Abstract
This study examined the adiponectin and leptin levels and insulin resistance (IR) in patients with inflammatory bowel disease (IBD) and the associations between these factors and IBD characteristics. Fasting serum leptin, adiponectin, glucose, and insulin levels, as well as inflammatory parameters, were measured in 105 patients with IBD (49 patients with Crohn’s disease [CD], 56 patients with ulcerative colitis [UC]) and 98 healthy controls [HC]. IR was evaluated using the Homeostatic Model Assessment of Insulin Resistance (HOMA-IR). Disease activity and severity in patients with UC were evaluated using the Truelove–Witts index, and patients with CD were evaluated using the Crohn’s Disease Activity Index. Serum adiponectin levels were found to be significantly lower in patients with CD and UC (p<0.001). Serum leptin levels were also found to be significantly higher in both the UC and CD groups (p<0.001). When HOMA-IR levels were compared, no significant difference was detected for either the CD or UC groups compared with the controls. In conclusion, it was shown that leptin levels increased and adiponectin levels decreased in patients with IBD, which is thought to be related to chronic inflammation. The effects of adipocytokines in patients with IBD with inflammatory and metabolic processes need to be investigated in further broader studies.
Introduction
Inflammatory bowel diseases (IBDs) are chronic inflammatory diseases of the gastrointestinal tract.Citation1 Crohn’s disease (CD) and ulcerative colitis (UC) are the main forms of IBD. Chronic inflammation plays a significant role in the development of insulin resistance (IR), metabolic syndrome (MS), and diabetes via various inflammatory mediators and adipocytokines, including adiponectin and leptin.Citation2–Citation4 In recent years, it has been found that adipose tissue actively participates in local and systemic immune responses via molecules called adipocytokines secreted by adipose tissue. Activation of inflammatory cells located in visceral adipose tissue, namely, macrophages, and secretion of adipocytokines such as leptin, adiponectin, and tumor necrosis factor-alpha (TNF-α) have been found to activate inflammatory pathways, which leads to the development of IR, steatohepatitis, and atherosclerosis.Citation5,Citation6 Mesenteric visceral adipose tissue hyperplasia, also known as creeping fat, is a pathognomonic finding of CD. These mesenteric fat deposits are secondary to intestinal inflammation and increased by inflammatory cytokines such as TNF-α, inflammatory cells such as macrophages, and preadipocytes.Citation7
The first investigated adipocytokine associated with intestinal inflammation was leptin. In experimental colitis models, intestinal inflammation was significantly reduced in leptin-deficient (ob/ob) mice.Citation8 On the other hand, intrarectal leptin administration has been shown to cause mucosal inflammation in mice.Citation9 It has been shown in experimental studies that leptin has an important function in many autoimmune diseases as a proinflammatory cytokine.Citation10 Leptin, together with interleukin 1 alpha (IL-1α), TNF-α, and interleukin 6 (IL-6), plays an active role in inflammatory processes.Citation11 Another function of leptin is to regulate food intake and energy balance. It has been shown that a lack of leptin or leptin resistance was correlated with the development of obesity, IR, and diabetes.Citation12
Adiponectin, another important adipocytokine synthesized in large quantities from adipocytes, has anti-inflammatory effects. In the experimental colitis model, adiponectin (−/−) mice showed a significant increase in colitis severity due to adiponectin deficiency, which suggested possible protective effects of adiponectin against colitis.Citation13 Studies in humans have reported conflicting results about systemic serum levels of adiponectin in patients with IBD. Some authors reported that serum adiponectin levels decrease in patients with IBD,Citation14,Citation15 whereas others reported no change or increased levels.Citation16–Citation18
Adiponectin also plays a significant role in energy homeostasis. Adiponectin is involved in glucose regulation and fatty acid catabolism. It decreases gluconeogenesis and increases glucose uptake in cells, beta oxidation, and fat clearance. Lower serum adiponectin levels are associated with IR and metabolic diseases in the general population.Citation19 Adiponectin may be regarded as a potential biomarker of MS.Citation20 It has been shown that cytokines lead to decreased adiponectin expression in fat tissue.
