Abstract
Although uremic pruritus (UP) is a common and annoying symptom for end-stage renal disease patients on hemodialysis (HD) and peritoneal dialysis, its pathogenesis is poorly understood. However, systemic inflammation is one of the possible pathogenesis of UP, and blood lead level (BLL) has been noted to be associated with inflammation and nutritional status in long-term HD patients. There might be an interaction or association, therefore, between BLL and UP through systemic inflammation. We analyzed cross-sectional data among 866 participants. All of the 866 patients in this study were stratified into groups with low-normal (<10 μg/dL), high-normal (10–20 μg/dL), and abnormal BLLs (>20 μg/dL). The associations between UP and BLL and the clinical data were analyzed. Multivariate logistic regression demonstrated that HD duration, non-anuria, log ferritin, serum low-density lipoprotein, log BLL, high-normal BLL, and high BLL were associated with UP. In conclusion, BLL was positively associated with UP.
Introduction
Uremic pruritus (UP) is a common and annoying symptom for end-stage renal disease (ESRD) patients on hemodialysis (HD) and peritoneal dialysis (PD). The prevalence of UP varies from 22% to 57%Citation1–Citation3 with a prevalence in Taiwan of 28.6%–62.6% in ESRD patients on HD or PD.Citation4–Citation6 Unfortunately, the pathogenesis of UP remains poorly understood. Proposed hypotheses include systemic inflammation,Citation7 imbalance between the expression of opioid receptors,Citation8 and other risk factors. Blood lead level (BLL) has been reportedly associated with inflammation and nutritional status in long-term HD patients with diabetes and might contribute to 1-year mortality in these patients.Citation9 All-cause, cardiovascular, and infection-related 18-month mortality in patients on maintenance HD were positively associated with high BLL.Citation10 Taking these findings into consideration, there might be an interaction or association between BLL and UP through systemic inflammation. The purpose of this study, therefore, was to find the possible association between BLL and UP.
Materials and methods
Methods
Chang Gung Memorial Hospital’s Institutional Review Board (IRB) Committee approved the study protocol (Code of IRB: 98–1937B). The methods in the study were performed in accordance with the approved guidelines. Because this was a cross-sectional retrospective-designed study, informed consent was not required, and this was approved by our IRB committee. We collected all primary data in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology guidelines. Data was de-identified to ensure patient confidentiality.
Patients
All patients were enrolled from three HD centers of Chang Gung Memorial Hospital, Lin-Kou Medical Center, including both the Taipei and Taoyuan branches. Maintenance hemodialysis (MHD) patients who were equal to or older than 18 years old and had undergone HD for at least 6 months were recruited in this study. Exclusion criteria were patients with malignancies, infectious diseases, and those who had been admitted or received surgery within 3 months of the study. Most patients received 4 hours of HD per session and HD 3 times a week. Dialysate with standard ionic composition and bicarbonate-based buffer was used for all patients. Cardiovascular diseases were recorded in these patients. Smoking behavior was also noted in this study. Diagnosis of pruritus was as follows: pruritus appearing after HD with/without antipruritics as visualized by trained dermatologists or nephrologists. The severity of pruritus was measured by visual analog scale (VAS), which consisted of a 10-cm horizontal line with 0 point (no pruritus) to 10 points (maximum intensity of pruritus).
Measurement of BLL
We measure BLLs at the end of the run-in phase using a previously described method.Citation11,Citation12 BLLs were measured by electrothermal atomic-absorption spectrometry (SpectrAA-200Z; Agilent Technologies, www.agilent.com) with Zeeman background correction and an L’vov platform.
