Abstract
Childhood-onset growth hormone deficiency (CO-GHD) is an endocrine condition associated with a broad range of health issues from childhood through to adulthood, which requires particular attention during the transition period from adolescence to young adulthood. There is uncertainty in the clinical practice of the management of CO-GHD during transition regarding the clinical assessment and management of individual patients during and after transition to obtain optimal follow-up and improved health outcomes. Despite the availability of clinical guidelines providing the framework for transition of young adults with CO-GHD, there remains substantial variation in approaching transitional care among pediatric and adult services. A well-structured and coordinated transitional plan with clear communication and direct collaboration between pediatric and adult health care to ensure optimal management of adolescents with CO-GHD during transition is needed.
Introduction
Transition is a planned process that aims to address the wider set of medical, psychological and educational requirements of young adults with chronic conditions as they move from pediatric to adult health services.Citation1 Increased survival of children with complex health conditions has led to growing attention on the importance of the quality of services provided for adolescents during transition. Childhood-onset growth hormone deficiency (CO-GHD) is one of a number of complex endocrine conditions that may have an impact throughout life, starting in childhood and continuing into adulthood. Transition of patients with CO-GHD that have been treated with recombinant human growth hormone (rhGH) during childhood is a challenging phase for health care providers. This phase occurs in CO-GHD from mid-late teens until approximately 6 or 7 years after final height has been reached.Citation2 This is a critical period for maximizing bone mass and muscle strength, which are important determinants for the risk of fractures related to osteoporosis occurring later in life.Citation3 The presented data largely support the positive role of rhGH therapy during the transition period of adolescence and beyond.Citation4,Citation5 The effects of CO-GHD and the benefits of rhGH therapy during transition have been previously described in several studies.Citation6–Citation10 Although there are numerous publications that have discussed transition in general with some specifically discussing CO-GHD,Citation2,Citation6,Citation11 a number of unanswered issues still remain.
Current practice during transition
Several clinical practice guidelines have described the theoretical framework for the transition of adolescents with CO-GHD.Citation2,Citation12–Citation15 However, in the clinical practice setting, both adult and pediatric endocrinologists lack consistency when managing the adolescent patients with CO-GHD during transition. The clinical experience in Scotland highlighted various areas of uncertainty in clinical practice:Citation16 the best method of evaluating the hypothalamic–pituitary axis, identification of patients to test, determining which test to use for diagnosing persistent growth hormone deficiency (GHD), identifying which patients would most benefit from continued rhGH treatment and methods of ascertaining if patients should continue treatment. In addition, it is unclear with whom the responsibility for the biochemical reevaluation of GHD lies, the pediatric endocrinologist or adult endocrinologist who will decide whether to continue rhGH therapy into adulthood and the involvement of patients and family in making the decision and management plan. Practice varies across the UK, particularly in cases when no organic cause for GHD is identified.Citation16–Citation18
The current guidelines classify CO-GHD according to the likelihood of persistent GHD.Citation2,Citation12,Citation13 A previous history of organic disease, deficits in two or more additional pituitary hormones,Citation19,Citation20 hypothalamic–pituitary structural abnormalities as well as tumour-related organic GHD are strong predictors of persistent GHD.Citation21–Citation24 Patients who are considered having a high probability of persistent GHD may not require their growth hormone (GH) status to be reevaluated and should continue rhGH without interruption. Those who are less likely to have persistent GHD should undergo reevaluation of the GH axis before resuming rhGH replacement therapy.Citation2 Although there are clearly stated criteria under both categories, other factors such as clinical observations and development of symptoms could influence the decision of how the patients are categorized and managed. In addition, there is no clear reevaluation pathway for those who exhibit a discordant GH and IGF-1 pattern.
The timing of retesting is another issue. According to guidelines, the decision when to reassess depends largely on attainment of adult height as defined (height velocity <2 cm/ year). The recommended washout interval between stopping rhGH and retesting is from 1 to 3 months,Citation2 although there are no existing data on the optimal shortest interval without rhGH for retesting reliability and health parameters. There is some evidence, however, that the longer interval and delay in reinstituting rhGH may have an impact on optimizing skeletal outcomes in these subjects,Citation25 as well as other parameters, such as cardiovascular and lipid profiles.Citation26 Moreover, a longer period off rhGH during reevaluation may increase the risk of losing patients to follow-up.Citation27
GH stimulation tests are used to reevaluate GH levels taking into account the appropriate cutoff limits with various assay measurements.Citation28,Citation29 The insulin tolerance test (ITT) is considered the gold standard to reevaluate secretion of GH in these patients. Assuming adequate hypoglycemia is achieved, this test can distinguish GH deficiency from the sufficient GH secretion that is linked with obesity and normal aging. This test, however, is contraindicated in patients with epilepsy or heart disease. Given this precaution, the only alternative reliable stimulation tests that can be used are glucagon/growth hormone-releasing hormone (GHRH)+arginine, taking into account appropriate cutoff limits.Citation2
Cutoff levels for peak GH responses to stimulation tests during the transition period are still arbitrary; studies use a peak GH cutoff <5.1 µg/L,Citation23 <6.1 µg/LCitation30 or <5.6 µg/L.Citation31 The European Society for Pediatric Endocrinology recommended a cutoff <5 µg/L, using the ITT as an appropriate criterion for GH replacement during transition.Citation2 In CO-GHD where persistent GHD is highly likely, confirmation of persistent GHD can be made by a low IGF-I level alone (<−2 SD for age and gender) measured after at least 1 month off rhGH therapy, without the need for any further stimulation testing.Citation5 illustrates the current proposal workup of diagnosing and reevaluating CO-GHD patients during transition,Citation2 highlighting the areas of uncertainty.
