Abstract
Background:
Periodontal disease has been associated with a number of systemic diseases. A high prevalence of periodontitis among individuals with chronic kidney diseases and end-stage renal disease has been reported. However, no association between periodontal diseases and glomerulonephritis has previously been investigated.
Objective:
The aim of this study was to assess the severity and possible role of periodontitis in a group of patients with unknown primary glomerulonephritis.
Methods:
Ten patients with unknown primary glomerulonephritis, and who had a renal biopsy with stable renal function and serum creatinine <1.6 mg/dL, were recruited. Severity of the periodontal disease was clinically measured with plaque index (PI), gingival index (GI), and periodontal pocket depth (PD). The subjects received appropriate dental treatments where indicated. The patients were also put on angiotensin-converting enzyme inhibitor or angiotensin receptor blockers for controlling blood pressure and proteinuria. Six months following appropriate periodontal treatment, renal function, degree of proteinuria, and level of C-reactive protein (CRP) were measured in each individual.
Results:
The median age of the patients was 30 (15.8) years. The median urine protein excretion was lower following the periodontal therapy (P=008). Prior to the dental and/or periodontal therapies, the median PI, PD, and GI were 57.5%, 4.3, and 1.1, respectively. The majority of the patients had advanced periodontal disease. In four patients, +2/+3 CRP turned negative after periodontal treatment.
Conclusions:
The present study revealed that a causative link might exist between periodontal disease and glomerulonephritis.
Introduction
Periodontitis, a chronic Gram-negative infection of dental supporting tissues, is considered one of the most frequent chronic infections in humans.Citation1 The interaction between major periodontal microbial pathogens and inflammatory cells leads to periodontal disease.Citation1 However, a number of studies have proposed an association between periodontal disease and endothelial dysfunction, atherosclerosis, coronary artery disease, and stroke.Citation2–Citation4 These have been attributed to the inflammatory nature of periodontitis and systemic dissemination of locally produced inflammatory mediators such as interleukin (IL)-1β, IL-6, and tumor necrosis factor α (TNF-α).Citation4–Citation6 Cell wall components of those microbial pathogens responsible for periodontitis might generate a response from the host.Citation7 In addition, other microbial products invading the gingival and deeper periodontal tissues may gain access to the systemic circulation.Citation8 These may, in turn, activate the immune system, which may deregulate the lipid metabolism and increase the expression of cytokine-mediated inflammatory responses. Interestingly, predictive roles of inflammatory biomarkers for a poor outcome in end-stage renal disease have previously been highlighted.Citation9–Citation11
Although a high prevalence of periodontitis among individuals at early stages of chronic kidney diseases and end-stage renal disease has recently been reported,Citation12 no association between periodontal diseases and glomerulonephritis has previously been investigated. Therefore, the present study aimed to assess the severity and possible role of periodontitis in a group of patients with unknown primary glomerulonephritis. We also examined the long-term effect of periodontal treatment on the renal condition of these patients.
Methods
This study was carried out between May 2005 and November 2008. Ten patients with unknown primary glomerulonephritis were recruited. Exclusion criteria were the presence of any possible causes of secondary glomerulonephritis, including immune complex disease, vasculitis, diabetes mellitus, or malignancies. To rule out the systemic disease (secondary glomerulonephritis), testing of the serum for the presence of various proteins, including human immunodeficiency virus (HIV), hepatitis B and C antigens, and antibodies (antiglomerular basement membrane, antiphospholipid, antineutrophil cytoplasmic antibodies, anti-DNA, cryoglobulins, anti-HIV, and antihepatitis B and C antibodies) and depletion of complement components (C3, C4, and CH50) were performed.Citation13 We included only those patients who had a renal biopsy with a stable renal function and serum creatinine <1.6 mg/dL. An informed consent was obtained from the patients prior to the study.
Periodontal examination was performed by a single examiner, who was unaware of the patients’ renal pathologies. Clinical measurements of the severity of the periodontal disease and/or health status were plaque index (PI),Citation14 gingival index (GI),Citation15 and periodontal pocket depth (PD). PI was defined as the amount of dental plaque on the tooth surfaces and scored based on a 0–100 scale. PI was performed by disclosing the soft debris on the tooth surfaces with a chewable tablet. GI was scored based on a 0–3 scale considering the assessment of gingival tissue inflammation and bleeding on stimulation; a GI score of 0 represents a healthy gingiva. Buccal surfaces of all the existing teeth were examined. Probing depth was performed using a periodontal probe (William’s Probe, Hu-Friedy, Chicago, IL, USA) at six sites in each tooth (mesial, central, and distal of the buccal and lingual aspects). The subjects received appropriate dental treatments where indicated. The dental treatments were aimed to eradicate the inflammatory/infectious diseases from the oral cavity by a wide variety of treatments, including oral hygiene instruction, nonsurgical or surgical periodontal therapy, systemic or topical medicaments, root canal therapy, and extraction, if needed.