Definition of low-normal, high-normal, and high blood lead levels
All of the studied patients were stratified into groups with low-normal (<10 μg/dL), high-normal (10–20 μg/dL), and abnormal BLLs (>20 μg/dL).Citation9
Laboratory parameters
We drew blood samples from the arterial end of the vascular access immediately after the initial 2-day interval of HD. Nutritional markers were recorded as serum creatinine levels, normalized protein catabolism rate (nPCR), and serum albumin levels. High-sensitivity C-reactive protein levels were used as marker of inflammation. Dialysis adequacy, Kt/Vurea, of HD patients was calculated by Daugirdas method. The nPCR of HD patients was calculated by validated equations.Citation13 The definition of anuria was a daily urine amount <100 mL.
Statistical analysis
Normal distribution was tested by the Kolmogorov–Smirnov test. Mean ± SD/median (interquartile range) was expressed for continuous variables, and numbers or percentages were expressed for categorical variables. The correlation between categorical variables was analyzed by chi-square or Fisher’s exact test. Mann–Whitney U-test or Student’s t-test was used to compare two groups. Univariate and multivariate logistic regression analyses were performed to evaluate the variables related to UP. Area under the receiver operating characteristic (AUROC) analysis was used to assess the discrimination. The best Youden index (sensitivity + specificity − 1) was used to calculate the cutoff points. Data were analyzed using SPSS, version 12.0 for Windows 95 (SPSS Inc, Chicago, IL, USA). The level of significance was set at P<0.05.
Results
Study population characteristics
The flow chart of HD patient recruitment was demonstrated in . Eight hundred and sixty-six patients were included (). They received an average HD duration of 6.96±5.35 years. Patients with high BLLs had a higher incidence of hepatitis C infection, UP, and hemodiafiltration, and higher HD durations, Kt/Vurea Daugirdes, levels of hemoglobin, and intact parathyroid hormone. There were 189 patients with UP. There was a significant difference in BLLs between HD patients with UP and HD patients without UP, (13.9 μg/dL [interquartile range: 10.45, 19.26] vs 9.48 μg/dL [interquartile range: 6.94, 12.76], respectively, P<0.001). The severity of UP was evaluated by VAS.Citation14,Citation15 The median of VAS was 6 (interquartile range: 4, 6.5). The correlation between log Pb and VAS was not significant with r=0.012 and P=0.869. The median VAS of patients with UP and BLL <10 μg/dL, 20> BLL ≥10 μg/dL, and BLL ≥20 μg/dL were 6.0 (interquartile range: 4, 7), 6.0 (interquartile range: 5, 6), and 6.0 (inter-quartile range: 4, 7), respectively. There was no difference of VAS between these three groups (P=0.681).
Figure 1 Flow chart of patient recruitment.
Table 1 Characteristics of studied MHD patients and comparison of patients with different blood lead levels
Predictors of uremic pruritus
Univariate logistic regression showed that 13 variables were associated with UP (). Multivariate logistic regression demonstrated that the following were associated with UP: HD duration (odds ratio [OR]: 1.092, 95% confidence interval [CI]: 1.056–1.130, P<0.001), non-anuria (OR: 0.557, 95% CI: 0.340–0.979, P=0.041), log ferritin (OR: 2.086, 95% CI: 1.388–3.134, P,<0.001), serum low-density lipoprotein (LDL) (OR: 1.009, 95% CI: 1.003–1.015, P=0.002), and log BLL (OR: 29.230, 95% CI: 11.512–574.214, P<0.001) (). After we divided BLLs into low-normal BLL, high-normal BLL, and high BLL, multivariate logistic regression showed that HD duration (OR: 1.088, 95% CI: 1.051–1.126, P<0.001), non-anuria (OR: 0.565, 95% CI: 0.333–0.960, P=0.035), log ferritin (OR: 2.153, 95% CI: 1.428–3.248, P<0.001), serum LDL (OR: 1.009, 95% CI: 1.003–1.015, P=0.003), high-normal BLL (low-normal BLL as reference) (OR: 3.286, 95% CI: 2.174–4.967, P<0.001), and high BLL (low-normal BLL as reference) (OR: 8.938, 95% CI: 4.942–16.166, P<0.001) were associated with UP ().
