Abstract
Background
A beneficial effect on cardiovascular risk may be obtained by improving lipid-related serum lipoprotein functions such as high-density lipoproteins (HDLs) cholesterol efflux capacity (CEC) and serum cholesterol loading capacity (CLC) and by reducing proprotein convertase subtilisin kexin type 9 (PCSK9), independently of lipoprotein concentrations.
Aim
We aimed to evaluate the effect of an innovative nutraceutical (NUT) combination containing red yeast rice (monacolin K 3.3 mg), berberine 531.25 mg and leaf extract of Morus alba 200 mg (LopiGLIK®), on HDL-CEC, serum CLC and on circulating PCSK9 levels.
Materials and methods
Twenty three dyslipidemic subjects were treated for 4 weeks with the above NUT combination. HDL-CEC was measured using specific cell-based radioisotopic assays; serum CLC and PCSK9 concentrations were measured fluorimetrically and by enzyme-linked immunosorbent assay, respectively.
Results
The NUT combination significantly reduced plasma level of the total cholesterol and low-density lipoprotein cholesterol (−9.8% and −12.6%, respectively). Despite no changes in HDL-cholesterol, the NUT combination improved total HDL-CEC in 83% of the patients, by an average of 16%, as a consequence of the increase mainly of the ATP-binding cassette A1-mediated CEC (+28.5%). The NUT combination significantly reduced serum CLC (−11.4%) while it did not change PCSK9 plasma levels (312.9±69.4 ng/mL vs 334.8±103.5 mg/L, before and after treatment, respectively).
Conclusion
The present NUT combination improves the serum lipoprotein functional profile providing complementary beneficial effects, without any detrimental increase of PCSK9 plasma levels.
Introduction
In moderate hypercholesterolemia, an available alternative to pharmacological therapy is the use of innovative nutritional compounds, the so-called nutraceuticals (NUTs), as the recent guidelines recommend.Citation1 Several NUTs have shown significant effects in improving plasma lipid profile, by reducing total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) and, in some cases, by moderately increasing high-density lipoprotein cholesterol (HDL-C), with a potential positive impact on cardiovascular (CV) risk.Citation2,Citation3
However, the mere improvement of quantitative lipid profile may not be the only target to obtain optimal CV benefits, as exemplified by the failure of the hypothesis that HDL-C raising therapy would reduce CV risk.Citation4 Therefore, recent research on HDL has moved to the evaluation of their functional properties, in particular their ability to remove excess cholesterol from macrophages of the artery wall, by promoting the process of cholesterol efflux to HDL. HDL cholesterol efflux capacity (CEC) has been inversely associated with the prevalence of coronary heart diseaseCitation5 and the incidence of CV events.Citation6,Citation7 In addition, it displayed the potential to improve risk stratification beyond the traditional factors, including plasma HDL-C concentrations.Citation8
Although HDL-CEC may explain the link between HDL function and atherosclerosis, it only reflects the movement of cholesterol out of cells, while lipid trafficking is bidirectional.Citation9 Serum capacity to promote cholesterol uptake and accumulation in macrophages (cholesterol loading capacity [CLC]) might represent, in this context, an additional functional lipid-related parameter that takes into account the movement of cholesterol from lipoproteins into the cells.Citation9 Such ability relies on the contribution of all lipoproteins present in the serum, in terms of both concentration and function, and it can be considered an index of the serum pro-atherogenic potential. In support of this idea, our research team and others have previously demonstrated that serum CLC is increased in pathological conditions associated to increased CV risk.Citation9–Citation11
An additional identified player of cholesterol homeostasis maintenance is the proprotein convertase subtilisin kexin type 9 (PCSK9), able to degrade the hepatic LDL receptor, resulting in elevated plasma LDL-C concentrations.Citation12 Nevertheless, the activity of PCSK9 goes beyond LDL-C modulation and it involves other aspects of cholesterol homeostasis relevant for the pathogenesis of atherosclerosis.Citation12,Citation13 The results of a cohort study indicate, in fact, that circulating levels of PCSK9 are associated with CV events,Citation14 atherosclerosis progression,Citation15 arterial stiffness,Citation16 independently of plasma LDL-C concentrations. Among the possible direct pro-atherogenic effects of PCSK9, there is the inhibition of the cholesterol efflux process promoted by the transporter ATP-binding cassette A1 (ABCA1) in macrophages, as we recently demonstrated.Citation17 Based on these observations, the increase of HDL-CEC, the inhibition of serum CLC and the reduction of PCSK9 plasma levels may be a multi-target strategy to counteract cholesterol accumulation in macrophage and foam cell formation.
