Abstract
Osteoarthritis (OA) is the most common form of articular joint arthritis and a cause of significant morbidity. In this review, we present the role of nerve growth factor (NGF) in pain generation, relationship between NGF and OA pain, and pathogenic factors (interleukin-1β, transforming growth factor-β1, mechanical loading, and adipokines) involved in OA development. Since NGF blocking is an efficient way to inhibit OA-associated pain, we summarize four categories of drugs that target NGF/tropomyosin receptor kinase A (TrkA) signaling. In addition, we discuss the future of NGF/TrkA antagonists and underline their potential for use in OA pain relief. A better understanding of the causes and treatment of OA will facilitate the development of more effective methods of OA pain management.
Introduction
Osteoarthritis (OA) is the most common form of articular joint arthritis, the primary symptom of which is pain.Citation1,Citation2 It causes significant morbidity in elderly individuals, and its age of onset is gradually decreasing.Citation3 It results in tissue disruption surrounding the affected joint, impairing its articular function and leading to disability.
Many etiologies contribute to OA development, including age, sex, hereditary factors, and trauma.Citation2 In recent years, the risk factors, molecular basis, and medications for OA have been investigated to prevent and treat OA.
Nerve growth factor (NGF) was found to be a sort of crucial protein for neurons’ growth and survival initially. But its role in mediating pain was identified later. NGF blocking was also effective in relieving OA pain.Citation4 In this article, we outline the molecular mechanisms underlying the role of NGF in OA pain and review evidence supporting the attenuation of OA pain by NGF blockade with the aim of promoting the discovery of new analgesics based on the antagonism of NGF signaling.
Relationship between NGF and pain
NGF was the first growth factor to be identified, and its discovery dates back to the 1950s. It belongs to a family of neurotrophic factors that include brain-derived neurotrophic factor, neurotrophin-3, and neurotrophin-4. Initially, it was identified as a soluble factor produced by tumor tissue, which promotes the growth and survival of sensory neurons. Subsequent studies showed that it functions in nervous system development and tissue pain.Citation4,Citation5 Hefti et alCitation6 reported that NGF levels are elevated during injury, inflammation, and chronic pain. In rats, inhibition of endogenous NGF degradation by matrix metalloproteinase (MMP)-2 and MMP-9 induces mechanical allodynia and thermal hyperalgesia.Citation7
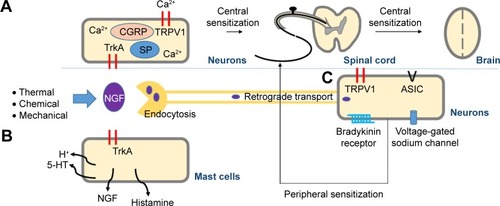
The molecular mechanisms underlying these actions of NGF can be explained as follows: Thermal, chemical, or mechanical stimuli activate nociceptors located in peripheral tissues, causing NGF production and release. NGF binds to NGF receptors, promoting multiple pain signaling pathways, leading to nociceptive pain.Citation8,Citation9 When NGF binds to tropomyosin receptor kinase (Trk) A, the complex is endocytosed and retrogradely transported to neuronal cell bodies, where it modulates the expression of bradykinin receptors, acid-sensing ion channel 2/3, voltage-gated sodium channels, and transient receptor potential vanilloid receptor 1 (TRPV1).Citation10 These cell surface receptors involved in nociception initiate peripheral sensitization and pain hypersensitivity.Citation11,Citation12 The NGF–TrkA complex causes rapid posttranslational changes in the pain receptor TRPV1, evoking sustained density and increased activity in peripheral and central neurons.Citation11 Once opened, TRPV1 facilitates Ca2+ influx and transmission of pain signals.Citation13 Central sensitization results from transcriptional changes triggered by NGF–TrkA signaling, including increased expression of calcitonin gene-related peptide and substance P.Citation12,Citation14 In addition, NGF stimulates mast cells to express histamine, serotonin/5-hydroxytryptamine, and protons, as well as NGF, forming a positive-feedback loop.Citation12,Citation14 Thus, NGF signaling not only modulates the expression of peripheral and central pain-related substances but also sensitizes adjacent nociceptive neurons in response to inflammation ().