IR is a factor that has significance in the development of atherosclerosis; it forms the basis for the development of MS and diabetes and indirectly affects morbidity and mortality. This study assessed the serum adiponectin and leptin levels and IR in adult patients with IBD and the associations between these factors and characteristics of IBD.
Patients and methods
Patient population
A total of 105 patients who were followed up after being diagnosed as having IBD (56 with UC [(22 females and 34 males)], mean age 40±11 years; 49 with CD [24 females and 25 males], mean age 38±11 years) and 98 healthy controls [HC], who had similar baseline demographic values, were enrolled in the study. Patients with diabetes mellitus, thyroid diseases, chronic obstructive pulmonary disease (COPD), coronary heart disease, malignant hypertension, or renal failure; those receiving hormone replacement therapy or corticosteroids in the past 6 months; who were pregnant; or those with liver cirrhosis were excluded. After obtaining written informed consent from the participants and approval from Istanbul University’s ethics committee (2012-4873), the study was conducted in accordance with the Declaration of Helsinki. Disease activity and severity were evaluated using the Truelove–Witts (TW) index in patients with UC and the Crohn’s Disease Activity Index (CDAI) in patients with CD. There were no patients with severe active colitis among patients with UC. Crohn’s patients were divided into two groups as CDAI <150 and CDAI ≥150 in terms of activity index. There were no patients with CDAI >450. Patients with UC were divided into two groups according to extension: left-sided, determined as limited distal of the splenic flexure, and extensive involvement exceeding the splenic flexure. Patients with CD were divided into two groups as ileal CD and ileocolonic CD.
Laboratory analysis
Blood samples were taken from antecubital vein using 20-gage needles between 8:30–10:30 am after fasting for 8–12 hours. The levels of glucose, insulin, cholesterol, triglyceride, low-density lipoprotein (LDL), high-density lipoprotein (HDL), very-low-density lipoprotein (VLDL), complete blood count, sedimentation, C-reactive protein (CRP), and hemoglobin A1c (HbA1c) levels were studied on the same day. Blood samples taken for the measurement of adiponectin were centrifuged for 6 minutes at 5,000 rpm, and the obtained 1–2 mL serum samples were stored at −80°C. All materials used during the study were in compliance with the specifications of kits identified in the operation manual, and the steps in the operating manual were carefully observed while performing each test. Serum adiponectin and leptin levels were evaluated by commercial enzyme-linked immunosorbent assay (ELISA) kits (AssayMax Human ELISA, AssayPro, St Charles, MO, USA).
Assessing IR
IR was calculated in the patient and HC group. The Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) index was used to assess IR. The HOMA-IR formula used is as follows: HOMA-IR = fasting plasma glucose (mmol/L) × fasting plasma insulin (mU/L)/22.5. HOMA ≥2.5 was accepted as IR.Citation21
Statistical methods
All data obtained at the end of the study were analyzed using the Statistical Package for the Social Sciences (SPSS), version 20 (IBM Corporation, Armonk, NY, USA). The analysis of variance (ANOVA) test and Student’s t-test were used to compare averages of variables in more than two groups. The calculation of differences between sexes was made using the chi-square test. For the assessment of correlation between the variables, Pearson’s and Spearman’s correlation analyses were conducted for parametric and nonparametric variables, respectively. A p-value of <0.05 was considered as statistically significant. The Shapiro–Wilk test and Kolmogorov–Smirnov test were used to evaluate the distribution of the variables. Categorical variables were presented as frequencies and percentages. Normally distributed continuous variables are expressed as mean ± standard deviation, and skewed distributed continuous variables are expressed as median (interquartile range).
Results
The demographic and laboratory data of patients with CD and UC and HC are presented in . When the patient and control groups were compared, there was no difference in terms of age, sex, or body mass index (BMI). In the UC group, serum glucose levels were found to be significantly higher than that of the control group (p=0.004), however in the CD group, cholesterol and LDL levels were found to be significantly lower than that of the control group (p=0.007, p=0.002 respectively, ).