Table 2 Univariate logistic regression analysis between uremic pruritus and clinical variables
Table 3 Multivariate logistic regression analysis (forward method) between uremic pruritus and clinical variables
Table 4 Multivariate logistic regression analysis (forward method) between uremic pruritus, and clinical variables
Calibration, discrimination, and correlation for the BLLs
Calibration of BLL was carried out as follows: Hosmer– Lemeshow; XCitation2=12.77, P=0.12. The BLLs had good calibration, as estimated by the Hosmer–Lemeshow goodness-of-fit test. The cutoff point calculated by obtaining the best Youden index (0.375) of BLLs to predict UP was 12.77 μg/dL (AUROC =0.792±0.023, 95% CI: 0.74–0.83, P<0.001, ) with a sensitivity of 78%, specificity of 66%, and overall correctness of 71.8%. Multivariate logistic regression showed that BLL ≥12.77 μg/dL (OR: 4.511, 95% CI: 3.128–6.505, P<0.001) was one of the predictors of UP (). The median VAS of patients with UP and BLL <12.77 μg/dL and BLL ≥12.77 μg/dL were 6.0 (interquartile range: 4, 6) and 6.0 (interquartile range: 4, 7), respectively. There was no difference of VAS between these two groups (P=0.279).
Figure 2 ROC curve of blood lead levels to predict uremic pruritus.
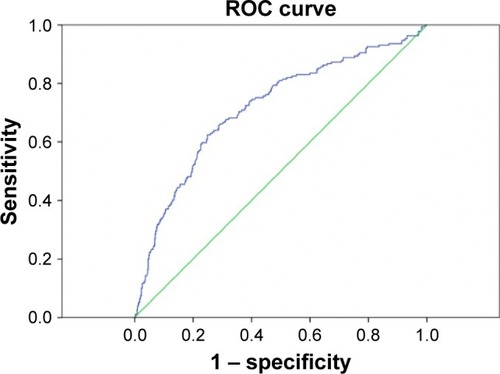
Table 5 Multivariate logistic regression analysis (forward method) between uremic pruritus and clinical variables
Discussion
UP has been proposed to be the result of systemic inflammation.Citation7,Citation16 BLLs have been previously shown to be associated with inflammation and poor nutrition in long-term HD patients.Citation9 Al Momen demonstrated a case of chronic lead exposure with the symptoms of hyperpigmentation of the skin, severe itching, muscle weakness, and thrombocytosis. After chelation therapy with dimercaptosuccinic acid, complete recovery of hyperpigmentation, itching, and thrombo-cytosis was noted.Citation17 Dongre et al showed that in the battery manufacture, workers with lead exposure had high blood pressure, disturbed calcium and phosphorous metabolism, and skin itching.Citation18 In the surveys of drinking water polluted by lead in Kerou and So-ava, people who consumed lead-polluted water had various symptoms including skin itching.Citation19,Citation20 Therefore, there was significant correlation between BLL and the occurrence of skin pruritus. There was no significant correlation between Log (Pb) and severity of UP in our study, but we showed that BLL ≥12.77 μg/dL was a cut-off point to predict UP. This may suggest that BLL ≥12.77 μg/dL may be a point to trigger pruritus symptom, and BLL above this point is not correlated with the severity of UP. And the severity sensation of UP is different in every patient and this may explain that the quantitative measure of pruritus did not correlate with BLLs.
Imbalances in the expression between mu and kappa opioid receptors, which will cause pruritus, have also been proposed as a hypothesis of UP.Citation21,Citation22 Lead treatment by using rat models has shown that lead alters the development of mu and delta receptors and biological responses to opioids.Citation23 Other studies have also shown that the dynorphin/kappa opioid system is less affected by lead than the mu and delta systems.Citation24 Therefore, it is reasonable to postulate that lead can cause UP via the opioid systems.