Given the relevance of the above parameters with respect to CV risk,Citation18,Citation19 as well as the poor data available on the impact of nutritional approaches on these lipoprotein functional properties and, even less, on plasma PCSK9 levels,Citation20–Citation24 the aim of the present work was to evaluate the effect of a novel NUT combinationCitation25 on HDL-CEC, serum CLC and circulating PCSK9 levels.
Materials and methods
Patients
LopiWEB is a randomized study (NCT02898805), expecting to enroll >600 subjects by physicians, designed to assess the efficacy of LopiGLIK® (Akademy Pharma, Milano, Italy) in real-life. Several regions of Italy are involved in the study and data are collected via electronic case report forms on the website www.lopiweb.it.
In this work, we studied a subgroup population of 23 subjects. The inclusion criteria were age between 18 and 70 years, the presence of metabolic syndrome or moderate hypercholesterolemia not requiring immediate statin treatment or statin intolerance. Pregnant, breastfeeding women and subjects taking hypolipidemic drugs were excluded from the study. The selected patients were not subjected to any kind of therapy before the NUT combination treatment. For this study, subjects received one pill/day containing a NUT combination of 625 mg of Berberis aristata cortex dry extract (corresponding to 531.25 mg of berberine), 220 mg of red yeast rice powder (corresponding to 3.3 mg of monacolin K) and 200 mg of Morus alba leaf dry extract (containing 4 mg of 1-deoxynojirimycin) for 16 weeks. During the study, blood withdrawal and serum isolation were planned at baseline and after 4 and 16 weeks of treatment. Our results refer to serum analyses at baseline (T1) and after 4 weeks (T2) of treatment.
Serum samples were collected at the University of Naples after obtaining written informed consent from participants and immediately stored at −80°C. Serum lipid profile was assessed by standard laboratory methods. The study was conducted in accordance with the guidelines of the Declaration of Helsinki and the study protocol was approved by the Ethics Committee of the Federico II University of Naples.
HDL-CEC
HDL-CEC was evaluated through a widely validated isotopic technique. In particular, we adopted different cellular models expressing the most important transporters involved in the promotion of cholesterol efflux to HDL: J774 murine macrophages in basal conditions were taken as a model of aqueous diffusion (AD); J774 macrophages incubated with 0.3 mM of a cAMP analog (8-(p-chlorophenylthio)-cAMP; Sigma-Aldrich Co, St Louis, MO, USA) to induce ABCA1 expression, were used as a model of total HDL-CEC as previously described.Citation5,Citation26 The ABCA1 contribution was measured as the difference between total HDL-CEC and AD-CEC.Citation27 Chinese hamster ovary cells transfected or not transfected with the human ABCG1 gene were used to evaluate ABCG1-mediated CEC. The specific ABCG1 contribution was calculated as the difference between CEC in ABCG1-transfected and not transfected cells.Citation11 In all assays, the cells were labeled with [1,2-3H]-cholesterol (PerkinEl-mer Inc, Waltham, MA, USA) for 24 hours in the presence of an inhibitor of the cholesterol esterifying enzyme, acyl-CoA:cholesterol acyltransferase, to ensure all cholesterol was present in the unesterified form. After an equilibration period in medium containing 0.2% bovine serum albumin (Sigma-Aldrich), the cells were exposed for 4 or 6 hours to 1% or 2% (v/v) (depending on the efflux pathway to be analyzed) of serum HDL fraction from patients before and after NUT combination treatment. HDLs were isolated from whole serum by precipitation of the apolipoprotein B (apoB) containing lipoproteins with polyethylene glycol as previously described.Citation28 HDL-CEC was expressed as a percentage of the radioactivity released into the medium over the total radioactivity incorporated by the cells. A pool of human normolipidemic sera was tested in each assay as reference standard 1 and its CEC was used to normalize the patient samples CEC values from the different experiments (n=3), to correct for the inter-assay variability. Each experiment was performed in triplicate.