Table 1 Demographic data for patients with CD, UC, and the HC group
Assessment of leptin, adiponectin, and HOMA-IR index in the study groups is shown in . CRP levels were significantly higher in patients with CD compared with the control group (p=0.04, ). Serum adiponectin levels were found to be significantly lower both in patients with CD and UC compared with the HC (p<0.001; ). Serum leptin levels were also found to be significantly higher in both the UC and CD groups compared with the HC group (p<0.001; ). When HOMA-IR levels were compared, no significant difference was detected for either the CD or UC group compared with the HC. The HOMA-IR index and BMI were clearly correlated (r=0.43, p<0.001 and r=0.43, p<0.001, respectively) in the IBD and control groups.
Figure 1 Distribution of serum adiponectin concentration.
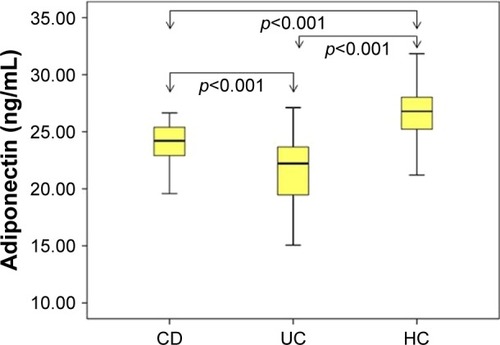
Figure 2 Distribution of serum leptin concentration.
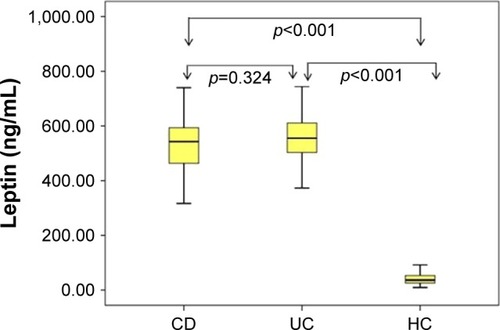
Table 2 Assessment of leptin, adiponectin, HOMA-IR index, and IR in the control group and patients with UC and CD
Serum leptin, adiponectin, and HOMA-IR index were assessed for patients with UC and CD with normal and high CRP levels, and no significant difference was found ().
Table 3 Comparison of serum leptin, adiponectin, and HOMA-IR index in groups with normal and high CRP levels, in terms of disease activity indexes and in terms of BMI in patients with IBD
In UC patients, serum adiponectin levels were significantly higher in patients with BMI <25 kg/m2 than BMI ≥25 kg/m2 (p=0.04). In CD patients, HOMA-IR levels were significantly increased in patients with BMI ≥25 kg/m2 compared with those of BMI <25 (p=0.03, ).
Disease activity and IR, HOMA-IR index, leptin, and adiponectin were assessed according to the disease activity in UC as mild and moderate TW index; no significant correlation with disease activity was detected. When patients with CD who had CDAI values <150 and ≥150 were compared in terms of adiponectin, leptin, and the HOMA-IR index, no significant difference was found ().
When patients with UC were divided into two groups according to localization as limited to the distal of splenic flexure (proctitis: 5, rectosigmoiditis: 15, left side: 12; n=32) and exceeding the splenic flexure (extensive: 4, pancolitis: 19; n=23), no significant difference was noted regarding serum leptin level (551±116 vs 590±150; p=0.38), adiponectin level (21.57±36 vs 20.80±38; p=0.53), and the HOMA-IR index (1.93±1.02 vs 2.09±1.60; p=0.85) between the two groups (data not shown).
Similarly, when patients with CD were divided into two groups according to the involvement areas as ileal involvement (n=21) and ileocolonic involvement (n=28), no significant difference was found in terms of serum leptin (526±155 vs 570±180; p=0.46), adiponectin (24.31±16 vs 23.78±22; p=0.46), and the HOMA-IR index (1.49±1.10 vs 1.56±0.95; p=0.82; data not shown).
In both IBD and control groups, HOMA-IR was found to be significantly correlated with BMI (r=0.43, p<0.001 and r=0.43, p<0.001, respectively). However, the HOMA-IR score was not found to be associated with adipocytokine levels (data not shown).