Animal studies have also demonstrated that chronic exposure to low-dose lead results in reactive oxygen species (ROS) generation.Citation25 ROS has also been noted to play a role in atopic dermatitis, which is a noncontagious, relapsing inflammatory skin disease characterized by eczema and pruritus.Citation26 Lead may thus cause UP through the induction of ROS. Exposure to low levels of lead could also induce lipid peroxidation.Citation27 Yago et al demonstrated that neurotropin significantly suppressed the C3a level and improved the condition of pruritic HD patients. They also found that neurotropin could lower the level of lipid peroxidase in plasma of HD patients.Citation28 Secondary hyperparathyroidism (SHPT) was also associated with UP.Citation29,Citation30 Chu et al showed that SHPT might result in an increased release of lead from bone stores because of high bone turn over; they found that parathyroidectomy effectively suppressed the elevated levels of blood lead.Citation31 Some evidence also indicates an association between beta-2 microglobulin levels and UP in HD patients,Citation32–Citation34 and rats given lead acetate in drinking water experienced an increase of beta-2 microglobulin excretion.Citation35 The association between BLLs and UP might occur through the effect of beta-2 microglobulin. However, we cannot confirm this because we did not regularly measure beta-2 microglobulin in our HD patients. According to the above discussion, BLLs in HD patients might increase the intensity of or have an addictive role of pruritus caused by other more common risk factors.
Lead compound can also cause crystallization of calcium phosphate in the body like bone and skin.Citation36 Elevated skin calcium phosphate content was associated with skin inflammation and UP in HD patients.Citation3,Citation37
In this study, we showed that even BLLs in the high normal rangeCitation38 were significantly associated with UP. The cutoff point of BLLs to predict UP in our study was 12.77 μg/dL, which had a high sensitivity, specificity, and overall correctness. A previous study also revealed that high BLL was associated with increased hazards for all-cause, cardiovascular-related, and infection-related 18-month mortality in patients on MHD.Citation39 The definition of high BLL was BLL >12.64 μg/dL in this study. Chronic low-level environmental lead exposure might inhibit urate excretion in the general population,Citation12 and accelerate progressive renal insufficiency in patients without diabetes who have chronic renal disease.Citation11 These patients all had normal lead burden, and chelation therapy could improve renal function and slow the progression of renal insufficiency. According to our findings and those from the abovementioned studies, the normal range of BLL in HD patients needs to be revised or there are no normal BLLs in HD patients.
Ferritin had a positive correlation with UP in our study, which was consistent with previous research.Citation40 BLLs have been noted to be negatively associated with serum ferritin levels.Citation10,Citation41,Citation42 In our study, both serum ferritin and BLL were two independent factors positively associated with UP. Blood lead-related systemic inflammation, therefore, appears to play an important role in the elevation of serum ferritin in our studied HD patients.
More than half (52.9%) of our recruited HD patients were in the high-normal or high BLL categories, which was higher than the average BLL in general Taiwanese adults (7.7 μg/dL).Citation43 The elevated BLL in patients on maintenance HD might have been due to a complete loss of renal function, which is a main route to excrete lead from the body, and the difficulty in removing lead through HD.Citation9 Our present study also showed that anuria was also a positive predictor of UP. Anuria definitely caused a decreased excretion of blood lead in HD patients compared to those with residual urine output. Therefore, lead from environmental lead exposure in maintenance HD patients cannot be excreted efficiently.
Conclusion
Our study is the first to show that BLL is positively correlated with UP and can predict it, and there may actually be no normal blood lead level in maintenance HD patients. Further randomized controlled study to use chelation therapy for lead removal is needed for the definite causal relationship between blood lead and UP.
Limitations
This is a cross-sectional study and further randomized controlled studies using chelation therapy to reduce BLL should be performed to confirm the relation between causal and effect of BLL and UP in HD patients.
