Abstract
Naftopidil, approved only in Japan, is an α1-adrenergic receptor antagonist (α1-blocker) used to treat lower urinary tract symptoms (LUTS) suggestive of benign prostatic hyperplasia (BPH). Different from tamsulosin hydrochloride and silodosin, in that it has higher and extremely higher affinity respectively, for the α1A-adrenergic receptor subtype than for the α1D type, naftopidil has distinct characteristics because it has a three times greater affinity for the α1D-adrenergic receptor subtype than for the α1A subtype. Although well-designed large-scale randomized controlled studies are lacking and the optimal dosage of naftopidil is not always completely determined, previous reports from Japan have shown that naftopidil has superior efficacy to a placebo and comparable efficacy to other α1-blockers such as tamsulosin. On the other hand, the incidences of ejaculatory disorders and intraoperative floppy iris syndrome induced by naftopidil may be lower than for tamsulosin and silodosin having high affinity for the α1A-adrenergic receptor subtype. However, it remains unknown if the efficacy and safety of naftopidil in Japanese is applicable to white, black and Hispanic men having LUTS/BPH in western countries.
Introduction
Benign prostatic hyperplasia (BPH) is a histological condition that is commonly observed in elderly men.Citation1 BPH is an adenoma composed of a mixture of increased numbers of epithelial and/or stromal cells. Benign enlargement of the prostate (BPE) due to BPH induces bladder outlet obstruction (BOO) and results in the development of lower urinary tract symptoms (LUTS). There are two mechanisms involved in the development of BOO: mechanical obstruction due to increased volume of the prostate; and functional obstruction due to increased tone of the prostatic smooth muscle. In addition, BOO secondarily affects bladder function. Either detrusor overactivity during the filling phase or detrusor underactivity during the voiding phase, or a combination of both conditions, modifies LUTS in elderly men with BOO.
There are two mainstays of medical treatment for LUTS suggestive of BPH (LUTS/BPH): 5α-reductase inhibitors (5ARI); and α1-adrenergic receptor (AR) antagonists (α1-blockers).Citation2 5ARI (finasteride, dutasteride) improve BOO through reduction of the prostate volume and contribute to gradual improvement of LUTS and long-term inhibition of disease progression. On the other hand, α1-blockers improve BOO through a decrease of tone of the prostatic smooth muscle. Several α1-blockers are clinically available, including those having nonspecific affinity for α1-AR subtypes (prazosin, terazosin, doxazosin, alfuzosin) and those having specific affinity for them (tamsulosin, naftopidil, silodosin). Although several large-scale studiesCitation3–Citation5 have demonstrated recently that combination of a 5 ARI and α1-blocker is superior to either 5 ARI or α1-blocker monotherapy in terms of improvement of subjective and objective urinary symptoms as well as the long-term inhibition of BPH-related events such as acute urinary retention and conversion to surgical treatment, α1-blockers are still essential as first-line medical treatments for patients having LUTS/BPH with problems, because symptomatic improvement can be achieved rapidly with α1-blockers.
Naftopidil, with three times greater affinity for α1D than for the α1A-AR subtype, is an α1-blocker that has been approved for clinical use for LUTS/BPH only in Japan since 1999.Citation6 Two Japanese clinical guidelines, the Clinical Guideline for Male Lower Urinary Tract SymptomsCitation7 and Clinical Guideline for Benign Prostatic Hyperplasia,Citation8 recommend the use of naftopidil for male LUTS/BPH as a grade A recommendation. At present, naftopidil has the second highest use, following tamsulosin, in Japan. However, due to the limited number of English reports on naftopidil and doctors being unable to use it in other countries, the clinical characteristics of this drug have not been sufficiently introduced. Although the English literature on naftopidil up to January 2009 was comprehensively summarized in a review article written by Garimella et al,Citation9 several important studies written in Japanese were missed because only reports published in English were reviewed. In this article, the efficacy and safety of naftopidil for LUTS/BPH are reviewed based on the English literature published up to March 2011 as well as several important Japanese reports such as the results of a randomized placebo-controlled trial conducted in Japan.
Pharmacological characteristics of naftopidil
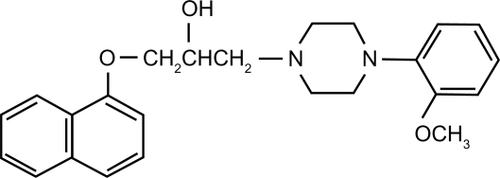
Naftopidil ((±)-1-[4-(2-methoxyphenyl)piperazinyl]-3-(1-naphthyloxy) propan-2-ol, ) is an α1-blocker produced by Boehringer Mannheim, Germany. In vitro study has shown that naftopidil binds to α1-AR in human BPH tissue.Citation10 It has a threefold higher affinity for α1D than for the α1A-AR subtype, whereas tamsulosin and silodosin have threefold and 56-fold higher affinities for the α1A-AR subtype than for the α1D subtype, respectively ().Citation6,Citation11 In an in vivo study using anesthetized male mongrel dogs, not only prostatic pressure but also blood pressure increased by phenylephrine was significantly decreased by intravenous administration of naftopidil in a dose-dependent manner. The inhibition of prostatic pressure was more remarkable than that of blood pressure.Citation6 Thus, as it appeared to be a promising α1-blocker with selectivity for the prostate, a phase I clinical study of naftopidil was conducted.Citation12 Naftopidil, 12.5 to 100 mg, was given to 15 healthy adult Japanese male volunteers. With a single administration of 50 mg of naftopidil after food, the means ± standard deviations of Tmax (hour), Cmax (ng/mL), T1/2β (hour), AUC0–∞ (ng · hour/mL) and Clt (L/hour) were 2.20 ± 1.04, 58.6 ± 24.2, 13.2 ± 5.4, 311.6 ± 54.3 and 164.2 ±26.9, respectively. The recovery of the intact form of naftopidil in urine was not more than 0.01%. Although there were no abnormal findings in clinical laboratory tests, 4 of the 5 subjects who received a single administration of 100 mg of naftopidil reported headaches, cold sweats, facial pallor, sleepiness and postural hypotension. Naftopidil is excreted into bile after being primarily metabolized by CYP2C9 and CYP3A4 in the liver microsomes.Citation13
Table 1 Affinities of naftopidil, tamsulosin and silodosin to cloned human α1-adrenergic receptorsCitation6,Citation11
Rationale for naftopidil treatment of LUTS suggestive of BPH
Different from tamsulosin and silodosin, which have higher and extremely higher affinity for the α1A-AR subtype than for the α1D-AR subtype, respectively, naftopidil has distinct characteristics because it has threefold affinity for the α1D subtype than for the α1A-AR subtype.Citation6,Citation11 Since the tissue of BPH shows nine- and threefold increased expression of mRNA of α1A and α1D-AR subtypes, respectively, compared to normal prostatic tissue,Citation14 it has been speculated that not only α1A but also α1D-AR contributes to contraction of prostatic smooth muscle. Recently, an interesting observation was reported by Kojima et al.Citation15 The expression of mRNA of the α1-AR subtype in the prostate biopsy specimens of 61 patients having LUTS/BPH was investigated using quantitative reverse transcriptase-polymerase chain reaction. Of the 61 prostates, 34 (55.7%) and 27 (44.3%) had dominant expression of α1a and α1d, respectively. As discussed in detail later, tamsulosin and naftopidil were more effective in patients with dominant expression of α1a and α1d, respectively. Thus, in approximately half of the patients having LUTS/BPH, the efficacy of the α1D/α1A blocker naftopidil may be more promising than that of the α1A/α1D blocker tamsulosin.