Figure 1 Schema for assessing the GH/IGF-1 axis during the transition period.
Abbreviations: GH, growth hormone; GHD, growth hormone deficiency; MRI, magnetic resonance imaging; PBM, peak bone mass.
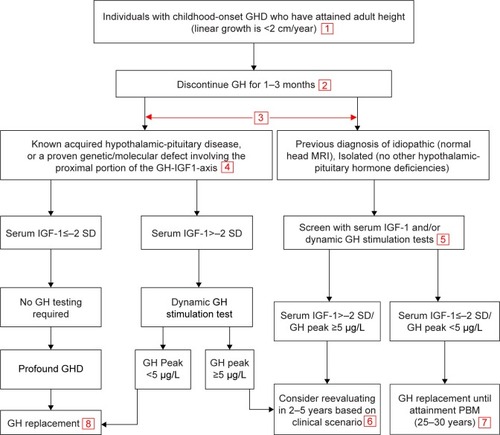
It is currently recommended to restart rhGH at a low dose (0.2–0.3 mg/day) and then titrate to attain IGF-1 levels that fall into the upper half of the normal range. The dose of rhGH is then adjusted during the transition to adult treatment, with young females receiving oral estrogen requiring higher rhGH doses. IGF-1 should be measured during dose adjustment at 1–2-month intervals and also a minimum of once a year during therapy in order for it to be kept at an age- and sex-appropriate level.Citation2
Benefits of rhGH during transition
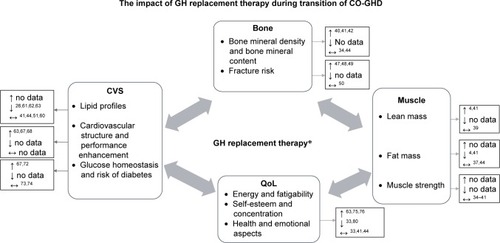
The broader benefits of rhGH for optimizing other health aspects during childhood and the period of the transition to adulthood are becoming more widely recognized ().Citation32,Citation33
Figure 2 Impact of GH replacement therapy during transition of CO-GHD.
Abbreviations: GH, growth hormone; CO-GHD, childhood-onset growth hormone deficiency; CVS, cardiovascular system; QoL, quality of life.
Bone and fracture risk
There is currently conflicting evidence on whether CO-GHD contributes to low bone mass and an increased risk of fracture in adulthood,Citation34–Citation36 although mechanisms and the pathophysiology of bone mass reduction are still far from fully understood. Indeed, many factors such as gender, height, age at onset, body composition, gonadal status, GHD severity and assessment methods may all contribute to inconsistent data on bone mass in patients with CO-GHD. Studies suggest that CO-GHD results in developmental bone mass deficits both at the time of diagnosisCitation37,Citation38 and at retesting when final height has been reached.Citation39 Reinstitution of rhGH replacement beyond the attainment of final height in young adults who have persistent CO-GHD has been reported to have a positive effect on bone density with a net benefit change in bone mineral density (BMD) of lumber spine ranging from 3% to 6% after 1 yearCitation35 or 2 years.Citation4,Citation40–Citation42 On the other hand, some studies did not find any change in BMD up to 2 years after either discontinuation of rhGHCitation43 or after reinstituting rhGH replacement therapy after final height has been reached.Citation34,Citation44 In addition, there are scarce data on the link between bone density and fracture risk in adolescents and young adults with CO-GHD that were observed in adults with GHD and hypopituitarism.Citation45,Citation46 Previous studies have reported increased fracture risk in adults with CO-GHDCitation47,Citation48 and only in women with CO-GHD in another study,Citation49 while another study did not find any difference in the risk of fracture in adolescents with CO-GHD compared with that in the normal population.Citation50 Therefore, there is no clear evidence that the CO-GHD results in low bone mass or increases the risk of fracture.
