations. In addition to the early diagnosed PHTN at the age of 15 days, she had right ventricular hypertrophy. Unfortunately, she died at 45 days of life.
Godambe et alCitation10 reported the fourth case in the literature. The patient was a sporadic case in the family. She had typical skin lesions and developed cyanotic spells shortly after birth which led to a diagnosis of PHTN. Further investigations revealed ophthalmological manifestations. Follow-up echocardiography showed dilated right ventricle and marked tricuspid and pulmonary regurgitation. She received prostaglandin infusion initially followed by nitric oxide inhalation. The patient improved temporarily, then deteriorated again. Her condition continued to worsen despite treatment, and she died at 26 days of life.
The latest case was reported in 2016 by Yasuda et al. The case presented early in life with typical skin lesions, seizures, and multiple cerebral infarcts and encephalomalacia. She was found to have congenital heart disease and pulmonary hypertension at 2 months of age. Her case was unresponsive to medical treatment (oral bosentan) and she died at 5 months of age owing to severe pulmonary hypertensive crisis.Citation26
Compared with previous cases, the patient in this case report did not manifest any neurological or ophthalmological complications of the disease. She shared clinical features of the fourth case in terms of the cyanotic spells.
Apparently, the patient studied in this report is the only case among the other reported cases that responded well to medical treatment and remained stable.
Although the exact explanation of PHTN associated with IP remains unknown, many proposed explanations have been attributed to dysfunction in the NF-κB pathway.Citation27–Citation29 However, the hypoplasia of the pulmonary arteries and the obstruction of the arterioles described in the cardiac catheterization for the patient in this study might indicate vasculopathy. This was proposed previously to be a cause of PHTN, CNS manifestations,Citation30 and retinopathy, as described by Hennel et al,Citation31 and Yasuda et alCitation26 proposed diffuse microvasculopathy. However, Maingay-de Groof et alCitation2 postulated macrovasculopathy in the medium and small arteries.
The variability in phenotype among IP patients with PHTN could be explained by lionization, proposed previously to explain the partial involvement pattern found in the skin and other organs in IP patients.Citation32
Conclusion
In conclusion, there is growing evidence that IP can be associated with pulmonary hypertension of variable severity, which can be attributed to the dysfunction of the NF-κB pathway, resulting in progressive vasculopathy.
Acknowledgments
Written informed consent has been provided by the parents to have the case details and any accompanying images published. We are grateful to the family and patient reported in this article for their genuine support.
Disclosure
The authors report no conflicts of interest in this work.
References
- SmahiACourtoisGVabresPGenomic rearrangement in NEMO impairs NF-kappaB activation and is a cause of incontinentia pigmenti. The International Incontinentia Pigmenti (IP) ConsortiumNature2000405678546647210839543
- Maingay-de GroofFLequinMHRoofthooftDWExtensive cerebral infarction in the newborn due to incontinentia pigmentiEur J Paediatr Neurol200812428428917950640
- JinDYJeangKTIsolation of full-length cDNA and chromosomal localization of human NF-kappaB modulator NEMO to Xq28J Biomed Sci19996211512010087442
- BeinkeSRobinsonMJHuguninMLeySCLipopolysaccharide activation of the TPL-2/MEK/extracellular signal-regulated kinase mitogen-activated protein kinase cascade is regulated by IkappaB kinase-induced proteolysis of NF-kappaB1 p105Mol Cell Biol200424219658966715485931
- ZhouHWertzIO’RourkeKBcl10 activates the NF-kappaB pathway through ubiquitination of NEMONature2004427697016717114695475
- HaydenMSGhoshSSignaling to NF-kappaBGenes Dev200418182195222415371334