Blood samples (5 mL) were taken through venipuncture and collected in sterile tiger top tubes and centrifuged at 3000 rpm for 10 min at 4°C. The supernatant was frozen in −80°C, lyophilized for 48 h, and stored for laboratory measurements. Prior to the dental procedures and 6 months later, serum C-reactive protein (CRP) was measured by the qualitative method of latex–CRP (ENiSon, ENiSon Lab, Tehran, Iran). Results of latex–CRP tests were reported according to the presence or absence of agglutination and size of agglutinated droplets on microscopic examination; no agglutination was considered negative, small-sized agglutinated droplets +1, medium-sized agglutinated droplets +2, and large-sized agglutinated droplets +3. Serum creatinine was determined using commercial reagents with an automated chemical analyzer (Abbott Analyzer, Abbott Laboratories, Abbott Park, Chicago, IL, USA). Total protein measurement on the 24-h urine sample by a biuret colorimetric assay (Cobas Integra Analyzer, F Hoffmann-La Roche, Basel, Switzerland) was performed on the same day as collections were completed. Urine protein levels were measured prior to and 6 months following the treatment.
The patients were also put on angiotensin-converting enzyme (ACE) inhibitor (enalapril, 10–20 mg/daily) or angiotensin II receptor blockers (ARB) (losartan, 25–50 mg/daily) for controlling both the blood pressure and the proteinuria. Six months following appropriate periodontal treatment, renal function and degree of proteinuria were measured in each individual.
Data were presented as median (interquartile range) or percentage. Statistical analysis was performed with Statistical Package of Social Science (SPSS Inc, Chicago, IL, USA) for Windows Version 16.0 using Wilcoxon signed-rank test. A P value <0.05 was considered statistically significant.
Results
Ten patients (five males and five females) were included in the current study. The median age of the patients was 30 (15.8) years. The secondary glomerulonephritis diagnostic work up (see Methods) was negative in all the patients. The median serum C3, C4, and CH50 levels were 118 mg/dL (27.5), 27.5 mg/dL (10), and 114% (44.5), respectively. The median urine protein excretion was lower following the dental treatments (3100 mg/day before the dental procedure vs 900 mg/day after treatment, P = 0.008, Wilcoxon signed-rank test, ). Prior to the dental and/or periodontal therapies, the median PI, PD, and GI were 57.5% (68), 4.3 (4.9), and 1.1 (1.2), respectively. The median serum creatinine level before and after the dental procedure was significantly different (0.95 vs 1.05 mg/dL, P = 0.048, Wilcoxon signed-rank test, ). Patients’ laboratory data are shown in . Light microscopic examination of the renal biopsies revealed mesangioproliferative glomerulonephritis in seven patients and membranoproliferative glomerulonephritis in two patients. C3 complement deposit was illustrated in five kidney biopsies (). The majority of our patients had advanced periodontal disease. In four patients (40%), +2/+3 CRP turned negative after periodontal treatment. Oral findings as well as the dental treatments are presented in .
Figure 1 The median A) urine protein excretion and B) serum creatinine level before and after the dental procedure (P <0.05).
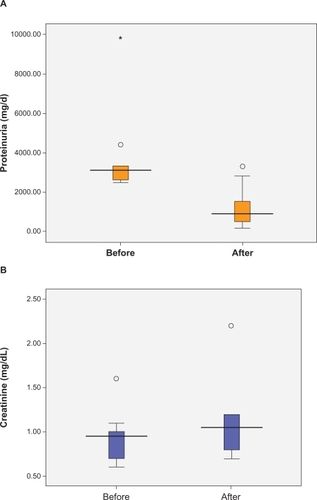
Table 1 Patients’ demographic and laboratory data (n = 10)Table Footnote1
Table 2 Kidney biopsy results of the patients (n = 10)
Table 3 Oral findings and dental treatments in the patients (n = 10)
Discussion
The present study showed a high rate of periodontal disease in a group of patients with unknown primary glomerulonephritis. Treatment of periodontal disease might have lowered urine protein in the present study; however, all patients were put on an ACE inhibitor or ARB, and these agents alone might be sufficient to explain the decrease in proteinuria. Although the current investigation did not include any control group in which periodontal diseases remained untreated, possible association between periodontitis and unknown primary glomerulonephritis might be concluded.