Body composition and muscle strength
During transition after withdrawal of childhood rhGH replacement therapy, several but not all studies have showed a significant decrease in lean mass (LM; −8%) parallel by increased fat mass (FM; 10%–17%) in patients with CO-GHD who were diagnosed with persistent GHD and had stopped rhGH for at least 2 years.Citation4,Citation34,Citation51,Citation52 Restarting rhGH therapy resulted in a notable improvement in body composition, with LM increasing by 14% and FM being reduced by 7% over 2 years of therapy.Citation4,Citation41 In terms of muscle strength, previous studies have reported a lower maximum isotonic strength in young adults with CO-GHD, as measured by hand grip force,Citation53,Citation54 but the effects on muscle strength in adolescents with CO-GHD during transition still remain to be completely elucidated.Citation55 There are some suggestions that reduced muscle mass was the cause of diminished muscle strength in GHD, as opposed to a reduction in contractile function.Citation56 Furthermore, it has been proposed that CO-GHD may initially result in a reduction in muscle mass and force, which would ultimately have an impact on bone density and geometry.Citation57 However, little research has been carried out into the relationship between muscle and bone strength in adult GHD patients,Citation58 and there has been no existing study targeting CO-GHD in children and adolescents.
Cardiovascular risks and glucose homeostasis
It has been reported in some studies that adolescents with CO-GHD have adverse lipid profiles after they discontinue rhGH treatment upon reaching final height,Citation26,Citation51,Citation52,Citation59 but not all studies.Citation41,Citation44,Citation51,Citation60 These parameters have been found to improve after restarting rhGH replacement therapy.Citation26,Citation61–Citation63 In addition, it has been noted that the longer the rhGH therapy is discontinued, the more abnormal the lipid profiles.Citation64 It is possible that the alterations in lipid profiles observed when rhGH is discontinued may be partly explained by the short-term effects of rhGH therapy and therefore could not be analyzed independently of treatment duration during childhood.Citation65 Studies of adults with GHD provided evidence for this assumption, revealing that only long periods of rhGH therapy (in the range of 5–10 years) were associated with improved lipid profiles in adult GHD patients.Citation66,Citation67 Further research is required to confirm this hypothesis in CO-GHD. In addition to aberrations in lipid profiles, direct impacts of CO-GHD on cardiac structure and function were reported in some echocardiographic studies. A study observed a decrease in all cardiac dimensions of adolescents with CO-GHD after withdrawal of rhGH during the transition period, when reinstituting rhGH results in a significant increase in left ventricular (LV) mass index and exercises capacity after 12–24 monthsCitation63,Citation68 with improvement in endothelial function within the first 6 months of restarting rhGH.Citation69
Regarding glucose homeostasis parameters, children with CO-GHD have been reported to be more insulin sensitive at the time of initial diagnosisCitation70 and after rhGH is withdrawn when they reach final height.Citation51,Citation71 However, adults with GHD have been found to have an increased likelihood of insulin resistance,Citation67,Citation72 but this is not the case for adolescents.Citation63 It is not clear whether changes in glucose homeostasis in these subjects can be attributed to GHD itself, body composition and adiposity or both, with no clear evidence for an increased risk of developing diabetes.Citation73,Citation74
Quality of life (QoL)
GH/IGF-1 axis may play a role in normal brain structure, cognitive function, psychology and thereby QoL.Citation75,Citation76 A study of children with isolated-GHD revealed a significant reduction in white matter, corpus callosum and neural volumes.Citation77 Given these findings, several clinical studies have been carried out to evaluate the effects of discontinuing and subsequently resuming rhGH treatment with regard to QoL elements in young adults with CO-GHD during transition from childhood to adulthood.