Figure 1 Molecular mechanisms of NGF function leading to pain.
Abbreviations: 5-HT, 5-hydroxytryptamine; ASIC, acid-sensing ion channel; CGRP, calcitonin gene-related peptide; NGF, nerve growth factor; SP, substance P; TrkA, tropomyosin receptor kinase A; TRPV1, transient receptor potential vanilloid receptor 1.
Relationship between NGF and OA pain
Pain is the defining symptom of OA, and NGF expression is evident in joints affected by the condition. In humans, elevated NGF levels are found in the synovial fluid of patients with inflammatory or degenerative rheumatic diseases, including rheumatoid arthritis, spondyloarthritis, and OA.Citation15 In clinical trials, anti-NGF therapy provided significant pain relief.Citation16 In a recent article,Citation17 NGF and bradykinin receptors were found to be significantly upregulated in the joints of mice with OA displaying pain-related behavior. In a systematic review of 13 multicenter placebo-controlled trials about hip or knee OA, Schnitzer and MarksCitation18 identified that NGF inhibition yielded extensive pain relief compared with placebos.
The OA process involves many chemical and physical factors. Next, we present the biologic mechanisms of the risk factors for OA, including interleukin (IL)-1β, transforming growth factor-β1 (TGF-β1), and mechanical stimulation with NGF.
IL-1β elevates NGF expression in the synovium and chondrocytes
Inflammatory factors, especially IL-1β, contribute to OA progression by downregulating the expression of cartilage extracellular matrix components and stimulating the synthesis of MMPs and other cytokines.Citation2,Citation19 Symptoms are usually linked to inflammation.Citation20
Interestingly, inflammation-induced NGF expression is evident in OA. In a controlled trial,Citation21 medial tibial plateaus and synovium samples were harvested from 29 asymptomatic donors (non-OA control group, postmortem) and 29 symptomatic donors (advanced OA group, during total knee replacement). The histopathologic analysis of samples indicated that patients with advanced OA presented more severe synovitis and increased synovial NGF. NGF staining was localized to fibroblasts and some macrophages.Citation21 Pecchi et alCitation19 examined whether proinflammatory cytokines influence NGF synthesis by chondrocytes. Human and mouse articular chondrocytes were cultured with IL-1β. Consequently, NGF mRNA expression was increased by IL-1β in a dose-dependent manner, paralleled by NGF protein release.Citation19 These findings are consistent with those of Blaney Davidson et al,Citation22 who found that IL-1β elevates NGF expression through TAK1 in the human chondrocyte cell line G6, murine H4 chondrocyte cell line, and bovine primary chondrocytes. This phenomenon was also observed in two other studies investigating the effects of IL-1β exposure on cartilage stem cells and synovial tissue.Citation23,Citation24
TGF-β1 induces NGF expression in the synovium and chondrocytes
Although it is established that pain is linked to inflammation, some patients with OA pain lack detectable inflammation.Citation25 Iannone et alCitation26 reported that the interplay between NGF and pain perception or other nervous system-related functions may directly influence the growth factors and cytokines such as tumor necrosis factor (TNF)-α and TGF-β1, the roles of which have been extensively studied in the pathophysiology of cartilage.Citation27 Using quantitative polymerase chain reaction, Blaney Davidson et alCitation22 identified that TGF-β1 induces NGF expression via the ALK5-Smad2/3 pathway in different cells, including human, murine, and bovine chondrocytes. This induction of NGF by TGF-β1 is more potent than IL-1β stimulus.Citation22 In a recent articleCitation17 that examined damaged joint cartilage from modified mice and pigs with OA, it was demonstrated that injury produces pro-algesic molecules, including NGF. The molecular basis of this is partly dependent on TGF-β-activated kinase 1.Citation17 Much evidence has been published on the promotion of the OA process by TGF-β1.Citation28–Citation30
Mechanical loading stimulates NGF expression by chondrocytes