The α1D-AR subtype may be involved in detrusor overactivity associated with BOO causing overactive bladder syndrome (OAB), since increased expression of mRNA of the α1D-AR subtype in human detrusor muscle and human lumbosacral spinal cord has been demonstrated.Citation16,Citation17 Several animal studies using rats and mice have demonstrated that the micturition reflex is facilitated by the α1D-AR subtype on urothelial cells.Citation18,Citation19 In addition, it has been demonstrated that naftopidil has an inhibitory effect on C-fiber afferents because the drug increases bladder capacity in rats with cerebral infarction, but not in C-fiber desensitized rats with cerebral infarction.Citation20 Thus, naftopidil, with a higher affinity for the α1D-AR subtype, may contribute to improving detrusor overactivity through the blockade of the α1D-AR subtype not only in the prostate but also in nonprostatic organs.
Dose-finding study and randomized placebo-controlled study conducted in Japan
After the phase I clinical study,Citation12 a late phase II clinical study was conducted to determine the efficacy, safety and optimal dosage of naftopidil.Citation21 In the study, naftopidil was given to 133 patients with LUTS/BPH. After a 1-week run-in period using a placebo, 25 mg/day naftopidil was given for 2 weeks, then the dosage was escalated to 50 mg/day and given for the next 2 weeks. If the efficacy of 50 mg/day naftopidil was insufficient based on the assessment of investigators, the dosage was escalated to 75 mg/day and given for an additional 2 weeks (n = 47). Dose-dependent improvements of LUTS and the maximum flow rate (Qmax) were observed. Although 3 patients (2.3%) reported adverse events, no serious adverse events were observed. The buzzing or lightheadedness in 2 patients with 25 mg/day naftopidil, and hypotension in 1 patient with 50 mg/day naftopidil spontaneously disappeared after termination or dose reduction of naftopidil. Thus, the optimal dosage is thought to be 25 mg to 75 mg/day.
Yasuda et al evaluated the urodynamic effect of naftopidil using a pressure-flow study.Citation22 Naftopidil, 25 to 75 mg/day, was given to 32 patients aged 52 to 78 years (mean 66.3 years) with LUTS/BPH for 4 to 6 weeks. Before and after treatment, uroflowmetry, determination of post-void residual urine (PVR), urethral pressure profile, filling cystometry and pressure-flow study were performed. The mean Qmax (n = 28) and PVR (n = 28) significantly increased from 9.9 to 14.3 mL/second (P < 0.001) and decreased from 48.1 to 19.3 mL (P < 0.05), respectively. The mean urethral pressure profile (n = 14) and cystometry (n = 23) showed a significant decrease of maximum urethral closure pressure from 69.0 to 58.8 cm H2O (P < 0.05) and an increase of the first desire to void from 193.5 to 238.7 mL (P < 0.05), respectively. Although the mean opening pressure and pressure at the maximum flow rate in the pressure-flow study (n = 14) did not change significantly, from 59.9 to 71.9 cm H2O and 73.5 to 71.5 cm H2O, respectively, the mean minimum urethral resistance significantly decreased from 1.7 to 0.9 (P < 0.05). Thus, it is likely that naftopidil urodynamically improves BOO induced by BPH.
A double-blind randomized placebo-controlled trial was conducted between 1993 and 1995 in Japan.Citation23 Although the results were reported in Japanese in 1997, it remains the only randomized placebo-controlled trial. Based on the results of a double-blind study comparison with prazosin hydrochloride,Citation24 naftopidil was approved by the Japanese Regulatory Authority for treatment for LUTS/BPH in 1999. In the double-blind randomized placebo-controlled trial,Citation23 333 patients having LUTS/BPH were randomly allocated into 4 groups after at least a 1-week run-in period using a placebo, a placebo for 4 weeks (placebo group, n = 79), 25 mg/day naftopidil for 4 weeks (25 mg group, n = 86), 25 mg/day naftopidil for 1 week followed by 50 mg/day naftopidil for 3 weeks (50 mg group, n = 86), and 25 mg/day naftopidil for 1 week followed by 75 mg/day naftopidil for 3 weeks (75 mg, n = 82). Although changes in LUTS were not evaluated by the International Prostatic Symptom Score (IPSS) because it was not validated in Japanese at that time, improvements of the subjective urinary symptoms and Qmax in the 50 mg and the 75 mg groups were significantly superior to the placebo group. There was no significant difference in the development of adverse events among the groups. Thus, 50 mg and 75 mg of naftopidil were recommended as the standard dosages for treatment of LUTS/BPH in Japan.
Randomized comparative studies of naftopidil compared with other α1-blockers and phytotherapy
There are 8 randomized studies of naftopidil compared with other α1-blockers (tamsulosin in 6, tansulosin and silodosin in 1) and phytotherapy (1) in the contemporary English literature using the IPSS for evaluation of LUTS after 2003 ().Citation25–Citation32 They consist of various inclusion criteria, study designs, treatment durations and analytical methods. A crossover design was applied to 3 studies.Citation25,Citation28,Citation29 Most of the studies recruited small numbers of patients without provision of the required sample size for the hypothesis test.