Some studies reported no changes in QoL in patients with CO-GHD, when measured at the time they discontinued rhGH after reaching final height and after 2 years remeasured while either off or resuming rhGH therapy.Citation33,Citation41,Citation44 Another study further reported no impairment in QoL in short-stature adolescents and children with GHD and without GHD,Citation78 which indicates that other unknown confounders could influence QoL other than height and GH levels.Citation79 On the other hand, other studies have stated that adolescent CO-GHD patients who had been treated with rhGH and then discontinued therapy after attaining height suffered from some cognitive and psychological impairment, particularly in domains of attention, memory, energy drive, emotional reactions and social isolation.Citation80,Citation81 These measures significantly improved when rhGH treatment was restarted. An inverse relationship has been reported between QoL and rhGH therapy duration; a longer period without rhGH was associated with a poorer QoL, whereas restarting rhGH treatment had a significantly positive effect on health-related QoL aspects.Citation64 There is also some evidence that discontinuing rhGH treatment resulted in decreased QoL within 6 months, which was improved in 3–6 months after recommencing rhGH therapy.Citation81,Citation82 Although individuals with GHD treated with rhGH reported having a higher self-esteem compared with short-stature and normal-stature children,Citation83,Citation84 the replacement of rhGH in adult CO-GHD patients resulted in less QoL improvement than in those with adult-onset GHD,Citation33 even after undergoing rhGH therapy for long-term periods of 4–10 years.Citation85
Challenges for future
The need for an appropriate transition service is increasingly recognized within health care systems. Generally, the purpose of transition is to offer adolescent patients continuity of care and promote mental and physical health through the exchange of information among health care workers in order to optimize their health and QoL aspects.Citation11 Although the existing guidelines have theoretically described how to reevaluate CO-GHD during transition, there is no clear pathway about how to organize and deliver transitional care from pediatric to adult services. Much of the transition literature has focused on the risk of untreated GHD during transition in terms of health and QoL outcomes; however, none of the studies have identified a clear transition plan, before and after reevaluation follow-up. Several studies have highlighted the risks of adolescents who drop out specialist endocrine care during the transition to adulthood care. The Scottish audit demonstrated 21% of patients stopped rhGH replacement without comprehensive reevaluation and 18% of patients did not attend follow-up while on treatment and never returned to endocrine clinics. This study has also highlighted issues about the follow-up of patients who no longer have GHD and particularly those patients with persistent GHD who have chosen not to resume adult rhGH treatment.Citation16 In another endocrine setting, transitional care was evaluated in patients with congenital adrenal hyperplasia reporting that 50% of the whole cohort were lost to follow-up following transfer to adult care,Citation86 with only 10% of the expected numbers still attending the adult endocrine service in another study.Citation87
The current transition of endocrine conditions including GHD has been reported to be of suboptimal quality in young adults with endocrine conditions.Citation88,Citation89 Such studies provide an insight into actual practice barriers and potential solutions for successful transition. The readiness of the patients and their caregivers, appropriate environmental setting and local resources were described as factors, which interfere with the precise format of the service and successful transition. There are various expert-based recommendations on how to improve transition and provide quality services for other chronic endocrine diseases, for example, type 1 diabetes mellitus,Citation90 turner syndrome,Citation91 and hypogonadismCitation92. Other reports suggest some strategies for successful transition including standardized referral systems and establishment of transition centers to ensure that the needs of young adults with CO-GHD are being met.Citation93,Citation94 In addition, for optimizing transitional care, it was recommended that pediatric and adult endocrinologists collaborate and share information while understanding that some overlap and a joint transition service would optimize the follow-up and transfer of adolescents,Citation94 although there are lack of data about patient attendance and follow-up after transition. The existing theoretical generic models have demonstrated structured processes of transitioning adolescents from pediatric to adult care.Citation95,Citation96 However, there appears to be clear difficulties in the feasibility of implementation of the all processes at every center and/or for every individual. Using the model designed by Gleeson et al, application of this model faces several challenges for effective transition of adolescents with CO-GHD as outlined in . In addition to the above highlighted areas of uncertainty in the schema of reevaluation and management of adolescents with CO-GHD, the model does not address issues concerning the readiness of patients and their caregiver to transition, transition clinic visits and frequency of appointments. The arrangement and duration to get the first adult care appointment, together with aspects to ensure the continuity of care, are also important factors. Therefore, there is a growing need to further review the current practices and develop more specific models for better planning and promoting the continuity of care during and after transition.
Table 1 Transition process from pediatric to adult care
Conclusion
Transition is a concept of a multidisciplinary approach that ensures continuity of health care while adolescents with complex health conditions are transferred from pediatric care to adult care. There is some evidence that recommencing rhGH treatment in CO-GHD during transition results in not only improved growth and bone health but also a better prognosis for metabolic and cardiovascular risks in the long term. Further studies are required on the current practice of assessing and managing CO-GHD, as well as research into the long-term follow-up of patients who have been confirmed to have persistent CO-GHD and continuous monitoring for optimizing somatic and psychological outcomes in patients. The major challenge during this stage, however, is appropriately identifying candidates for adult GH therapy. The current guidelines need to be reviewed, addressing areas of uncertainly as evidenced by variation in clinical practice. Hence, clearly a structured transition protocol is a vital key to establish the best practice of transitioning adolescents with CO-GHD.
Disclosure
MA was supported by the Government of Libya. SFA has received speaker fees from Novo Nordisk and Sandoz and consultancy fees from Pfizer. MGS has received speaker fees from Pfizer and educational grants from Novo Nordisk, Pfizer and Sandoz. The authors report no other conflicts of interest in this work.