Discussion
In this study, it was shown that serum adiponectin levels in adult patients with IBD were significantly lower than those in the HC group. Adiponectin levels in patients with UC were also significantly lower than those in patients with CD. As another important adipocytokine, serum leptin levels were found significantly higher in both the UC and CD groups than in the HC group. When patients with IBD were evaluated for serum leptin levels, adiponectin levels, and HOMA-IR index, no significant correlation was found between disease localization and disease activity.
Capristo et alCitation22 first reported that entire body glucose uptake and oxidation were similar in patients with IBD, irrespective of disease activity. In the study by Nagahori et al,Citation23 MS prevalence in Japanese patients with IBD was found to be considerably higher in patients with UC compared with patients with CD. In the study by Yorulmaz et al,Citation24 the incidence of MS was found to be higher in patients with UC than in patients with CD. In our study, the incidence of IR in patients with UC was 21% and in those with CD was 18%. There were no significant differences between the IBD subgroups and control group.
In a study by Chouliaras et alCitation17 in which glucose metabolism and adipocytokines were assessed for pediatric patients with UC and CD, it was reported that IR was considerably higher in patients with both UC and CD. Bregenzer et alCitation25 assessed IR and β-cell activity using the HOMA-IR test in 17 patients with CD, and IR was detected higher in the patient group compared with the control group. It was thought that IR could be correlated with chronic inflammation.
Dagli et alCitation26 assessed risk factors in IBD regarding atherosclerotic diseases and included 40 patients with IBD and 40 controls in their study. In patients with IBD, carotid artery stiffness, CRP, HOMA-IR, and homocysteine were found to be considerably higher compared with the control group. In our study, the HOMA-IR index was found to be significantly higher in patients with BMI ≥25 kg/m2 in both groups and the control group. In the UC group, serum adiponectin levels were higher in patients with BMI <25 kg/m2 compared with patients who had BMI ≥25 kg/m2. Higher IR and lower serum adiponectin levels were the expected findings in patients who were overweight–obese. However, in our study, the absence of significant elevations in the IR index may be due to the absence of patients with severe active disease.
In experimental models, a decrease in adiponectin levels was demonstrated to be associated with the development of IR and diabetes.Citation27 Serum adiponectin was also regarded as a biomarker of MS, and it was shown that a decrease in serum adiponectin level led to an increase in IR and was an independent predictor for the development of diabetes.Citation28–Citation31
In the study by Valentini et al, 44 patients with UC and 49 patients with CD were examined and IR and adiponectin levels were evaluated. In their study, serum adiponectin levels were found to be significantly lower in the patient group compared with the HC group and hyperinsulinemia was detected.Citation14
Adiponectin has been demonstrated in experimental animal studies in relation to intestinal inflammation. In an experimental colitis model, it was shown that the severity of colitis was significantly increased in adiponectin-deficient mice and that adiponectin had protective effects against colitis.Citation13,Citation32 In contrast, another study showed that the experimental colitis model regulates inflammatory cytokines but does not affect disease severity.Citation33
Yamamoto et alCitation34 found that adiponectin expression in mesenteric fat tissues increased in patients with CD. On the other hand, Rodrigues et alCitation15 showed that adiponectin levels significantly decreased in patients with active CD, but adiponectin levels did not change in those in remission. Weigert et alCitation35 found higher adiponectin levels in patients with UC than in those with CD and HC. Adiponectin levels were found to be similar for all three groups (UC, CD, and HC) in the studies of Karmiris et alCitation18 and Waluga et al.Citation16 In our study, serum adiponectin levels were detected to be significantly lower in patients with CD and UC compared with those in the control group. Although serum adiponectin levels decreased in the entire IBD group, the decrease was more notable in patients with UC than in patients with CD. As mentioned in the earlier studies, serum adiponectin levels are variable in patients with IBD and their role in the pathogenesis of the disease is still unclear.