Table 2 Contemporary randomized trials of naftopidil for LUTS/BPH conducted in Japan
Ikemoto et al reported the efficacy of tamsulosin and naftopidil for 96 patients having LUTS/BPH with IPSS ≥ 8 and Qmax < 12 mL/second.Citation25 The 96 patients were randomly allocated into 2 groups. The naftopidil-to-tamsulosin group (N-T group, n = 43) received 50 mg/day naftopidil for 8 weeks (25 mg/day for the first 2 weeks) immediately followed by 0.2 mg/day tamsulosin, which is the approved optimal dosage for treatment of LUTS suggestive of BPH in Japan, for 8 weeks. The tamsulosin-to-naftopidil group (T-N group, n = 53) received 0.2 mg/day tamsulosin for 8 weeks and then 50 mg/day naftopidil for 8 weeks (25 mg/day for the first 2 weeks) without a washout period between the 2 drugs. Twelve (28%) and 18 patients (34%) in the N-T and the T-N groups were withdrawn for several reasons including non-attendance at hospital appointments and adverse events. There was no significant difference in the incidence of adverse events between the 2 groups (3% versus 2%). The mean IPSS significantly decreased from 17.0 at baseline to 8.5 at 16 weeks and 17.5 at baseline to 9.2 at 16 weeks in the N-T and the T-N groups, respectively. Storage symptoms such as daytime frequency, urgency, and nocturia were significantly improved only by naftopidil monotherapy, whereas voiding symptoms such as intermittency and straining were improved only by tamsulosin monotherapy. The QOL index and Qmax showed similar improvements in both groups. In 28 patients who reported “not effective” or “worse” for the initial treatment, the IPSS was improved by crossover to the other drug in 10 of the 12 patients in the N-T group and 16 of the 16 patients in the T-N group. Thus, their study demonstrated that a second α1-blocker may be effective even if the first α1-blocker is ineffective.
Yamanishi et al compared the clinical and urodynamic effects of naftopidil to those of phytotherapy with eviprostat.Citation26 Forty-nine patients (mean age 67.9 years) having LUTS/BPH with IPSS ≥8, Qmax ≤12 mL/second, prostate volume (PV) ≥ 15 mL, and an obstructive or equivocal condition in a pressure-flow study were included in the study. After a 1-week run-in period, the patients were randomly allocated into naftopidil (n = 36) and eviprostat (n = 13) groups at the ratio 3:1. In the naftopidil group, naftopidil was given at a dose of 25 mg/day for 2 weeks, followed by 50 mg/day for 2 weeks, and then 75 mg/day for another 2 weeks if the patients were not satisfied with the improvement of LUTS. In the other group with eviprostat, which is a mixture of plant extracts from Phila umbellata, Populus tremula and Pulsatilla pratensis, and wheat-germ oil, 6 tablets/day were given for 6 weeks. Symptomatic improvements evaluated by the IPSS and QOL index were significantly better in the naftopidil group than in the eviprostat group. Although improvement of Qmax tended to be better in the naftopidil group, there was no significant difference (P = 0.0886). In the pressure-flow study, there was no improvement of detrusor pressure at the maximum flow rate by the treatments (naftopidil group, −8.9 cm H2O, P = 0.0865; eviprostat group, −10.2 cm H2O, P = 0.2015). There was no significant difference in the change of detrusor pressure at the maximum flow rate between the groups (P = 0.8879). On the other hand, the Abrams-Griffiths number was significantly decreased by naftopidil treatment (−14.6, P = 0.0422) but not by eviprostat treatment (−10.8, P = 0.1441), although no significant intergroup difference was observed (P = 0.7262). In the 19 patients having detrusor overactivity at baseline, it disappeared in 3 of the 14 patients (21%) in the naftopidil group but in none of the 5 patients in the eviprostat group. Thus, naftopidil was more effective than eviprostat in terms of symptomatic improvement of LUTS/BPH.
Gotoh et al compared the efficacy and safety of naftopidil and tamsulosin in a multicenter randomized trial.Citation27 Men aged ≥50 years having LUTS/BPH with IPSS ≥8, Qmax < 15 mL/second, a voided volume of ≥150 mL, and PV ≥ 20 mL, were enrolled in the study. Of the 185 enrolled patients, 144 were eligible for efficacy analysis and randomly allocated into groups receiving 0.2 mg/day tamsulosin for 12 weeks or 25 mg/day naftopidil for 2 weeks followed by 50 mg/day for 10 weeks. The IPSS and QOL index significantly improved in both groups (IPSS, −8.4 in the tamsulosin group, P < 0.001, −5.9 in the naftopidil group, P < 0.001; QOL index, −1.4 in the tamsulosin group, P < 0.001, −1.3 in the naftopidil group, P < 0.001). There was no significant difference in the change of the IPSS between the groups at 12 weeks (P = 0.060), although the change of the IPSS in the tamsulosin group tended to be larger than with naftopidil. No intergroup differences were observed in any IPSS index after treatment. Qmax was significantly improved by 2.1 mL/second in both groups (P < 0.001 and P = 0.001) and no intergroup difference after treatment was observed (P = 0.709). The adverse events were comparable, with no significant differences in systolic and diastolic blood pressure after treatment between the groups. Thus, the authors concluded that 50 mg of naftopidil was as effective and safe as 0.2 mg of tamsulosin.
Nishino et al investigated the efficacies of naftopidil and tamsulosin in a crossover design.Citation28 Thirty-four patients (mean age 72.4 years) having LUTS/BPH with IPSS ≥ 8 and Qmax < 15 mL/second were enrolled in the study. Seventeen patients were initially prescribed 50 mg/day naftopidil for 4 weeks, followed by 0.2 mg/day tamsulosin for 4 weeks after a 1-week washout period. For another 17 patients, tamsulosin was initially prescribed followed by 1 week of washout, and then naftopidil. Thus, all 34 patients received both naftopidil for 4 weeks and tamsulosin for 4 weeks. There was no significant difference in the mean IPSS after treatment between naftopidil (8.9) and tamsulosin monotherapies (9.3, P = 0.265). Although there was no significant difference in the mean IPSS voiding symptom subscores after treatment between the groups (3.1 versus 2.6, P = 0.134), the mean IPSS storage symptom subscore after treatment was higher with tamsulosin monotherapy (5.5) than with naftopidil monotherapy (4.8, P = 0.007). Of the storage symptoms after treatment, only nocturia showed a significant difference between the groups (2.3 versus 3.1, P < 0.001). Although no significant difference in the mean Qmax after treatment was observed between naftopidil monotherapy (13.5 mL/second) and tamsulosin monotherapy (13.2 mL/second), the mean detrusor pressure at the maximum flow rate in the pressure-flow study after treatment was lower for tamsulosin monotherapy (61.2 cm H2O) than for naftopidil monotherapy (64.3 cm H2O, P = 0.002). On the other hand, the mean first desire to void and mean maximum desire to void were significantly higher with naftopidil monotherapy (188.4 mL, 339.4 mL) than with tamsulosin monotherapy (174.1 mL, P < 0.001; 334.9 mL, P = 0.036). Detrusor overactivity disappeared in 2 patients with naftopidil but not in those with tamsulosin. The authors concluded that naftopidil was better than tamsulosin for improvement of storage symptoms, especially nocturia, as a result of the disappearance of detrusor overactivity and increase in the first desire to void.