Although pathogenesis of glomerulonephritis often remains unknown, idiopathic or primary glomerulonephritis, many types of insult have been found to initiate the glomerulonephritis. Endogenous processes, including autoimmunity and malignancies, and exogenous factors, such as infectious organisms and drugs, have been attributed to the development of glomerulonephritis.Citation16 The mechanisms of glomerulonephritis have been extensively studied and reviewed by Chadban and Atkins,Citation16 Cunard and Kelly,Citation17 Naicker et al,Citation18 and Hricik et al.Citation19 Briefly, antibody deposition within the kidney is known to be an early process of glomerular inflammation in several forms of glomerulonephritis, including Goodpasture’s disease and cryoglobulinemia.Citation16,Citation18,Citation20,Citation21 Moreover, evidence from immunofluorescence studies has revealed that complement deposition within glomeruli is a key feature of many forms of glomerulonephritis (eg, lupus nephritis).Citation16,Citation18 Similarly, decreased levels of complement components in sera of patients suffering from complement-mediated glomerular injury imply a pathogenetic role of complement deposition in glomerulonephritis.Citation16 Additionally, the role of inflammatory leukocytes within both the glomerular and the interstitial compartments of the kidney has been highlighted in numerous studies. Recruitment of the inflammatory cells, predominantly T lymphocytes and macrophages, from the circulation is attracted to the kidney by local release of chemokines.Citation16,Citation22 The processes following mononuclear cell infiltration give rise to the production of more cytokines and proteases that can cause further kidney damage.Citation16,Citation23 Nevertheless, no association between periodontal diseases and glomerulonephritis has been hitherto reported. In the present study, a high rate of periodontal disease in patients with unknown primary glomerulonephritis might reveal probable association between periodontitis and primary glomerulonephritis through direct effects of periodontal pathogens and/or immune-mediated processes (see below).
Periodontitis is an important cause of low-grade infection that is commonly asymptomatic and remains undiagnosed for a long period of time. Meanwhile, periodontal pathogens are able to invade the systemic circulation.Citation1,Citation2 For instance, Porphyromonas gingivalis, a periodontal pathogen, has showed an invasive trend toward endothelial cell cultures.Citation1,Citation24 Hence, periodontal pathogens may directly affect the glomeruli. On the one hand, further studies on renal biopsy specimens to find specific periodontal bacteria or antigens seem to be conclusive. On the other hand, periodontitis is associated with increased levels of inflammatory cytokines including IL-6 and TNF-α.Citation3 Moreover, lipopolysaccharides of periodontal pathogens could directly activate the toll-like receptors of oral epithelial cells and lead to production of inflammatory cytokines.Citation25 Mitogenic effects of IL-1, IL-6, and TNF-α have been detected on mesangial cell and epithelial cells which, in turn, induce glomerular inflammation.Citation26,Citation27 TNF-α also activates the synthesis of IL-1β and IL-6 by glomerular mesangial cells.Citation28 Inflammatory cytokines could also activate the complement system.Citation29 It has also been shown that intensive periodontal therapy reduces local and systemic inflammation.Citation30
In conclusion, it seems that periodontitis may be a neglected and treatable cause of glomerulonephritis which needs more consideration. The authors believe that the causative link between peritoneal disease and glomerulonephritis might be plausible through both invasion of the glomeruli by periodontal pathogens, directly, and systemic inflammatory burden of chronic periodontitis, indirectly. Further investigations are recommended to be orchestrated in order to discover the periodontal pathogens and/or the level of systemic inflammatory cytokines before and after periodontal treatment in individuals with unknown primary glomerulonephritis.
Acknowledgements
This study was financially supported by a grant from the Tuberculosis and Lung Disease Research Center, Tabriz University of Medical Sciences.
Disclosure
The authors report no conflicts of interest in this work.