Author contributions
CH W and WH H wrote the main manuscript text. CW H and CC H prepared figures and tables. CH W, TH Y, and MJ C collected the data. All authors reviewed the manuscript. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
Cheng-Hao Weng was funded by research grants from the Chang Gung Memorial Hospital, Linkou (CMRPG3A1271, CMRPG5D0081).
Disclosure
The authors report no conflicts of interest in this work.
References
- Pauli-MagnusCMikusGAlscherDMNaltrexone does not relieve uremic pruritus: results of a randomized, double-blind, placebo-controlled crossover studyJ Am Soc Nephrol200011351451910703675
- PisoniRLWikstromBElderSJPruritus in haemodialysis patients: International results from the Dialysis Outcomes and Practice Patterns Study (DOPPS)Nephrol Dial Transplant200621123495350516968725
- NaritaIAlchiBOmoriKEtiology and prognostic significance of severe uremic pruritus in chronic hemodialysis patientsKidney Int20066991626163216672924
- WuHYPengYSChenHYA Comparison of Uremic Pruritus in Patients Receiving Peritoneal Dialysis and HemodialysisMedicine (Baltimore)2016959e293526945400
- KoMJPengYSChenHYInterleukin-31 is associated with uremic pruritus in patients receiving hemodialysisJ Am Acad Dermatol201471611511159e115125270263
- ChiuYLChenHYChuangYFAssociation of uraemic pruritus with inflammation and hepatitis infection in haemodialysis patientsNephrol Dial Transplant200823113685368918515654
- KimmelMAlscherDMDunstRThe role of micro-inflammation in the pathogenesis of uraemic pruritus in haemodialysis patientsNephrol Dial Transplant200621374975516249205
- IkomaASteinhoffMStanderSYosipovitchGSchmelzMThe neurobiology of itchNat Rev Neurosci20067753554716791143
- LinJLLin-TanDTYenTHBlood lead levels, malnutrition, inflammation, and mortality in patients with diabetes treated by long-term hemodialysisAm J Kidney Dis200851110711518155539
- LinJLLin-TanDTHsuCWAssociation of blood lead levels with mortality in patients on maintenance hemodialysisAm J Med2011124435035821435426
- LinJLLin-TanDTHsuKHYuCCEnvironmental lead exposure and progression of chronic renal diseases in patients without diabetesN Engl J Med2003348427728612540640
- LinJLTanDTHoHHYuCCEnvironmental lead exposure and urate excretion in the general populationAm J Med2002113756356812459402
- SargentJAControl of dialysis by a single-pool urea model: the National Cooperative Dialysis StudyKidney Int Suppl198313S19S256345894
- ReichAHeisigMPhanNQVisual analogue scale: evaluation of the instrument for the assessment of pruritusActa Derm Venereol201292549750122102095
- ChenYCChiuWTWuMSTherapeutic effect of topical gammalinolenic acid on refractory uremic pruritusAm J Kidney Dis2006481697616797388
- MettangTPauli-MagnusCAlscherDMUraemic pruritus – new perspectives and insights from recent trialsNephrol Dial Transplant20021791558156312198205
- Al MomenAThrombocytosis secondary to chronic lead poisoningPlatelets201021429729920196630
- DongreNNSuryakarANPatilAJHundekariIADevarnavadagiBBBiochemical effects of lead exposure on battery manufacture workers with reference to blood pressure, calcium metabolism and bone mineral densityIndian J Clin Biochem2013281657024381424
- GillesEKLucKPatientGEvalution of the exposure to pollutants in the drinking water of So-ava municipality using biomarkers: epidemiological studyJ Biodiversity Environ Sci20144215
- ElegbedeBEdorhPAAïssiAKBlood Lead Levels and Bio-markers of Lead Toxicity via the Consumption of Drinking Water in Kerou (Benin) in Watershed of the NigerInt J Environ Protect2012266