Momose et al also reported the results of crossover between naftopidil and tamsulosin.Citation29 Forty-five patients (mean age 66.9 years) having LUTS/BPH were included in the study. There were no symptomatic severity criteria for inclusion. The patients were randomly assigned to the N-T group (n = 20, 50 mg of naftopidil for 4 weeks followed by 0.2 mg/day tamsulosin for 4 weeks) and T-N group (n = 25, 0.2 mg/day tamsulosin for 4 weeks followed by 50 mg of naftopidil for 4 weeks). Although the IPSS was similarly improved during the first treatment period in both groups (19.6 to 12.9 in the N-T group, P < 0.01; 18.4 to 11.1 in the T-N group, P < 0.01), after crossover the IPSS was slightly improved in the N-T group (12.9 to 11.1) whereas it slightly deteriorated in the T-N group (11.1 to 12.8). Tamsulosin was more effective than naftopidil for intermittency, nocturia, and the QOL index. The authors concluded that the therapeutic effects of 0.2 mg of tamsulosin on storage and voiding symptoms were superior to those of naftopidil.
Ukimura et al directly compared the efficacy of naftopidil and tamsulosin in patients having LUTS/BPH associated with nocturia.Citation30 Patients aged ≥50 years having LUTS/BPH with nocturia ≥ 2, IPSS ≥ 8, QOL index ≥ 3, Qmax < 15 mL/second, with a voided volume of ≥ 150 mL, PVR < 50 mL and PV < 50 mL were randomized into two groups, 50 mg/day naftopidil for 6–8 weeks (n = 31) and 0.2 mg/day tamsulosin for 6–8 weeks (n = 28). The IPSS was significantly improved in both groups, from 17.2 to 7.8 (P = 0.0000) in the naftopidil group and from 18.9 to 9.2 (P = 0.0001) in the tamsulosin group without a significant intergroup difference at the end of the observation period (P = 0.98). The sum of day frequency and nocturia was improved earlier in the naftopidil group (7.0 at baseline, 4.4 at 2 weeks, 3.2 at 6–8 weeks) than in the tamsulosin group (6.8 at baseline, 4.9 at 2 weeks [versus naftopidil, P = 0.0489], 3.7 at 6–8 weeks [versus naftopidil, P = 0.10]). There were no significant differences in changes of Qmax and PVR between the 2 groups. The authors concluded that naftopidil provided early improvements of day frequency and nocturia compared with tamsulosin.
Masumori et al performed a head-to-head comparison of the efficacies of naftopidil and tamsulosin, although the study was conducted to investigate differences in the incidence of ejaculatory disorders as a primary endpoint. Ninety-five men aged ≥50 years (mean 64 years) having LUTS/BPH with IPSS ≥ 8 were enrolled in the study and randomly assigned to receive 50 mg/day of naftopidil (n = 48) or 0.2 mg/day of tamsulosin (n = 47) for 12 weeks.Citation31 Although there were no significant differences in the changes in the Qmax and PVR caused by the treatments between the 2 groups, the changes in the IPSS were significantly higher in the tamsulosin group (from 17.8 to 10.6, −7.2) than in the naftopidil group (from 15.0 to 11.2, −3.8, P = 0.013). Similarly, the improvement in the QOL index in the tamsulosin group (from 4.7 to 2.8, −1.9) was larger than that in the naftopidil group (from 4.2 to 3.2, −1.0, P = 0.013). Thus, the authors speculated that the efficacy of 0.2 mg of tamsulosin might be better than that of 50 mg of naftopidil.
More recently, Yokoyama et al directly compared the effects of three different types of α1-blockers; naftopidil, tamsulosin, and silodosin, on LUTS, erectile dysfunction, and ejaculatory dysfunction.Citation32 A total of 136 patients aged 50–80 years having LUTS/BPH with IPSS ≥ 8 were enrolled in the study. They were randomly assigned to 3 groups, naftopidil at 50 mg once a day (n = 46), tamsulosin at 0.2 mg once a day (n = 45), or silodosin at 4 mg twice a day (n = 45) for 12 weeks. The mean IPSS was significantly improved by treatment for 12 weeks in all 3 groups, from 17.4 to 11.3 in the naftopidil group; 18.0 to 10.7 in the tamsulosin group, and from 18.7 to 13.8 in the silodosin group. No significant difference was observed in the IPSS at 12 weeks among the groups. Similarly, there were no significant differences in the mean QOL index, Qmax, and PVR at 12 weeks among the groups. Thus, the authors concluded that the three α1-blockers similarly provided objective and subjective improvements in LUTS/BPH in the population they investigated.
Based on the results of the previous randomized studies comparing the efficacy of naftopidil to other α1-blockers, mainly tamsulosin, some reports demonstrated the comparative efficacy,Citation25,Citation27,Citation28,Citation30,Citation32 whereas others did not,Citation29,Citation31 although no study had sufficient statistical power to draw a solid conclusion. Kojima et al clearly showed that the dominant α1-AR subtype varied among individuals.Citation15 They performed prostate biopsies for 61 patients having LUTS/BPH and the expression of mRNA in the α1-AR subtype in the specimens was analyzed. Naftopidil, 50 mg/day and tamsulosin, 0.2 mg/day were randomly given to 28 and 33 patients for 12 weeks each. In the naftopidil group, α1a- and α1d-AR were dominant in the prostates of 12 and 16 patients, respectively. In the tamsulosin group, α1a- and α1d-AR were dominant in the prostates of 22 and 11 patients, respectively. There were no significant differences in the efficacies such as the IPSS, QOL index, Qmax, and PVR between the naftopidil and tamsulosin groups. However, in the naftopidil group, improvements of subjective and objective urinary symptoms were more remarkable in the patients with the α1d-AR dominant subtype than in those in whom α1a-AR was dominant. Conversely, in the tamsulosin group, striking symptomatic improvements were observed in the patients with the α1a-AR dominant subtype. Thus, the response to α1-blockers may depend on the ratio of the expression level of the α1-AR subtype rather than on the type of α1-blocker.