- NelsonDLNEMO, NFkappaB signaling and incontinentia pigmentiCurr Opin Genet Dev200616328228816647846
- LandySJDonnaiDIncontinentia pigmenti (Bloch–Sulzberger syndrome)J Med Genet199330153598423608
- MinicSTrpinacDObradovicMIncontinentia pigmenti diagnostic criteria updateClin Genet201485653654223802866
- GodambeSMcNamaraPRajguruMHellmannJUnusual neonatal presentation of incontinentia pigmenti with persistent pulmonary hypertension of the newborn: a case reportJ Perinatol200525428929215789024
- SteffannJRaclinVSmahiAA novel PCR approach for prenatal detection of the common NEMO rearrangement in incontinentia pigmentiPrenat Diagn200424538438815164415
- NakataSImaiYTakanashiYA new method for the quantitative standardization of cross-sectional areas of the pulmonary arteries in congenital heart diseases with decreased pulmonary blood flowJ Thorac Cardiovasc Surg19848846106196482493
- CourtoisGSmahiAIsraelANEMO/IKK gamma: linking NF-kappa B to human diseaseTrends Mol Med200171042743011597506
- BuinauskaiteEBuinauskieneJKucinskieneVStrazdieneDValiukevicieneSIncontinentia pigmenti in a male infant with Klinefelter syndrome: a case report and review of the literaturePediatr Dermatol201027549249520807362
- PoziomczykCSRecueroJKBringhentiLIncontinentia pigmentiAn Bras Dermatol2014891263624626645
- FuscoFPaciollaMNapolitanoFGenomic architecture at the Incontinentia Pigmenti locus favours de novo pathological alleles through different mechanismsHum Mol Genet20122161260127122121116
- BerlinALPallerASChanLSIncontinentia pigmenti: a review and update on the molecular basis of pathophysiologyJ Am Acad Dermatol2002472169187 quiz 188–19012140463
- OkanFYapiciZBulbulAIncontinentia pigmenti mimicking a herpes simplex virus infection in the newbornChilds Nerv Syst200824114915117632728
- van LeeuwenRLWintzenMvan PraagMCIncontinentia pigmenti: an extensive second episode of a “first-stage” vesicobullous eruptionPediatr Dermatol200017170
- Hadj-RabiaSFroidevauxDBodakNClinical study of 40 cases of incontinentia pigmentiArch Dermatol200313991163117012975158
- PhanTAWargonOTurnerAMIncontinentia pigmenti case series: clinical spectrum of incontinentia pigmenti in 53 female patients and their relativesClin Exp Dermatol200530547448016045670
- FuscoFPaciollaMConteMIIncontinentia pigmenti: report on data from 2000 to 2013Orphanet J Rare Dis201499324961275
- TrikiCDevictorDKahSCerebral complications of incontinentia pigmenti. A clinicopathological study of a caseRev Neurol (Paris)1992148127737761303572
- MitevaLNikolovaAIncontinentia pigmenti: a case associated with cardiovascular anomaliesPediatr Dermatol2001181545611207974
- HayesIMVarigosGUpjohnEJOrchardDCPennyDJSavarirayanRUnilateral acheiria and fatal primary pulmonary hypertension in a girl with incontinentia pigmentiAm J Med Genet A2005135330230315884011
- YasudaKMinamiNYoshikawaYTaketaniTFukudaSYamaguchiSFatal pulmonary arterial hypertension in an infant girl with incontinentia pigmentiPediatr Int201658539439627173419
- BelaibaRSBonelloSZahringerCHypoxia up-regulates hypoxia-inducible factor-1alpha transcription by involving phosphatidylinositol 3-kinase and nuclear factor kappaB in pulmonary artery smooth muscle cellsMol Biol Cell200718124691469717898080
- HamidRNewmanJHEvidence for inflammatory signaling in idiopathic pulmonary artery hypertension: TRPC6 and nuclear factor-kappaBCirculation2009119172297229819414653
- WoodsMWoodEGBardswellSCRole for nuclear factor-kappaB and signal transducer and activator of transcription 1/interferon regulatory factor-1 in cytokine-induced endothelin-1 release in human vascular smooth muscle cellsMol Pharmacol200364492393114500749
- RidderDAWenzelJMullerKBrain endothelial TAK1 and NEMO safeguard the neurovascular unitJ Exp Med2015212101529154926347470
- HennelSJEkertPGVolpeJJInderTEInsights into the pathogenesis of cerebral lesions in incontinentia pigmentiPediatr Neurol200329214815014580659
- RottHDExtracutaneous analogies of Blaschko linesAm J Med Genet199985433834110398255