References
- WillisERMcdonaghJETransition from children’s to adults’ services for young people using health or social care services (NICE Guideline NG43)Arch Dis Child Educ Pract Ed Epub20171221
- ClaytonPECuneoRCJuulAMonsonJPShaletSMTauberMEuropean Society of Paediatric Endocrinology. Consensus statement on the management of the GH-treated adolescent in the transition to adult careEur J Endocrinol2005152216517015745921
- RizzoliRBianchiMLGarabédianMMckayHAMorenoLAMaximizing bone mineral mass gain during growth for the prevention of fractures in the adolescents and the elderlyBone201046229430519840876
- AttanasioAFShavrikovaEBlumWFContinued growth hormone (GH) treatment after final height is necessary to complete somatic development in childhood-onset GH-deficient patientsJ Clin Endocrinol Metab200489104857486215472176
- MolitchMEGrowth hormone treatment in adults with growth hormone deficiency: the transitionJ Endocrinol Invest201134215015421270511
- Chamberlain SheaHLevyRATransition care of patients with growth hormone deficiency from pediatric endocrinologists to adult endocrinologistsEndocr Pract201218225626822068249
- NguyenVTMisraMTransitioning of children with GH deficiency to adult dosing: changes in body compositionPituitary200912212513518373203
- GleesonHTransition from Puberty to AdulthoodHoKGrowth Hormone Related Diseases and Therapy. Contemporary EndocrinologyNew YorkHumana Press2011187210
- AttanasioAFShaletSMGrowth hormone and the transition from puberty into adulthoodEndocrinol Metab Clin North Am200736118720117336740
- AhmidMPerryCGAhmedSFShaikhMGGrowth hormone deficiency during young adulthood and the benefits of growth hormone replacementEndocr Connect201653R1R1127129699
- GleesonHTurnerGTransition to adult servicesArch Dis Child Educ Pract Ed2012973869221979963
- RadovickSDivallSApproach to the growth hormone-deficient child during transition to adulthoodJ Clin Endocrinol Metab20079241195120017409338
- HoKKConsensus guidelines for the diagnosis and treatment of adults with GH deficiency II: a statement of the GH Research Society in association with the European Society for Pediatric Endocrinology, Lawson Wilkins Society, European Society of Endocrinology, Japan Endocrine Society, and Endocrine Society of AustraliaEur J Endocrinol2007157669570018057375
- CookDMRoseSRA review of guidelines for use of growth hormone in pediatric and transition patientsPituitary201215330131022271255
- GrimbergAAllenDBGrowth hormone treatment for growth hormone deficiency and idiopathic short stature: new guidelines shaped by the presence and absence of evidenceCurr Opin Pediatr201729446647128525404
- AhmidMFisherVGravelingAJAn audit of the management of childhood-onset growth hormone deficiency during young adulthood in ScotlandInt J Pediatr Endocrinol20162016626985190
- MurrayPGDattaniMTClaytonPEControversies in the diagnosis and management of growth hormone deficiency in childhood and adolescenceArch Dis Child201610119610026153506
- ChinoyAMurrayPGDiagnosis of growth hormone deficiency in the paediatric and transitional ageBest Pract Res Clin Endocrinol Metab201630673774727974187
- JuulAHolmKKastrupKWFree insulin-like growth factor I serum levels in 1430 healthy children and adults, and its diagnostic value in patients suspected of growth hormone deficiencyJ Clin Endocrinol Metab1997828249725029253324
- HartmanMLCroweBJBillerBMHoKKClemmonsDRChipmanJJHyposCCS Advisory BoardU.S. HypoCCS Study GroupWhich patients do not require a GH stimulation test for the diagnosis of adult GH deficiency?J Clin Endocrinol Metab200287247748511836272
- TillmannVTangVWPriceDAHughesDGWrightNBClaytonPEMagnetic resonance imaging of the hypothalamic-pituitary axis in the diagnosis of growth hormone deficiencyJ Pediatr Endocrinol Metab20001391577158311154153
- KalinaMAKalina-FaskaBGruszczyńskaKBaronJMałecka-TenderaEUsefulness of magnetic resonance findings of the hypothalamic-pituitary region in the management of short children with growth hormone deficiency: evidence from a longitudinal studyChilds Nerv Syst201228112112721935593
- BonfigWBechtoldSBachmannSReassessment of the optimal growth hormone cut-off level in insulin tolerance testing for growth hormone secretion in patients with childhood-onset growth hormone deficiency during transition to adulthoodJ Pediatr Endocrinol Metab200821111049105619189699
- QuigleyCAZagarAJLiuCCUnited States multicenter study of factors predicting the persistence of GH deficiency during the transition period between childhood and adulthoodInt J Pediatr Endocrinol201320131623406437