Dose modification study
Even though 50 mg and 75 mg/day naftopidil were recommended as standard dosages according to the phase III clinical study,Citation23 no previous randomized studies allowed dose escalation up to 75 mg or a dosage of 75 mg as the starting dose. Yokoyama et al performed a randomized study to compare the efficacy and safety of 25 mg and 75 mg of naftopidil as the starting doses for patients aged 50–80 years having LUTS/BPH with IPSS ≥ 8, QOL index ≥ 2, nocturia ≥ 3, and PV ≥ 20 mL ().Citation33 Naftopidil, 25 mg (n = 72) or 75 mg (n = 67) was randomly administered once a day for 4 weeks. There were no differences in changes of the IPSS and QOL index between the groups. On the other hand, Qmax at the endpoint was significantly higher in the 75 mg group than in the 25 mg group (P < 0.05). In the patients having moderate symptomatic severity, the degree of improvement in voiding symptoms was greater in the 75 mg group than in the 25 mg group (P < 0.05). Since there was no significant difference in the incidence of adverse events between 25 mg (3.9%) and 75 mg (2.6%), they concluded that starting administration of 75 mg/day naftopidil should be considered for patients with moderate symptomatic severity. Kadekawa et al supported the clinical efficacy and safety of a naftopidil dose of 75 mg once a day for 4 weeks.Citation34 Interestingly, Tsuritani et al demonstrated in a randomized trial that improvement of the mean IPSS after 8 weeks of treatment was significantly higher for 75 mg once daily in the evening (9.2) than with 25 mg thrice daily (11.3, P = 0.0347) and that this was probably due to a difference in Cmax between them.Citation35 A recent study demonstrated that dose escalation to 75 mg in patients with an insufficient response to 50 mg once a day for 12 weeks (defined as improvement of the IPSS < 5) showed further improvement of subjective and objective urinary symptoms.Citation36 On the other hand, Oh-oka reported the results of a dose-reduction study.Citation37 For 100 patients having LUTS/BPH, naftopidil was administrated once in the morning at a dose 75 mg for 6 weeks; then, following washout for 1 week, a reduced dose of 50 mg was administered for another 6 weeks. There was no significant difference in the mean IPSS after treatment between 75 mg (5.2) and 50 mg (5.3), although a significant improvement in urgency was found only after administration of 50 mg. In addition, there were no significant differences in objective measurements, including the parameters in a pressure-flow study, except for bladder compliance. Thus, 75 mg of naftopidil is not always superior to 50 mg of naftopidil. It is highly likely that the optimal dosage of naftopidil varies among individuals based on different α1A/α1D-AR subtype ratios.
Table 3 Other contemporary randomized trials of naftopidil for LUTS/BPH conducted in Japan
Effect of naftopidil on overactive bladder symptoms and nocturia
Several single-arm studies focused on the efficacy of naftopidil for OAB and nocturia associated with BPH.Citation38–Citation41 Oh-oka showed that 75 mg of naftopidil was effective to decrease nocturnal frequency in 122 patients with remaining nocturia ≥ 3 even after treatment using 0.2 mg of tamsulosin for 6 weeks.Citation38 Although detrusor overactivity was found in 40 patients, it disappeared in 31 patients after 6 weeks of naftopidil administration. Takahashi et al demonstrated the efficacy of 50–75 mg/day naftopidil for 6 weeks for 81 patients (mean age 69 years) having LUTS/BPH with urgency/day ≥ 1, IPSS ≥ 8 and any of the scores for 3 items of the IPSS (day frequency, nocturia, urgency) ≥3.Citation39 The mean scores for urgency on the IPSS, day frequency and nocturia on the 2-day frequency volume chart decreased from 3.1 to 1.4 (P < 0.0001), 9.3 to 8.0 (P < 0.0001), and from 2.7 to 2.0 (P < 0.0001), respectively. Significant improvement of nocturia was observed in both the patients with, and without, nocturnal polyuria. Interestingly, a tendency for nocturnal urine volume to decrease was observed in the patients with nocturnal polyuria (from 919 to 766 mL, P = 0.101). Thus, naftopidil was effective for nocturia in patients with LUTS regardless of the existence of nocturnal polyuria. Yokoyama et al reported that nocturia was well-controlled by naftopidil treatment, with decreased sleep disturbances.Citation40 In addition, they demonstrated that nocturnal urine volume significantly decreased in the sleep disturbance group, perhaps through the central action of naftopidil on vasopressin secretion.
In general, α1-blocker monotherapy is effective to control not only voiding symptoms but also storage symptoms. However, α1-blocker monotherapy does not always control the storage symptoms. Recently, solid evidence to support the efficacy and safety of combination of α1-blockers and anticholinergic agents for male LUTS associated OAB has been reported.Citation42–Citation45 Maruyama et al compared the efficacy and safety of naftopidil monotherapy with combination therapy using naftopidil and anticholinergic agents for patients having LUTS/BPH with storage symptoms.Citation46 Either monotherapy with 25–75 mg of naftopidil (n = 45) or combination therapy using 25–75 mg of naftopidil and an anticholinergic agent (10–20 mg of propiverine hydrochloride or 2–6 mg of oxybutynin hydrochloride, n = 41) was randomly given to 101 patients with LUTS/BPH with IPSS ≥ 8, QOL index ≥ 3, a score for day frequency ≥ 3, and a score for nocturia ≥ 2 for 12 weeks. There were no significant differences in the IPSS, QOL index, and Qmax between the groups after treatment. PVR was significantly worse in the combination group than in the monotherapy group. Thus, clinical usefulness of combination therapy was not found in the study. Yokoyama et al performed a randomized study to compare the efficacy and safety of naftopidil monotherapy (n = 19), propiverine monotherapy (n = 18) and combination therapy (n = 21) for patients having LUTS/BPH associated with OAB (mean age 69.1 years). The IPSS did not improve with the propiverine monotherapy. On a frequency volume chart, although daytime frequency with the naftopidil monotherapy and nighttime frequency with the propiverine monotherapy did not improve, both significantly improved with the combination therapy. On the other hand, PVR significantly increased with the propiverine monotherapy and the combination therapy.
Thus, information on the efficacy and safety of combination therapy using naftopidil and anticholinergic agents is too limited to draw a definitive conclusion. In addition, no studies investigated add-on effects of anticholinergic agents for patients having remaining OAB after naftopidil monotherapy.
Long-term efficacy of naftopidil
Several studies reported on the long-term efficacy of α1-blockers over 3 years. Narayan et al demonstrated that improvement of LUTS and the flow rate lasted up to 6 years when using 0.4 mg or 0.8 mg of tamsulosin in an open-label extension study.Citation48 Schulman et al also reported long-term efficacy of up to 4 years with 0.4 mg of tamsulosin in Europe.Citation49 The MTOPS study and the CombAT study also demonstrated the long-term efficacy of doxazosin monotherapy and tamsulosin monotherapy, respectively.Citation3–Citation5 However, the long-term efficacy of naftopidil over 1 year has not been investigated well. In addition, information on the rate of treatment failure during long-term follow-up after administration of naftopidil is extremely limited.