Mechanical overloading is considered the archetypal cause of cartilage damage and pain.Citation31 Consequently, the influence of mechanical compression on NGF release in cartilage explants from joints with OA has become a focus of investigation. The accumulation of endogenous NGF was found to induce mechanical allodynia in rats, and similar evidence exists in humans and other models of OA.Citation7 Recently, high-extension silicone rubber membranes were used on primary chondrocytes to examine the effect of low-frequency, high-magnitude mechanical strain.Citation32 Mechanical strain was applied for the first 8 hours, followed by 16 hours’ rest, and then another 8 hours’ mechanical strain. Subsequently, NGF mRNA expression was significantly increased. Medium from the treated group was collected and applied to cultured PC12 cells, which are known to respond to NGF by sprouting axon-like neurites. The PC12 cells exposed to the mechanical strain-conditioned medium resembled the NGF-treated group, but differed from vehicle-treated controls or static conditioned medium-treated cells.Citation32 Although 4 hours’ dynamic compression did not increase the NGF levels, the concentration of NGF in the medium surrounding murine costal cartilage explants after 24 hours had significantly increased (4.7-fold versus the control). These findings indicate that NGF is a mechanosensitive gene in chondrocytes.Citation19 These findings are consistent with data obtained in neurocytes.Citation33
Other related stimuli
Increased levels of TNF-α and adipokines are found in joints with OA and are thought to play a role in OA progression.Citation34 In isolated synovial cells, synovial fibroblasts and macrophages form a model of OA in which the NGF gene expression increases significantly when exogenous TNF-α is added.Citation24 However, the role of TNF-α in OA pain is controversial because anti-TNF-α therapy is effective in the early, but not the late, phases of OA.Citation35
Anti-NGF therapies
Based on the mechanisms underlying OA, four categories of drugs have been developed and applied to relieve OA pain (). These drugs include agents that eliminate free NGF, molecules that prevent NGF from binding to the receptor, and drugs that inhibit TrkA activation. Desensitization or blocking of TRPV1 is used to prevent TrkA activation.
Table 1 The four categories of drugs that have been applied to relieve OA pain
The NGF-capturing agent RN624, now known as tanezumab, is a highly selective humanized immunoglobulin G2 monoclonal antibody. It can bind NGF directly and neutralize NGF bioactivity. Tanezumab has demonstrated promising therapeutic potential for the treatment of pain, including pain related to cancerCitation36 and OA,Citation37 in animalsCitation38–Citation40 and in clinical trialsCitation37 involving acute and chronic pain.Citation39,Citation41 In a proof-of-concept study by Lane et al,Citation37 450 patients with or without moderate-to-severe knee OA were examined. With clinically significant reductions ranging from 45% to 62%, tanezumab improved knee pain, stiffness, and limitations of physical function.Citation37 Other antibodies that have been evaluated in Phase III clinical trials include fasinumab (Regeneron Pharmaceuticals, Inc., Tarrytown, NY, USA) and fulranumab (Janssen [Beerse, Belgium] and Regeneron Pharmaceuticals, Inc.) and PG110 (PanGenetics BV, Utrecht, the Netherlands). In recent a systematic review, Schnitzer and MarksCitation18 reviewed the efficacy of these antibodies. They were effective at relieving OA pain when compared with placebos, and of them, tanezumab performed the best.Citation18,Citation42
Molecules targeting NGF-binding sites have been developed as a result of identification of NGF–TrkA interaction domains.Citation43 They interfere with these domains and block receptor activation. One such molecule, known as ALE0540, inhibits NGF binding to TrkA and p75NTR and is reportedly effective for the treatment of rat neuropathic and inflammatory pain.Citation44 The identity of the molecules that bind to NGF or its receptors is an issue of debate. Recently, the development of surface plasmon resonance technology has enabled evaluation of the inhibitory potential of ALE-0540, PD90780, Ro 08-2750, and PQC083. These compounds bind NGF, rather than the TrkA and p75NTR receptors. PD90780 is the most effective, inhibiting both the NGF–TrkA and NGF–p75NTR interactions, whereas ALE-0540 only inhibits NGF–TrkA binding. Four molecules and novel bivalent naphthalimide derivatives of ALE-0540 also reportedly inhibit the binding of proNGF to the p75NTR receptor.Citation45,Citation46
It took a long time to develop a drug with high levels of homology for TrkA, TrkB, and TrkC. In mice, the anti-TrkA monoclonal antibody MNAC13 demonstrates a significant antiallodynic effect on neuropathic pain, promoting functional recovery.Citation47 Intrathecal administration of antisense oligodeoxynucleotides for TrkA cause a dose-related reduction in hyperalgesia.Citation48 In a recent study by Nwosu et al,Citation49 AR786, a selective TrkA inhibitor, reduced pain behavior in intra-articular monosodium-iodoacetate injection-induced and meniscal transection-induced models of OA. Thus, selective inhibitors of TrkA exhibit the potential for OA pain relief in the future.