Leptin has metabolic, endocrinologic, and also immuno-logic effects. Leptin stimulates T lymphocytes by regulating the release of various cytokines. The direct role assumed by leptin in intestinal inflammation was indicated in experimental models.Citation10 Leptin mRNA overexpression in mesenteric adipose tissue was found in patients with IBD.Citation36 Leptin receptors were detected in inflamed colonic epithelium of patients with IBD, and leptin levels were found to be considerably high.Citation37 It was indicated in another study that colonic lavage leptin concentrations were 15 times higher in patients with UC and CD compared with the control group. It has also been demonstrated that the increase in leptin level was correlated with severity of disease and inversely correlated with the number of T regulator cells.Citation9 Tuzun et alCitation38 showed that leptin levels were substantially higher in patients with active UC. On the other hand, Waluga et alCitation16 found that leptin levels were very high in patients with CD during the posttreatment period. In our study, leptin levels were also detected to be higher both in patients with UC and CD. It was found to be high in both mild-to-moderate active patients, independent of disease activity. However, leptin levels were reported to be lower in the study by Karmiris et al.Citation18 In the study of Nishi et al,Citation39 there was no significant difference in leptin levels in patients with CD compared with those in the control group. Similarly, in the study by Rodrigues et al,Citation15 leptin levels in patients with CD did not change. In a recently published study by Ghomraoui et al,Citation40 leptin levels were found to be lower in patients with IBD compared with those in the control group, but no significant difference in leptin levels was found between the control group and patients with IBD. These conflicting results regarding leptin levels merit further studies on this issue.
Conclusion
A significant increase in leptin levels and a decrease in adiponectin levels were found in patients with IBD. These findings suggest that adipocytokines may play a role in inflammatory and metabolic processes in patients with IBD. Further studies are needed to investigate the possible effects of adipocytokines on inflammatory and metabolic processes in patients with IBD.
Disclosure
The authors report no conflicts of interest in this work.
References
- Sanchez-MunozFADominguez-LopezAYamamoto-FurushoJKRole cytokines in inflammatory bowel diseaseWorld J Gastroenterol200814274280428818666314
- ReavenGMBanting lecture 1988. Role of insulin resistance in human diseaseDiabetes19883712159516073056758
- HotamisligilGSPeraldiPBudavariAEllisRWhiteMFSpiegelmanBMIRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistanceScience199627152496656688571133
- SethiJKHotamisligilGSThe role of TNF alpha in adipocyte metabolismSemin Cell Dev Biol1999101192910355025
- TilgHMoschenARInflammatory mechanisms in the regulation of insulin resistanceMol Med2008143–422223118235842
- Al-DokhiLMAdipokines and etiopathology of metabolic disordersSaudi Med J20093091123113219750255
- KredelLISiegmundBAdipose-tissue and intestinal inflammation – visceral obesity and creeping fatFront Immunol2014546225309544
- SiegmundBLehrHAFantuzziGLeptin: a pivotal mediator of intestinal inflammation in miceGastroenterology200212272011202512055606
- SitaramanSLiuXCharrierLColonic leptin: source of a novel proinflammatory cytokine involved in IBDFASEB J200418669669814977884
- OteroMLagoRGomezRTowards a pro-inflammatory and immunomodulatory emerging role of leptinRheumatology200645894495016720637
- GrunfeldCZhaoCFullerJEndotoxin and cytokines induce expression of leptin, the ob gene product, in hamstersJ Clin Invest1996979215221578621806
- PlattTLBeckettTLKohlerKNiedowiczDMMurphyMPObesity, diabetes, and leptin resistance promote tau pathology in a mouse model of diseaseNeuroscience201631516217426701291
- NishiharaTMatsudaMArakiHEffect of adiponectin on murine colitis induced by dextran sulfate sodiumGastroenterology2006131385386116952554
- ValentiniLWirthEKSchweizerUCirculating adipokines and the protective effects of hyperinsulinemia in inflammatory bowel diseaseNutrition200925217218118849144