A second pool of human normolipidemic sera as reference standard 2 was tested in each assay and its CEC was the index of the inter-assay variability.
Serum CLC
THP-1-derived human macrophages were cultured in 24-well plates in the presence of 50 ng/mL of phorbol 12-myristate 13-acetate (Sigma-Aldrich) for 72 hours to allow the cells to differentiate into macrophages. Cells were exposed for 24 hours to serum collected before and after 4 weeks of treatment with the NUT combination. Whole serum dilution in this model was 10% (v/v). Cell cholesterol content was measured by fluorometric detection in cell lysates.Citation10 An aliquot of the cell lysates was used to measure cell protein by the modified colorimetric Lowry method.Citation29 CLC was defined as macrophage cholesterol content in the cell lysates after exposure of cells to serum and was expressed as micrograms of cholesterol per milligrams of protein.
Determination of apolipoprotein A (apoA)-I sera levels
To evaluate apoA-I concentration in the HDL fraction, sera were depleted of the apoB containing lipoproteins by precipitation with polyethylene glycol.Citation28 apoA-I was measured by enzyme-linked immunosorbent assay (ELISA; Mabtech, Sweden) following the manufacturer’s instructions. The sensitivity ranged from 0.63 to 40 ng/mL with a minimum detectable concentration of 0.2 ng/mL.
Circulating levels of PCSK9
Sera PCSK9 levels were measured by ELISA (R&D Systems, Minneapolis, MN, USA) as previously described.Citation30 The sensitivity ranged from 0.030 to 0.219 ng/mL with a minimum detectable concentration of 0.096 ng/mL.
Statistical analysis
The sample size was calculated a priori by using the G*Power software (Düsseldorf, Germany) (selecting two-tailed t-test, difference between two dependent means [matched pairs], and a priori power analysis), by fixing a power of 0.95 and a significance level of 0.05. Statistical analyses were performed using GraphPad Prism version 6.0 (GraphPad Software Inc, La Jolla, CA, USA). Each sample was run in triplicate. Data were expressed as mean ± SD.
Specific pathway-mediated CEC and CLC values at baseline were compared within treatment groups by using the two-tailed Student’s t-test for paired samples, after verifying equal variance with the F-test and normal distribution of the parameters with the D’Agostino and Pearson omnibus normality test. Significant differences were defined as p<0.05.
Results
Patients characteristics
The 23 patients enrolled for the study displayed a mean age of 55.39±12.7 years and 56.5% of them were males. Baseline demographic and characteristics of the study population are reported in . Overall, subjects had clinical and laboratory parameters within a range of normality except for a slight increase of TC and LDL-C, according to the inclusion criteria.
Table 1 Baseline subjects’ characteristics
shows the lipid profile at baseline and after treatment with the NUT combination. The NUT combination significantly reduced the plasma level of TC and of LDL-C of about 9.2% and 12.6%, respectively.
Table 2 Effect of NUT combination on serum lipid profile
Conversely, no differences were observed after treatment on plasma HDL-C, triglycerides levels and plasma remnants cholesterol calculated by subtracting LDL-C and HDL-C from TCCitation31 (25±12.5 mg/dL before treatment vs 23±11.7 mg/dL after treatment; not significant [ns]).
apoA-I concentration was measured in serum samples after precipitation of apoB- containing lipoproteins with polyethylene glycol. The NUT combination did not show any significant effect on apoA-I concentrations.
HDL-CEC
First, we evaluated the effect of the NUT combination on total HDL-CEC of sera from the enrolled subjects in a model of cAMP-stimulated J774 macrophages. In these conditions, release of cholesterol from cells mainly occurs through AD and the ABCA1-mediated processes. Compared to baseline, the NUT combination increased total HDL-CEC by an average of 16.1% (), improving it in 83% of the patients. Since the major contributions to total HDL-CEC are given by AD and ABCA1, we analyzed these two cholesterol efflux pathways individually. We found that the NUT combination significantly increased HDL-CEC via both ABCA1 and AD, with ABCA1 being increased at a higher extent compared to AD (+28.5% and +8.1%, respectively) ().