Once opened, TRPV1 promotes Ca2+ influx and calcitonin gene-related peptide and substance P expression, leading to central sensitization; therefore, agonists of TRPV1 may be effective for the treatment of OA pain. Anandamide is a TRPV1 agonist that desensitizes TRPV1, reducing Ca2+ influx.Citation50 However, it failed to show a benefit in clinical trials.Citation51 Recently, interfering with TRPV1 subunit association using a plasma membrane-tethered peptide was found to relieve mechanical and thermal hypersensitivity in mouse models. This may offer a new method of analgesic treatment.Citation52 Kelly et alCitation53 identified the clinical and mechanistic rationale for intra-articular application of TRPV1 antagonists (JNJ-17203212) to treat OA pain.
We reviewed the relationship between NGF and OA pain. It convinced us that targeting NGF/TrkA signaling is an effective method to treat OA pain. In the future, other mechanisms between NGF expression and related pro-algesic mediators will be identified, and specific NGF/TrkA antagonists will play a key role in OA pain relief.
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
We thank Professor Wenjie Jin for his help and guidance. This work was supported by the Scientific Research Fund of Shanghai Municipal Commission of Healthy and Family Planning (grant number 201540274).
Disclosure
The authors report no conflicts of interest in this work.
References
- MillerREMillerRJMalfaitAMOsteoarthritis joint pain: the cytokine connectionCytokine201470218519325066335
- MalfaitAMOsteoarthritis year in review 2015: biologyOsteoarthritis Cartilage2016241212626707989
- Glyn-JonesSPalmerAJAgricolaROsteoarthritisLancet2015386999137638725748615
- MillerREBlockJAMalfaitAMNerve growth factor blockade for the management of osteoarthritis pain: what can we learn from clinical trials and preclinical models?Curr Opin Rheumatol201729111011827672741
- ChangDSHsuEHottingerDGCohenSPAnti-nerve growth factor in pain management: current evidenceJ Pain Res2016937338327354823
- HeftiFFRosenthalAWalickePANovel class of pain drugs based on antagonism of NGFTrends Pharmacol Sci2006272859116376998
- OsikowiczMLongoGAllardSCuelloACRibeiro-da-SilvaAInhibition of endogenous NGF degradation induces mechanical allodynia and thermal hyperalgesia in ratsMol Pain201393723889761
- CohenSPMaoJNeuropathic pain: mechanisms and their clinical implicationsBMJ2014348f765624500412
- WheelerMAHeffnerDLKimSTNF-alpha/TNFR1 signaling is required for the development and function of primary nociceptorsNeuron201482358760224811380
- MizumuraKMuraseSRole of nerve growth factor in painHandb Exp Pharmacol2015227577725846614
- EskanderMARuparelSGreenDPPersistent nociception triggered by nerve growth factor (NGF) is mediated by TRPV1 and oxidative mechanismsJ Neurosci201535228593860326041925
- McKelveyLShortenGDO’KeeffeGWNerve growth factor-mediated regulation of pain signaling and proposed new intervention strategies in clinical pain managementJ Neurochem2013124327628923157347
- MoranMMXuHClaphamDETRP ion channels in the nervous systemCurrent opinion in neurobiology200414336236915194117
- MantyhPWKoltzenburgMMendellLMTiveLSheltonDLAntagonism of nerve growth factor-TrkA signaling and the relief of painAnesthesiology2011115118920421602663
- AloeLTuveriMACarcassiULevi-MontalciniRNerve growth factor in the synovial fluid of patients with chronic arthritisArthritis Rheum19923533513551536673