- TritosNAHamrahianAHKingDA longer interval without GH replacement and female gender are associated with lower bone mineral density in adults with childhood-onset GH deficiency: a KIMS database analysisEur J Endocrinol2012167334335122711759
- LanesRPaoliMCarrilloEVillaroelOPalaciosACardiovascular risk of young growth-hormone-deficient adolescents. Differences in growth-hormone-treated and untreated patientsHorm Res200360629129614646407
- CourtillotCBaudoinRDu SouichTMonocentric study of 112 consecutive patients with childhood onset GH deficiency around and after transitionEur J Endocrinol2013169558759623939920
- de BoerHvan der VeenEAWhy retest young adults with childhood-onset growth hormone deficiency?J Clin Endocrinol Metab1997827203220369215268
- GascoVCorneliGBeccutiGRetesting the childhood-onset GH-deficient patientEur J Endocrinol2008159Suppl 11S45S5218805914
- MaghnieMAimarettiGBelloneSDiagnosis of GH deficiency in the transition period: accuracy of insulin tolerance test and insulin-like growth factor-I measurementEur J Endocrinol2005152458959615817915
- SeccoAdi IorgiNNapoliFReassessment of the growth hormone status in young adults with childhood-onset growth hormone deficiency: reappraisal of insulin tolerance testingJ Clin Endocrinol Metab200994114195420419837937
- ClaytonPGleesonHMonsonJPopovicVShaletSMChristiansenJSGrowth hormone replacement throughout life: insights into age-related responses to treatmentGrowth Horm IGF Res200717536938217560153
- AttanasioAFShavrikovaEPBlumWFShaletSMQuality of life in childhood onset growth hormone-deficient patients in the transition phase from childhood to adulthoodJ Clin Endocrinol Metab20059084525452915899956
- BootAMvan der SluisIMKrenningEPde Muinck Keizer-SchramaSMBone mineral density and body composition in adolescents with childhood-onset growth hormone deficiencyHorm Res200971636437119506395
- DrakeWMCarrollPVMaherKTThe effect of cessation of growth hormone (GH) therapy on bone mineral accretion in GH-deficient adolescents at the completion of linear growthJ Clin Endocrinol Metab20038841658166312679453
- AttanasioAFHowellSBatesPCBody composition, IGF-I and IGFBP-3 concentrations as outcome measures in severely GH-deficient (GHD) patients after childhood GH treatment: a comparison with adult onset GHD patientsJ Clin Endocrinol Metab20028773368337212107251
- BootAMEngelsMABoermaGJKrenningEPde Muinck Keizer-SchramaSMKeizerschramaSChanges in bone mineral density, body composition, and lipid metabolism during growth hormone (GH) treatment in children with GH deficiencyJ Clin Endocrinol Metab1997828242324289253311
- SchweizerRMartinDDHaaseMSimilar effects of long-term exogenous growth hormone (GH) on bone and muscle parameters: a pQCT study of GH-deficient and small-for-gestational-age (SGA) childrenBone200741587588117826368
- AimarettiGCorneliGRovereSCroceCGGhigoEProcopioMIs GH therapy useful to preserve bone mass in transition-phase patients with GH deficiency?J Endocrinol Invest20052810 Suppl2832
- ConwayGSSzarras-CzapnikMRaczKTreatment for 24 months with recombinant human GH has a beneficial effect on bone mineral density in young adults with childhood-onset GH deficiencyEur J Endocrinol2009160689990719324976
- UnderwoodLEAttieKMBaptistaJGenentech Collaborative Study GroupGrowth hormone (GH) dose-response in young adults with childhood-onset GH deficiency: a two-year, multicenter, multiple-dose, placebo-controlled studyJ Clin Endocrinol Metab200388115273528014602761
- ShaletSMShavrikovaECromerMEffect of growth hormone (GH) treatment on bone in postpubertal GH-deficient patients: a 2-year randomized, controlled, dose-ranging studyJ Clin Endocrinol Metab20038894124412912970274
- ForsHBjarnasonRWirénLCurrently used growth-promoting treatment of children results in normal bone mass and density. A prospective trial of discontinuing growth hormone treatment in adolescentsClin Endocrinol2001555617624
- MaurasNPescovitzOHAlladaVMessigMWajnrajchMPLippeBTransition Study GroupLimited efficacy of growth hormone (GH) during transition of GH-deficient patients from adolescence to adulthood: a phase III multicenter, double-blind, randomized two-year trialJ Clin Endocrinol Metab20059073946395515855257
- WüsterCAbsRBengtssonBAKIMS Study Group and the KIMS International BoardPharmacia & Upjohn International Metabolic Database. The influence of growth hormone deficiency, growth hormone replacement therapy, and other aspects of hypopituitarism on fracture rate and bone mineral densityJ Bone Miner Res200116239840511204440
- RosenTWilhelmsenLLandin-WilhelmsenKLappasGBengtssonBIncreased fracture frequency in adult patients with hypopituitarism and GH deficiencyEur J Endocrinol199713732402459330587