Masumori et al examined treatment failure during a 4-year follow-up period after administration of 50 mg/day naftopidil for 247 patients (mean age 67.6 years) having LUTS/BPH with IPSS ≥ 8.Citation50 Of the 247, treatment failure defined as conversion to other medical treatment or surgery occurred in 42 patients (17.0%) during the 4-year follow up period. The 4-year treatment failure rate on the Kaplan–Meier curve was 35.0%. Among the parameters at baseline, PV was the only significant determinant of treatment failure. Patients with a PV of 35 mL or larger had a 2.1-fold hazard of treatment failure compared to those with a PV of smaller than 35 mL (95%CI; 1.06–4.33, P = 0.03). Those patients having severe IPSS at 12 weeks after administration of naftopidil had a 3.5-fold higher hazard than those having mild/moderate IPSS (95%CI; 1.34–9.26, P = 0.01). By 4 years, 200 patients (81%) had quit taking naftopidil because of adverse events, treatment failure, loss to follow-up, etc. Thus, only 19% were known to continue the same medication for 4 years in real-life clinical practice. Although similar results were reported by de la Rosette et al, indicating that the discontinuation rates were 64% after 3 years and 79% after 5 years of follow-up after administration of α1-blockers such as terazosin, alfuzosin, and tamsulosin, the discontinuation rate was significantly different according to the type of α1-blocker.Citation51 Kawachi et al retrospectively compared the 5-year failure rates of 25–75 mg/day naftopidil (n = 78) and 0.2 mg of tamsulosin (n = 53) for 131 patients (mean age 66.4 years) having LUTS/BPH with IPSS ≥ 8 and a QOL index ≥ 3.Citation52 No significant difference in failure rates was observed since 28 patients (35.9%) using naftopidil and 22 (41.4%) using tamsulosin discontinued treatment during the follow up period. In addition, there were no differences in reasons for discontinuation between the groups. Thus the differences in the long-term outcomes and discontinuation rates should be investigated prospectively.
Kojima et al investigated changes of expression levels of α1-AR subtypes in 15 prostates having LUTS/BPH before and after administration of 50 mg/day naftopidil for 12 weeks.Citation53 Naftopidil down-regulated the expression of α1a-and α1b-AR subtypes whereas it up-regulated the expression of the α1d-AR subtype without a change in the total α1-AR mRNA expression level. Although there was no correlation between the changes of α1-AR subtype expression levels and the short-term efficacy of naftopidil, its long-term use may induce therapeutic tolerance because up-regulation of the α1d-AR subtype is considered a compensatory adaptation to administration of the chronic α1D-AR antagonist naftopidil.
Sexual dysfunction and intraoperative floppy iris syndrome
One of the adverse events induced by α1-blockers is ejaculatory disorders. The induction of ejaculatory disorders by α1-blockers may be determined by the selectivity for the α1A-AR subtype. Hisasue et al demonstrated that α1A is the dominant subtype in human seminal vesicles (α1a, 75.0%; α1d, 13.3%; α1b, 11.7%).Citation54 In addition, the study of 17 healthy Japanese volunteers showed that 0.2 or 0.4 mg of tamsulosin for 3 days resulted in a reduced amount of semen, whereas 50 or 100 mg of naftopidil for 3 days did not. Since no sperm were detected in the postejaculate midstream urine, the ejaculatory dysfunction induced by tamsulosin is thought to be due to inhibition of seminal emission rather than retrograde ejaculation. Thus, a drug that has higher selectivity for the α1A-AR subtype seems to easily cause ejaculatory dysfunction, mainly through the inhibition of seminal emission.
Masumori et al performed a randomized prospective study to investigate the incidence of ejaculatory disorders caused by 50 mg of naftopidil (n = 48) and 0.2 mg of tamsulosin (n = 47). Among men who had sexual activity during the 12 weeks, the proportion who reported an abnormal feeling on ejaculation was higher in the tamsulosin group (16.7%) than in the naftopidil group (7.4%), although the difference was not significant (P = 0.402). The proportion of men who reported reduced ejaculatory volume after treatment was significantly higher in the tamsulosin group (96.0%) than in the naftopidil group (73.1%, P = 0.0496). Although the improvement of erectile function by α1-blockers has been reported,Citation55,Citation56 no significant change in the International Index of Erectile Function (IIEF)-5 score caused by either drug was observed in this small study. On the other hand, Yokoyama et al showed that the mean IIEF-5 score improved in a nafopidil group (7.0 at baseline to 7.6 at 3 months, P = 0.013) but not in a silodosin group (6.2 at baseline to 5.0 at 3 months, P = 0.682) or tamsulosin group (6.6 at baseline to 5.2 at 3 months, P = 0.342).Citation32 The proportion of newly developed reduced volume of ejaculation was relatively high in the silodosin group (10/41, 24.4%). However, there was no significant difference between naftopidil (1/42, 2.4%) and tamsulosin (1/39, 2.6%). Thus, naftopidil may be applicable for relatively young patients who are sexually active and want to avoid ejaculatory disorders.
Intraoperative floppy iris syndrome (IFIS) has been reported as one of the adverse events of α1-blockers. The FDA issued a labeling change for tamsulosin warning of the possibility of IFIS in 2005. There is a prospective multicenter study that investigated the incidence of IFIS.Citation57 In 2,643 consecutive eyes of 1,968 patients (1,015 eyes of 762 male patients and 1,628 eyes of 1,206 female patients) who received cataract surgery, IFIS was observed in 29 eyes (1.1%) of 25 male patients, all of whom were receiving systemic α1 blockers. Of the 58 eyes of 50 patients who took tamsulosin, 25 eyes (43.1%) developed IFIS. Of the 21 eyes of 19 patients receiving naftopidil, IFIS was observed in 4 eyes (19.0%). Thus, the incidence of IFIS was significantly higher with tamsulosin than with naftopidil (P = 0.042). On the other hand, no IFIS was observed in patients using nonselective α1-blockers such as prazosin and terazosin. Thus, α1-blockers having higher affinity for the α1A-AR subtype are likely to cause IFIS as well as ejaculatory disorders.
QOL and naftopidil
Two studies mainly investigated QOL using a questionnaire. Komiya et al demonstrated that LUTS/BPH impaired generic QOL evaluated by the Short Form-8 (SF-8), that was improved by naftopidil treatment.Citation58 Awa et al showed that naftopidil significantly improved 7 domains, though not general health perceptions and social limitations, in the King’s Health Questionnaire (KHQ) having 9 domains consisting of 21 questions.Citation59
Conclusions
Naftopidil is clinically available only in Japan. There are no data derived from white or black men living in western countries. Thus, it remains unknown if the efficacy and safety of naftopidil in the Japanese and Asian population are applicable to others. In addition, well-designed prospective large-scale clinical studies having adequate statistical power to draw solid conclusions are lacking. On the other hand, the possible low incidence of sexual dysfunction caused by naftopidil is attractive. Further comparative short-term and long-term studies including evaluation of sexual function are mandatory. In addition, studies in real life clinical practice are necessary to apply the results obtained by randomized controlled studies to the general population.
Disclosure
The author reports no conflict of interest in this work.