- SeidelMFHerguijuelaMForkertROttenUNerve growth factor in rheumatic diseasesSemin Arthritis Rheum201040210912619481238
- DriscollCChanalarisAKnightsCNociceptive sensitizers are regulated in damaged joint tissues, including articular cartilage, when osteoarthritic mice display pain behaviorArthritis Rheumatol201668485786726605536
- SchnitzerTJMarksJAA systematic review of the efficacy and general safety of antibodies to NGF in the treatment of OA of the hip or kneeOsteoarthritis Cartilage201523Suppl 1S8S1725527221
- PecchiEPriamSGossetMInduction of nerve growth factor expression and release by mechanical and inflammatory stimuli in chondrocytes: possible involvement in osteoarthritis painArthritis Res Ther2014161R1624438745
- Van SpilWENairSCKindsMBSystemic biochemical markers of joint metabolism and inflammation in relation to radiographic parameters and pain of the knee: data from CHECK, a cohort of early-osteoarthritis subjectsOsteoarthritis Cartilage2015231485625205017
- StoppielloLAMappPIWilsonDHillRScammellBEWalshDAStructural associations of symptomatic knee osteoarthritisArthritis Rheumatol201466113018302725049144
- Blaney DavidsonENvan CaamAPVittersELTGF-β is a potent inducer of Nerve Growth Factor in articular cartilage via the ALK5-Smad2/3 pathway. Potential role in OA related pain?Osteoarthritis Cartilage201523347848625529198
- JiangYHuCYuSCartilage stem/progenitor cells are activated in osteoarthritis via interleukin-1beta/nerve growth factor signalingArthritis Res Ther20151732726577823
- TakanoSUchidaKMiyagiMNerve growth factor regulation by TNF-alpha and IL-1beta in synovial macrophages and fibroblasts in osteoarthritic miceJ Immunol Res20162016570635927635406
- MyersSLBrandtKDEhlichJWSynovial inflammation in patients with early osteoarthritis of the kneeJ Rheumatol19901712166216692084242
- IannoneFPerniolaSLopalcoGCantariniLLapadulaGRole of nerve growth factor and tropomyosin receptor kinase A in the pathogenesis of osteoarthritis. Might nerve growth factor be the link interwinding obesity and osteoarthritis?Ann Rheum Dis20157412e7026386127
- LaiYBaiXZhaoYADAMTS-7 forms a positive feedback loop with TNF-alpha in the pathogenesis of osteoarthritisAnn Rheum Dis20147381575158423928557
- ZhenGWenCJiaXInhibition of TGF-beta signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritisNat Med201319670471223685840
- CuiZCraneJXieHHalofuginone attenuates osteoarthritis by inhibition of TGF-beta activity and H-type vessel formation in subchondral boneAnn Rheum Dis20167591714172126470720
- VinatierCMerceronCGuicheuxJOsteoarthritis: from pathogenic mechanisms and recent clinical developments to novel prospective therapeutic optionsDrug Discov Today201621121932193727616187
- SaxbyDJLloydDGOsteoarthritis year in review 2016: mechanicsOsteoarthritis Cartilage201725219019828100420
- RosenzweigDHQuinnTMHaglundLLow-frequency high-magnitude mechanical strain of articular chondrocytes activates p38 MAPK and induces phenotypic changes associated with osteoarthritis and painInt J Mol Sci2014158144271444125196344
- RanaORSchauertePHommesDMechanical stretch induces nerve sprouting in rat sympathetic neurocytesAuton Neurosci20101551–2253220122881
- GomezRCondeJScoteceMGomez-ReinoJJLagoFGualilloOWhat’s new in our understanding of the role of adipokines in rheumatic diseases?Nat Rev Rheumatol20117952853621808287