- BouillonRKoledovaEBezlepkinaOBone status and fracture prevalence in Russian adults with childhood-onset growth hormone deficiencyJ Clin Endocrinol Metab200489104993499815472196
- AbsRMattssonAFBengtssonBAKIMS Study GroupIsolated growth hormone (GH) deficiency in adult patients: baseline clinical characteristics and responses to GH replacement in comparison with hypopituitary patients. A sub-analysis of the KIMS databaseGrowth Horm IGF Res200515534935916168692
- HolmerHSvenssonJRylanderLFracture incidence in GH-deficient patients on complete hormone replacement including GHJ Bone Miner Res200722121842185017725379
- BaroncelliGIBertelloniSSodiniFSaggeseGLumbar bone mineral density at final height and prevalence of fractures in treated children with GH deficiencyJ Clin Endocrinol Metab20028783624363112161486
- CarrollPVDrakeWMMaherKTComparison of continuation or cessation of growth hormone (GH) therapy on body composition and metabolic status in adolescents with severe GH deficiency at completion of linear growthJ Clin Endocrinol Metab20048983890389515292323
- BechtoldSBachmannSPutzkerSDalla PozzaRSchwarzHPEarly changes in body composition after cessation of growth hormone therapy in childhood-onset growth hormone deficiencyJ Clin Densitom201114447147721723762
- SartorioANariciMContiAMonzaniMFagliaGQuadriceps and hand-grip strength in adults with childhood-onset growth hormone deficiencyEur J Endocrinol1995132137417850008
- SartorioAAgostiFde ColAMuscle Strength and Power, Maximum Oxygen Consumption, and Body Composition in Middle-Aged Short-stature Adults with Childhood-onset Growth Hormone DeficiencyArch Med Res2008391788318067999
- ModestoMJAmerNMErichsenOMuscle strength and body composition during the transition phase in patients treated with recombinant GH to final heightJ Pediatr Endocrinol Metab2014279–1081382024756044
- ChikaniVHoKKYKkHAction of GH on skeletal muscle function: molecular and metabolic mechanismsJ Mol Endocrinol2014521R107R12324163428
- HöglerWShawNChildhood growth hormone deficiency, bone density, structures and fractures: scrutinizing the evidenceClin Endocrinol2010723281289
- KlefterOFeldt-RasmussenUIs increase in bone mineral content caused by increase in skeletal muscle mass/strength in adult patients with GH-treated GH deficiency? A systematic literature analysisEur J Endocrinol2009161221322119439507
- CapalboDEspositoAdi MaseRUpdate on early cardiovascular and metabolic risk factors in children and adolescents affected with growth hormone deficiencyMinerva Endocrinol201237437938923235193
- AttanasioAFHowellSBatesPCConfirmation of severe GH deficiency after final height in patients diagnosed as GH deficient during childhoodClin Endocrinol2002564503507
- MeazzaCElsedfyHHPaganiSBozzolaEEl KholyMBozzolaMMetabolic parameters and adipokine profile in growth hormone deficient (GHD) children before and after 12-month GH treatmentHorm Metab Res201446321922324297484
- CapalboDMattace RasoGEspositoACluster of cardiometabolic risk factors in children with GH deficiency: a prospective, case-control studyClin Endocrinol2014806856862
- ColaoAdi SommaCPivonelloRThe cardiovascular risk of adult GH deficiency (GHD) improved after GH replacement and worsened in untreated GHD: a 12-month prospective studyJ Clin Endocrinol Metab20028731088109311889170
- Kołtowska-HäggströmMGeffnerMEJönssonPDiscontinuation of growth hormone (GH) treatment during the transition phase is an important factor determining the phenotype of young adults with nonidiopathic childhood-onset GH deficiencyJ Clin Endocrinol Metab20109562646265420335451
- ProdamFSavastioSGenoniGEffects of growth hormone (GH) therapy withdrawal on glucose metabolism in not confirmed GH deficient adolescents at final heightPLoS One201491e8715724498035
- MaisonPGriffinSNicoue-BeglahMHaddadNBalkauBChansonPMetaanalysis of Blinded, Randomized, Placebo-Controlled Trials. Impact of growth hormone (GH) treatment on cardiovascular risk factors in GH-deficient adults: a Metaanalysis of Blinded, Randomized, Placebo-Controlled TrialsJ Clin Endocrinol Metab20048952192219915126541
- GazzarusoCGolaMKaramouzisIGiubbiniRGiustinaACardiovascular risk in adult patients with growth hormone (GH) deficiency and following substitution with GH – an updateJ Clin Endocrinol Metab2014991182924217903
- FeinbergMSScheinowitzMLaronZCardiac dimension and function in patients with childhood onset growth hormone deficiency, before and after growth hormone retreatment in adult ageAm Heart J2003145354955312660681
- AmatoGCarellaCFazioSBody composition, bone metabolism, and heart structure and function in growth hormone (GH)-deficient adults before and after GH replacement therapy at low dosesJ Clin Endocrinol Metab199377167116768263158