References
- AbramsPD’AnconaCGriffithsSLower urinary tract symptom: etiology, patient assessment and predicting outcome from therapyMcConnellJAbramsPDenisLKhourySRoehrbornCMale Lower Urinary Tract Dysfunction Evaluation and ManagementEd 21ParisHealth Publications200669142
- ChappleCArtibaniWBergesRNew medical developments in the management of LUTS in adult menMcConnellJAbramsPDenisLKhourySRoehrbornCMale Lower Urinary Tract Dysfunction Evaluation and ManagementEd 21ParisHealth Publications2006143194
- McConnellJDRoehrbornCGBautistaOMThe long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasiaN Engl J Med2003349252387239814681504
- RoehrbornCGSiamiPBarkinJThe effects of dutasteride, tamsulosin and combination therapy on lower urinary tract symptoms in men with benign prostatic hyperplasia and prostatic enlargement: 2-year results from the CombAT studyJ Urol2008179261662118082216
- RoehrbornCGSiamiPBarkinJThe effects of combination therapy with dutasteride and tamsulosin on clinical outcomes in men with symptomatic benign prostatic hyperplasia: 2-year results from the CombAT studyEur Urol201057112313119825505
- TakeiR-IIkegakiIShibataKTsujimotoGAsanoTNaftopidil, a novel α1-adrenoceptor antagonist, displays selective inhibition of canine prostatic pressure and high affinity binding to cloned human α1-adrenoceptorsJpn J Pharmacol199979444745410361884
- HommaYArakiIIgawaYJapanese Society of Neurogenic Bladder. Clinical guideline for male lower urinary tract symptomsInt J Urol2009161077579019811547
- HommaYIshizukaOOzonoSClinical Guideline for Benign Prostatic HyperplasiaTokyoRichHill Medical Inc2011
- GarimellaPSFinkHAMacDonaldRWiltTJNaftopidil for the treatment of lower urinary tract symptoms compatible with benign prostatic hyperplasiaCochrane Database Syst Rev20091074CD00736019821408
- YamadaSSuzukiMKatoYBinding characteristics of naftopidil and α1-adrenoceptor antagonists to prostatic α-adrenoceptors in benign prostatic hypertrophyLife Sci19925021271351370569
- ShibataKFoglarRHorieKKMD-3213, a novel, potent, alpha 1a-adrenoceptor-selective antagonist: characterization using recombinant human alpha 1-adrenoceptors and native tissuesMol Pharmacol19954822502587651358
- NakashimaMKanamaruMUematsuTPhase I clinical study of naftopidil (KT-611), a new α1-adrenoceptor blockerJ Clin Therap Med19928Suppl 36381
- MiyanagaHSakamotoFSuzukiTAdachiYShimadaNIdentification of human P450 isozymes involved in the metabolism of naftopidil (KT-611) and effect of naftopidil (KT-611) on the enzyme activities of human P450Iyakuhin Kenkyu199930447456
- NasuKMoriyamaNKawabeKQuantification and distribution of α1-adrenoceptor subtype mRNAs in human prostate: comparison of benign hypertrophied tissue and non-hypertrophied tissueBr J Pharmacol199611957978038922723
- KojimaYSasakiSKubotaYExpression of α1-adrenoceptor subtype mRNA as a predictor of the efficacy of subtype selective α1-adrenoceptor antagonists in the management of benign prostatic hyperplasiaJ Urol200817931040104618206918
- MalloyBJPriceDTPriceRRAlpha 1 adrenergic receptor subtypes in human detrusorJ Urol19981603 Pt 19379439720591
- SmithMSSchambraUBWilsonKHPageSOSchwinnDAAlpha 1-adrenergic receptors in human spinal cord: specific localized expression of mRNA encoding alpha 1-adrenergic receptor subtypes at four distinct levelsMol Brain Res19996322542619878769
- IshihamaHMomotaYYanaseHWangXde GroatWCKawataniMActivation of alpha 1D adrenergic receptors in the rat urothelium facilitates the micturition reflexJ Urol2006175135836416406942
- WangXMatsumoto-MiyaiKYoshizumiMUrothelial α1D receptor is predominantly involved in the adrenergic facilitation of micturition reflexLUTS20091Suppl 1S10S14
- YokoyamaOYusupAOyamaNImprovement of bladder storage function by a1-blocker depends on the suppression of C-fiber afferent activity in ratsNeurourol Urodyn20062546146716673377
- FukayaYShiraiwaYYamaguchiODose-finding study of naftopidil (KT-611) for bladder outlet obstruction caused by benign prostatic hypertrophyNishinihon J Urol199254697710
- YasudaKYamanishiTTojoMNagashimaKAkimotoSShimazakiJEffect of naftopidil on urethral obstruction in benign prostatic hyperplasia: assessment by urodynamic studiesProstate199425146527517541
- YamaguchiOFukayaYShiraiwaYDose-dependent effects and clinical usefulness of naftopidil (KT-611) on urinary obstruction caused by benign prostatic hyperplasia: double-blind comparative study compared with placeboClin Rep19973113151360
- YamaguchiOFukayaYShiraiwaYClinical evaluation of naftopidil (KT-611) on urinary obstruction caused by benign prostatic hypertrophy: double-blind comparative study compared with prazosin hydrochlorideJ Clin Ther Med19928699722
- IkemotoIKiyotaHOhishiYUsefulness of tamsulosin hydrochloride and naftopidil in patients with urinary disturbance caused by benign prostatic hyperplasia: a comparative, randomized, two-drug crossover studyInt J Urol2003101158759414633083
- YamanishiTYasudaKKamaiTSingle-blind, randomized controlled study of the clinical and urodynamic effects of an alpha-blocker (naftopidil) and phytotherapy (eviprostat) in the treatment of benign prostatic hyperplasiaInt J Urol200411750150915242359
- GotohMKamihiraOKinukawaTOnoYOhshimaSOrigasaHComparison of tamsulosin and naftopidil for efficacy and safety in the treatment of benign prostatic hyperplasia: a randomized controlled trialBJU Int200596458158616104914
- NishinoYMasueTMiwaKTakahashiYIshiharaSDeguchiTComparison of two alpha1-adrenoceptor antagonists, naftopidil and tamsulosin hydrochloride, in the treatment of lower urinary tract symptoms with benign prostatic hyperplasia: a randomized crossover studyBJU Int200697474775116536766
- MomoseHHosokawaYKishinoTOnoTOyamaNCrossover comparison study on the therapeutic effects of tamsulosin hydrochloride and naftopidil in lower urinary tract symptoms associated with benign prostatic hyperplasiaDrugs Today(Barc)200743Suppl A11017401467
- UkimuraOKanazawaMFujiharaAKamoiKOkiharaKMikiTNaftopidil versus tamsulosin hydrochloride for lower urinary tract symptoms associated with benign prostatic hyperplasia with special reference to the storage symptom: a prospective randomized controlled studyInt J Urol200815121049105419120513