- YosipovitchGGreavesMWSchmelzMItchLancet2003361935869069412606187
- UmeuchiHTogashiYHondaTInvolvement of central mu-opioid system in the scratching behavior in mice, and the suppression of it by the activation of kappa-opioid systemEur J Pharmacol20034771293514512095
- KitchenILead toxicity and alterations in opioid systemsNeurotoxicology1993142–31151248247386
- McDowellJKitchenIPerinatal lead exposure alters the development of delta- but not mu-opioid receptors in rat brainBr J Pharmacol19889439339372846111
- VaziriNDMechanisms of lead-induced hypertension and cardiovascular diseaseAm J Physiol Heart Circ Physiol20082952H454H46518567711
- SivaranjaniNRaoSVRajeevGRole of reactive oxygen species and antioxidants in atopic dermatitisJ Clin Diagn Res20137122683268524551611
- DingYGonickHCVaziriNDLead promotes hydroxyl radical generation and lipid peroxidation in cultured aortic endothelial cellsAm J Hypertens2000135 Pt 155255510826409
- YagoHFujitaYKakuHStudy on pruritus in hemodialysis patients and the antipruritic effect of neurotropin: plasma levels of C3a, C5a, bradykinin and lipid peroxidesNihon Jinzo Gakkai Shi19893110106110672615018
- MassrySGPopovtzerMMCoburnJWMakoffDLMaxwellMHKleemanCRIntractable pruritus as a manifestation of secondary hyperparathyroidism in uremia. Disappearance of itching after subtotal parathyroidectomyN Engl J Med1968279136977005670911
- ChouFFHoJCHuangSCSheen-ChenSMA study on pruritus after parathyroidectomy for secondary hyperparathyroidismJ Am Coll Surg20001901657010625234
- ChuPWuCCChenCCLuKCParathyroidectomy leads to decreased blood lead levels in patients with refractory secondary hyperparathyroidismBone20125051032103822373954
- BerneBVahlquistAFischerTDanielsonBGBerneCUV treatment of uraemic pruritus reduces the vitamin A content of the skinEur J Clin Invest19841432032066432548
- CharlesworthENBeltraniVSPruritic dermatoses: overview of etiology and therapyAm J Med2002113Suppl 9A25S33S
- De MarchiSCecchinEVillaltaDSepiacciGSantiniGBartoliERelief of pruritus and decreases in plasma histamine concentrations during erythropoietin therapy in patients with uremiaN Engl J Med1992326159699741545849
- VyskocilASemeckyVFialaZCizkovaMViauCRenal alterations in female rats following subchronic lead exposureJ Appl Toxicol19951542572627594193
- BarrereFvan BlitterswijkCAde GrootKBone regeneration: molecular and cellular interactions with calcium phosphate ceramicsInt J Nanomedicine20061331733217717972
- BlachleyJDBlankenshipDMMenterAParkerTF3rdKnochelJPUremic pruritus: skin divalent ion content and response to ultraviolet phototherapyAm J Kidney Dis1985552372414003393
- DavenportAMurcuttGWhitingSCross-sectional audit of blood lead levels in regular outpatient haemodialysis patients dialysing in north LondonNephrology (Carlton)200914547648119674316
- LinJLLin-TanDTChenKHBlood lead levels association with 18-month all-cause mortality in patients with chronic peritoneal dialysisNephrol Dial Transplant20102551627163320031932
- VirgaGVisentinILa MiliaVBonadonnaAInflammation and pruritus in haemodialysis patientsNephrol Dial Transplant200217122164216912454228
- GoyerRALead toxicity: current concernsEnviron Health Perspect19931001771878354166
- SchwelaDAir pollution and health in urban areasRev Environ Health2000151–2134210939084
- LiouSHWuTNChiangHCThree-year survey of blood lead levels in 8828 Taiwanese adultsInt Arch Occup Environ Health199668280878720277