- HusbandsSOngKKGilbertJWassJADungerDBIncreased insulin sensitivity in young, growth hormone deficient childrenClin Endocrinol20015518792
- NørrelundHVahlNJuulAContinuation of growth hormone (GH) therapy in GH-deficient patients during transition from childhood to adulthood: impact on insulin sensitivity and substrate metabolismJ Clin Endocrinol Metab20008551912191710843174
- GiavoliCPorrettiSRonchiCLLong-term monitoring of insulin sensitivity in growth hormone-deficient adults on substitutive recombinant human growth hormone therapyMetabolism200453674074315164321
- ChildCJZimmermannAGScottRSCutlerGBBattelinoTBlumWFGeNeSIS International Advisory BoardPrevalence and incidence of diabetes mellitus in GH-treated children and adolescents: analysis from the GeNeSIS observational research programJ Clin Endocrinol Metab2011966E1025E103421490076
- CutfieldWSWiltonPBennmarkerHIncidence of diabetes mellitus and impaired glucose tolerance in children and adolescents receiving growth-hormone treatmentLancet2000355920461061310696981
- NybergFHallbergMGrowth hormone and cognitive functionNat Rev Endocrinol20139635736523629538
- Schneider-RivasSRivas-ArancibiaSVázquez-PereyraFVázquez-SandovalRBorgonio-PérezGModulation of long-term memory and extinction responses induced by growth hormone (GH) and growth hormone releasing hormone (GHRH) in ratsLife Sci19955622PL433PL4417746092
- WebbEAO’ReillyMAClaydenJDEffect of growth hormone deficiency on brain structure, motor function and cognitionBrain2012135121622722120144
- BullingerMKołtowska-HäggströmMSandbergDHealth-related quality of life of children and adolescents with growth hormone deficiency or idiopathic short stature – part 2: available results and future directionsHorm Res2009722748119690424
- EiserCMorseRQuality-of-life measures in chronic diseases of childhoodHealth Technol Assess2001541157
- WirénLJohannssonGBengtssonBAA prospective investigation of quality of life and psychological well-being after the discontinuation of GH treatment in adolescent patients who had GH deficiency during childhoodJ Clin Endocrinol Metab20018683494349811502769
- StouthartPJDeijenJBRoffelMDelemarrevan de WaalHAQuality of life of growth hormone (GH) deficient young adults during discontinuation and restart of GH therapyPsychoneuroendocrinology200328561262612727130
- van NieuwpoortICDrentMLCognition in the adult with childhood-onset GH deficiencyEur J Endocrinol2008159Suppl 1S53S5718787050
- GeislerALassNReinschNQuality of life in children and adolescents with growth hormone deficiency: association with growth hormone treatmentHorm Res Paediatr2012782949922907471
- ChaplinJEKriströmBJonssonBImprovements in behaviour and self-esteem following growth hormone treatment in short prepubertal childrenHorm Res Paediatr201175429130321304250
- SpielhagenCSchwahnCMöllerKThe benefit of long-term growth hormone (GH) replacement therapy in hypopituitary adults with GH deficiency: results of the German KIMS databaseGrowth Horm IGF Res201121111021093334
- GleesonHDavisJJonesJO’SheaEClaytonPEThe challenge of delivering endocrine care and successful transition to adult services in adolescents with congenital adrenal hyperplasia: experience in a single centre over 18 yearsClin Endocrinol20137812328
- ArltWWillisDSWildSHUnited Kingdom Congenital Adrenal Hyperplasia Adult Study Executive (CaHASE)Health status of adults with congenital adrenal hyperplasia: a cohort study of 203 patientsJ Clin Endocrinol Metab201095115110512120719839
- Hokken-KoelegaAvan der LelyA-JHauffaBBridging the gap: metabolic and endocrine care of patients during transitionEndocr Connect201656R44R5427803155
- GleesonH‘Part of the problem, part of the solution’ – adult physicians’ role in adolescent and young adult healthClin Med2015155413414
- PetersALaffelLAmerican Diabetes Association Transitions Working GroupDiabetes Care201134112477248522025785
- LucaccioniLWongSCSmythATurner syndrome – issues to consider for transition to adulthoodBr Med Bull20151131455825533182
- DwyerAAPhan-HugFHauschildMElowe-GruauEPitteloudNTransition in endocrinology: Hypogonadism in adolescenceEur J Endocrinol20151731R15R2425653257
- CrowleyRWolfeILockKMckeeMImproving the transition between paediatric and adult healthcare: a systematic reviewArch Dis Child201196654855321388969
- DowningJGleesonHKClaytonPEDavisJRWalesJKCalleryPTransition in endocrinology: the challenge of maintaining continuityClin Endocrinol20137812935
- GleesonHMccartneySLidstoneV‘Everybody’s business’: transition and the role of adult physiciansClin Med2012126561567
- KennedyASawyerSTransition from pediatric to adult services: are we getting it right?Curr Opin Pediatr200820440340918622194