- MasumoriNTsukamotoTIwasawaAFuruyaRSonodaTMoriMEjaculatory disorders caused by alpha-1 blockers for patients with lower urinary tract symptoms suggestive of benign prostatic hyperplasia: comparison of naftopidil in a randomized multicenter studyUrol Int2009831495419641359
- YokoyamaTHaraRFukumotoKEffects of three types of alpha-1 adrenoceptor blocker on lower urinary tract symptoms and sexual function in males with benign prostatic hyperplasiaInt J Urol201118322523021272091
- YokoyamaTKumonHHasuYTakamotoHWatanabeTComparison of 25 and 75 mg/day naftopidil for lower urinary tract symptoms associated with benign prostatic hyperplasia: a prospective, randomized controlled studyInt J Urol200613793293816882058
- KadekawaKSugayaKAshitomiKNishijimaSClinical efficacy of α1-adrenergic receptor antagonist naftopidil 75 mg/day in patients with benign prostatic hyperplasiaLUTS20102106112
- TsuritaniSNozakiTOkumuraAKimuraHKazamaTA prospective, randomized, controlled, multicenter study of naftopidil for treatment of male lower urinary tract symptoms associated with benign prostatic hyperplasia: 25 mg once daily in the evening compared to 25 mg thrice dailyUrol Int2010851808720516676
- FunahashiYHattoriRMatsukawaYKomatsuTSassaNGotohMClinical efficacy of a loading dose of naftopidil for patients with benign prostatic hyperplasiaWorld J Urol201129222523120309563
- Oh-okaHUsefulness of naftopidil for dysuria in benign prostatic hyperplasia and its optimal dose: comparison between 75 and 50 mgUrol Int200982213614219321997
- Oh-okaHEffect of naftopidil on nocturia after failure of tamsulosinUrology20087251051105518817955
- TakahashiSTajimaAMatsushimaHKawamuraTTominagaTKitamuraTClinical efficacy of an alpha1 A/D-adrenoceptor blocker (naftopidil) on overactive bladder syndromes in patients with benign prostatic hyperplasiaInt J Urol2006131152016448426
- YokoyamaOAokiYTsujimuraATakaoTNamikiMOkuyamaAα1-adrenoceptor blocker naftopidil improves sleep disturbance with reduction in nocturnal urine volumeWorld J Urol201129223323820387069
- KakizakiHTanakaHMitsuiTNonomuraKClinical efficacy of α1-blocker naftopidil in patients with overactive bladder associated with benign prostatic hyperplasiaLUTS200913539
- AthanasopoulosAGyftopoulosKGiannitsasKFisfisJPerimenisPBarbaliasGCombination treatment with an alpha-blocker plus an anticholinergic for bladder outlet obstruction: a prospective, randomized, controlled studyJ Urol200316962253225612771763
- LeeKSChooMSKimDYCombination treatment with propiverine hydrochloride plus doxazosin controlled release gastrointestinal therapeutic system formulation for overactive bladder and coexisting benign prostatic obstruction: a prospective, randomized, controlled multicenter studyJ Urol20051744 Pt 11334133816145414
- KaplanSARoehrbornCGRovnerESCarlssonMBavendamTGuanZTolterodine and tamsulosin for treatment of men with lower urinary tract symptoms and overactive bladder: a randomized controlled trialJAMA2006296192319232817105794
- RovnerESKrederKSussmanDOEffect of tolterodine extended release with or without tamsulosin on measures of urgency and patient reported outcomes in men with lower urinary tract symptomsJ Urol200818031034104118639297
- MaruyamaOKawachiYHanzawaKNaftopidil monotherapy vs naftopidil and an anticholinergic agent combined therapy for storage symptoms associated with benign prostatic hyperplasia: a prospective randomized controlled studyInt J Urol200613101280128517010005
- YokoyamaTUematsuKWatanabeTSasakiKKumonHNagaiANaftopidil and propiverine hydrochloride for treatment of male lower urinary tract symptoms suggestive of benign prostatic hyperplasia and concomitant overactive bladder: a prospective randomized controlled studyScand J Urol Nephrol200943430731419396723
- NarayanPEvansCPMoonTLong-term safety and efficacy of tamsulosin for the treatment of lower urinary tract symptoms associated with benign prostatic hyperplasiaJ Urol20031702 Pt 149850212853808
- SchulmanCCLockTMTWBuzelinJ-MLong-term use of tamsulosin to treat lower urinary tract symptoms/benign prostatic hyperplasiaJ Urol200116641358136311547074
- MasumoriNHashimotoJItohNTsukamotoTShort-term efficacy and long-term compliance/treatment failure of the alpha1 blocker naftopidil for patients with lower urinary tract symptoms suggestive of benign prostatic hyperplasiaScand J Urol Nephrol200741542242917853040
- De la RosetteJJMCHKortmannBBMRossiCSonkeGSFloratosDLKiemeneyLong-term risk of re-treatment of patients using alpha-blockers for lower urinary tract symptomsJ Urol200216741734173911912399
- KawachiYSakuraiTSugimuraSLong-term treatment and prognostic factors of a1-blockers for lower urinary tract symptoms associated with benign prostatic hyperplasia: a pilot study comparing naftopidil and tamsulosin hydrochlorideScand J Urol Nephrol2010441384520095868
- KojimaYSasakiSShinomuraHChange of expression levels of alpha 1-adrenoceptor subtypes by administration if alpha 1d-adrenoceptor-subtype selective antagonist naftopidil in benign prostatic hyperplasia patientsProstate200767121285129217626248
- HisasueSFuruyaRItohNKobayashiKFuruyaSTsukamotoTEjaculatory disorder caused by alpha-1 adrenoceptor antagonists is not retrograde ejaculation but a loss of seminal emissionInt J Urol200613101311131617010010
- KirbyRSO’LearyMPCarsonCEfficacy of extended-release doxazosin and doxazosin standard in patients with concomitant benign prostatic hyperplasia and sexual dysfunctionBJU Int200595110310915638905
- Van MoorselaarRJAHartungREmbertonMAlfuzosin 10 mg once daily improves sexual function in men with lower urinary tract symptoms and concomitant sexual dysfunctionBJU Int200595460360815705088
- OshikaTOhashiYInamuraMIncidence of intraoperative floppy iris syndrome in patients on either systemic or topical α1-adrenoceptor antagonistAm J Ophthalmol2007143115015117188051
- KomiyaASuzukiHAwaYClinical effect of naftopidil on the quality of life of patients with lower urinary tract symptoms suggestive of benign prostatic hyperplasia: a prospective studyInt J Urol201017655556220370847
- AwaYSuzukiHHamanoSClinical effect of alpha 1D/A adrenoceptor inhibitor naftopidil on benign prostatic hyperplasia: an International Prostate Symptom Score and King’s Health Questionnaire assessmentInt J Urol200815870971518662175