References
- PihlstromBLMichalowiczBSJohnsonNWPeriodontal diseasesLancet200536694991809182016298220
- TonettiMSD’AiutoFNibaliLTreatment of periodontitis and endothelial functionN Engl J Med2007356991192017329698
- LoosBGSystemic markers of inflammation in periodontitisJ Periodontol20057611 Suppl2106211516277583
- OffenbacherSBeckJDA perspective on the potential cardioprotective benefits of periodontal therapyAm Heart J2005149695095415976771
- BeckJDOffenbacherSSystemic effects of periodontitis: epidemiology of periodontal disease and cardiovascular diseaseJ Periodontol20057611 Suppl2089210016277581
- DemmerRTDesvarieuxMPeriodontal infections and cardiovascular disease: the heart of the matterJ Am Dent Assoc2006137Suppl14S20S17012731
- HettneKMWeeberMLaineMLAutomatic mining of the literature to generate new hypotheses for the possible link between periodontitis and atherosclerosis: lipopolysaccharide as a case studyJ Clin Periodontol200734121016102418028194
- LoescheWJGrossmanNSPeriodontal disease as a specific, albeit chronic, infection: diagnosis and treatmentClin Microbiol Rev200114472775211585783
- ZimmermannJHerrlingerSPruyAMetzgerTWannerCInflammation enhances cardiovascular risk and mortality in hemodialysis patientsKidney Int19995526486589987089
- Pecoits-FilhoRBárányPLindholmBHeimbürgerOStenvinkelPInterleukin-6 is an independent predictor of mortality in patients starting dialysis treatmentNephrol Dial Transplant20021791684168812198224
- ShlipakMGFriedLFCrumpCElevations of inflammatory and procoagulant biomarkers in elderly persons with renal insufficiencyCirculation20031071879212515748
- CraigRGInteractions between chronic renal disease and periodontal diseaseOral Dis20081411718173441
- ArdalanMRSadreddiniSNoshadHRenal involvement in Behcet’s diseaseSaudi J Kidney Dis Transpl200920461862219587503
- O’LearyTJDrakeRBNaylorJEThe plaque control recordJ Periodontol1972431384500182
- LoeHSilnessJPeriodontal disease in pregnancy. I. Prevalence and severityActa Odontol Scand19632153355114121956
- ChadbanSJAtkinsRCGlomerulonephritisLancet200536594731797180615910953
- CunardRKellyCJ18. Immune-mediated renal diseaseJ Allergy Clin Immunol20031112 SupplS637S64412592309
- NaickerSFabianJNaidooSWadeeSPagetGGoetschSInfection and glomerulonephritisSemin Immunopathol200729439741417846774
- HricikDEChung-ParkMSedorJRGlomerulonephritisN Engl J Med1998339138888999744974
- ClynesRDumitruCRavetchJVUncoupling of immune complex formation and kidney damage in autoimmune glomerulonephritisScience19982795353105210549461440
- JohnsonRJGretchDRYamabeHMembranoproliferative glomerulonephritis associated with hepatitis C virus infectionN Engl J Med199332874654707678440
- Pérez de LemaGMaierHNietoEChemokine expression precedes inflammatory cell infiltration and chemokine receptor and cytokine expression during the initiation of murine lupus nephritisJ Am Soc Nephrol20011271369138211423566
- KanekoYSakatsumeMXieYMacrophage metalloelastase as a major factor for glomerular injury in anti-glomerular basement membrane nephritisJ Immunol200317063377338512626598
- DornBRBurksJNSeifertKNProgulske-FoxAInvasion of endothelial and epithelial cells by strains of Porphyromonas gingivalisFEMS Microbiol Lett2000187213914410856647
- EskanMABenakanakereMRRoseBGInterleukin-1beta modulates proinflammatory cytokine production in human epithelial cellsInfect Immun20087652080208918332211
- EgidoJGómez-ChiarriMOrtízARole of tumor necrosis factor-alpha in the pathogenesis of glomerular diseasesKidney Int Suppl199339S59S648385721
- TeschGHLanHYAtkinsRCNikolic-PatersonDJRole of interleukin-1 in mesangial cell proliferation and matrix deposition in experimental mesangioproliferative nephritisAm J Pathol199715111411509212740
- YouMFlickLMYuDFengGSModulation of the nuclear factor kappa B pathway by Shp-2 tyrosine phosphatase in mediating the induction of interleukin (IL)-6 by IL-1 or tumor necrosis factorJ Exp Med2001193110111011136824
- Pezelj-RibarićSMagasićKPrpićJMiletićIKarlovićZTumor necrosis factor-alpha in peripical tissue exudates of teeth with apical periodontitisMediators Inflamm200720076941618320014
- D’AiutoFParkarMAndreouGPeriodontitis and systemic inflammation: control of the local infection is associated with a reduction in serum inflammatory markersJ Dent Res200483215616014742655