- RodriguesVSMilanskiMFagundesJJSerum levels and mesenteric fat tissue expression of adiponectin and leptin in patients with Crohn’s diseaseClin Exp Immunol2012170335836423121676
- WalugaMHartlebMBoryczkaGKuklaMZwirska-KorczalaKSerum adipokines in inflammatory bowel diseaseWorld J Gastroenterol201420226912691724944482
- ChouliarasGPanayotouIMargoniDCirculating leptin and adiponectin and their relation to glucose metabolism in children with Crohn’s disease and ulcerative colitisPediatr Res201374442042623823177
- KarmirisKKoutroubakisIEXidakisCPolychronakiMVoudouriTKouroumalisEACirculating levels of leptin, adiponectin, resistin, and ghrelin in inflammatory bowel diseaseInflamm Bowel Dis200612210010516432373
- StatnickMABeaversLSConnerLJDecreased expression of apM1 in omental and subcutaneous adipose tissue of humans with type 2 diabetesInt J Exp Diabetes Res200012818811469400
- HugCLodishHFThe role of the adipocyte hormone adiponectin in cardiovascular diseaseCurr Opin Pharmacol20055212913415780820
- MatthewsDRHoskerJPRudenskiASNaylorBATreacherDFTurnerRCHomeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in manDiabetologia19852874124193899825
- CapristoEMingroneGAddoloratoGGrecoAVGasbarriniGGlucose metabolism and insulin sensitivity in inactive inflammatory bowel diseaseAliment Pharmacol Ther199913220921710102952
- NagahoriMHyunSBTotsukaTPrevalence of metabolic syndrome is comparable between inflammatory bowel disease patients and the general populationJ Gastroenterol201045101008101320414788
- YorulmazEAdaliGYorulmazHUlasogluCTasanGTuncerIMetabolic syndrome frequency in inflammatory bowel diseasesSaudi J Gastroenterol201117637638222064334
- BregenzerNHartmannAStrauchUSchölmerichJAndusTBollheimerLCIncreased insulin resistance and beta cell activity in patients with Crohn’s diseaseInflamm Bowel Dis2006121535616374259
- DagliNPoyrazogluOKDagliAFIs inflammatory bowel disease a risk factor for early atherosclerosis?Angiology201061219820419398421
- HottaKFunahashiTBodkinNLCirculating concentrations of the adipocyte protein adiponectin are decreased in parallel with reduced insulin sensitivity during the progression to type 2 diabetes in rhesus monkeysDiabetes20015051126113311334417
- RyoMNakamuraTKiharaSAdiponectin as a biomarker of the metabolic syndromeCirc J2004681197598115502375
- DaimonMOizumiTSaitohTFunagata StudyDecreased serum levels of adiponectin are a risk factor for the progression to type 2 diabetes in the Japanese population: the Funagata studyDiabetes Care20032672015202012832305
- SnehalathaCMukeshBSimonMViswanathanVHaffnerSMRamachandranAPlasma adiponectin is an independent predictor of type 2 diabetes in Asian IndiansDiabetes Care200326123226322914633806
- YamauchiTKamonJWakiHThe fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesityNat Med20017894194611479627
- SaxenaAFletcherELarsenBBaligaMSDurstineJLFayadREffect of exercise on chemically-induced colitis in adiponectin deficient miceJ Inflamm20122130
- GoveMEPiniMFayadRCabayRJFantuzziGAdiponectin deficiency modulates adhesion molecules expression and cytokine production but does not affect disease severity in the transfer model of colitisCytokine200947211912519520591
- YamamotoKKiyoharaTMurayamaYProduction of adiponectin, an anti-inflammatory protein, in mesenteric adipose tissue in Crohn’s diseaseGut200554678979615888786
- WeigertJObermeierFNeumeierMCirculating levels of chemerin and adiponectin are higher in ulcerative colitis and chemerin is elevated in Crohn’s diseaseInflamm Bowel Dis201016463063719714754
- BarbierMVidalHDesreumauxPOverexpression of leptin mRNA in mesenteric adipose tissue in inflammatory bowel diseasesGastroenterol Clin Biol20032798799114732844
- BarrenetxeJVillaroACGuembeLDistribution of the long leptin receptor isoform in brush border, basolateral membrane, and cytoplasm of enterocytesGut200250679780212010881
- TuzunAUygunAYesilovaZLeptin levels in the acute stage of ulcerative colitisJ Gastroenterol Hepatol200419442943215012781
- NishiYIsomotoHUenoHPlasma leptin and ghrelin concentrations in patients with Crohn’s diseaseWorld J Gastroenterol200511467314731716437634
- GhomraouiFAAlotaibiSTAlharthiMAPlasma ghrelin and leptin in patients with inflammatory bowel disease and its association with nutritional statusSaudi J Gastroenterol201723319920528611344