- McNameeKEBurleighAGompelsLLTreatment of murine osteoarthritis with TrkAd5 reveals a pivotal role for nerve growth factor in non-inflammatory joint painPain2010149238639220350782
- SopataMKatzNCareyWEfficacy and safety of tanezumab in the treatment of pain from bone metastasesPain201515691703171325919474
- LaneNESchnitzerTJBirbaraCATanezumab for the treatment of pain from osteoarthritis of the kneeN Engl J Med2010363161521153120942668
- SheltonDLZellerJHoWHPonsJRosenthalANerve growth factor mediates hyperalgesia and cachexia in auto-immune arthritisPain20051161–281615927377
- LaBrancheTPBendeleAMOmuraBCNerve growth factor inhibition with tanezumab influences weight-bearing and subsequent cartilage damage in the rat medial meniscal tear modelAnn Rheum Dis201776129530227381034
- DjouhriLPG110, A humanized anti-NGF antibody, reverses established pain hypersensitivity in persistent inflammatory pain, but not peripheral neuropathic pain, rat modelsPain Med201617112082209426917622
- CattaneoATanezumab, a recombinant humanized mAb against nerve growth factor for the treatment of acute and chronic painCurr Opin Mol Ther20101219410620140821
- CohenELeeYCA mechanism-based approach to the management of osteoarthritis painCurr Osteoporos Rep201513639940626419467
- WiesmannCde VosAMNerve growth factor: structure and functionCell Mol Life Sci2001585–674875911437236
- OwolabiJBRizkallaGTehimACharacterization of antiallodynic actions of ALE-0540, a novel nerve growth factor receptor antagonist, in the ratJ Pharmacol Exp Ther199928931271127610336516
- SheffieldKSKennedyAEScottJARossGMCharacterizing nerve growth factor-p75(NTR) interactions and small molecule inhibition using surface plasmon resonance spectroscopyAnal Biochem2016493212626435172
- SheffieldKSVohraRScottJARossGMUsing surface plasmon resonance spectroscopy to characterize the inhibition of NGF-p75(NTR) and proNGF-p75(NTR) interactions by small molecule inhibitorsPharmacol Res201610329229926675716
- UgoliniGMarinelliSCovaceuszachSCattaneoAPavoneFThe function neutralizing anti-TrkA antibody MNAC13 reduces inflammatory and neuropathic painProc Natl Acad Sci U S A200710482985299017301229
- SummerGJPuntilloKAMiaskowskiCDinaOAGreenPGLevineJDTrkA and PKC-epsilon in thermal burn-induced mechanical hyperalgesia in the ratJ Pain200671288489117157774
- NwosuLNMappPIChapmanVWalshDABlocking the tropomyosin receptor kinase A (TrkA) receptor inhibits pain behavior in two rat models of osteoarthritisAnn Rheum Dis20167561246125426286016
- LizaneczEBagiZPasztorETPhosphorylation-dependent desensitization by anandamide of vanilloid receptor-1 (TRPV1) function in rat skeletal muscle arterioles and in Chinese hamster ovary cells expressing TRPV1Mol Pharmacol20066931015102316338989
- HugginsJPSmartTSLangmanSTaylorLYoungTAn efficient randomized, placebo-controlled clinical trial with the irreversible fatty acid amide hydrolase-1 inhibitor PF-04457845, which modulates endocannabinoids but fails to induce effective analgesia in patients with pain due to osteoarthritis of the kneePain201215391837184622727500
- FlynnRChapmanKIftincaMAboushoushaRVarelaDAltierCTargeting the transient receptor potential vanilloid type 1 (TRPV1) assembly domain attenuates inflammation-induced hypersensitivityJ Biol Chem201428924166751668724808184
- KellySChapmanRJWoodhamsSIncreased function of pronociceptive TRPV1 at the level of the joint in a rat model of osteoarthritis painAnn Rheum Dis201574125225924152419