Figure 1 Effect of NUT combination on serum HDL-CEC.
Abbreviations: CEC, cholesterol efflux capacity; HDL, high-density lipoproteins; ABCA1, ATP-binding cassette transporter A1; AD, aqueous diffusion; NUT, nutraceutical.
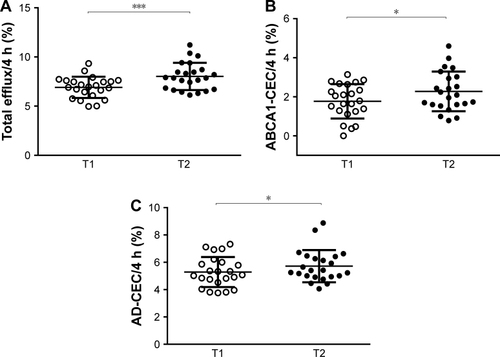
When we evaluated HDL-CEC mediated by ABCG1, we could not observe any significant changes induced by the NUT combination (before treatment 5.43%±2.21%, after treatment 5.07%±2.30%; p= ns). CEC values for all the analyzed pathways did not correlate with plasma HDL-C levels neither before nor after the consumption of the NUT combination (data not shown).
Serum CLC
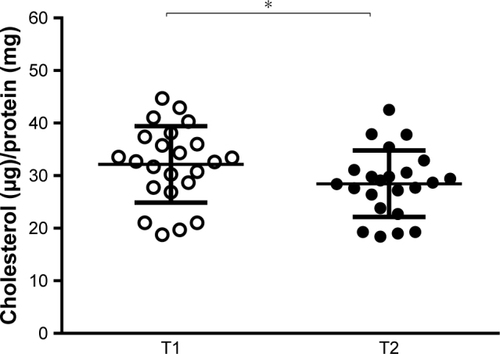
Cell cholesterol content is the result of both cholesterol efflux and influx.Citation32 To study the overall serum ability to modulate cellular cholesterol content, we evaluated the effect of the NUT combination on serum CLC in THP-1-derived human macrophages. As shown in , the NUT combination significantly reduced serum capacity of loading cells with cholesterol (from 32.15±7.28 to 28.47±6.32 µg of cholesterol/mg protein, p<0.05). No correlation was found between CLC values and neither plasma LDL-C levels nor plasma remnants cholesterol, both before and after the NUT combination (data not shown).
Figure 2 Effect of NUT combination on serum CLC.
Abbreviations: NUT, nutraceutical; CLC, cholesterol loading capacity.
Effect of NUT combination on circulating levels of PCSK9
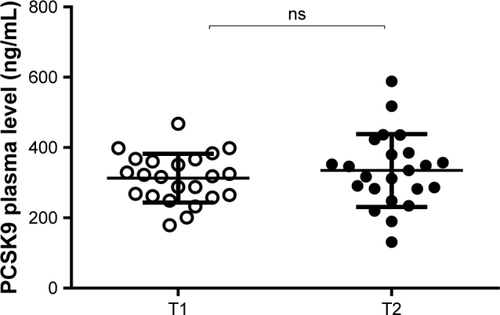
Since different components of the NUT combination are predicted to have opposite effects on PCSK9, berberine should decrease while monacolin K should increase PCSK9 plasma levels, we measured the PCSK9 serum concentrations by ELISA assay. The NUT combination did not show any significant effect on PCSK9 levels (312.9±69.4 and 334.8±103.5 ng/mL, respectively; p= ns) ().
Figure 3 Effect of NUT combination on plasma levels of PCSK9.
Abbreviations: NUT, nutraceutical; PCSK9, proprotein convertase subtilisin kexin type 9 (PCSK9); ns, not significant.
Discussion
In this study, we investigated the effect of a novel NUT combination of berberine, monacolin K, and M. alba extract (LopiGLIK®) on serum lipid-related factors involved in the protective process of reverse cholesterol transport. Serum CEC, serum CLC and serum level of PCSK9 represent factors that may directly modulate cholesterol homeostasis in macrophages, the main cellular component of atheroma.Citation33 The evaluation of these parameters provides a serum functional lipid profile related to CV risk independent of serum lipid concentrations.
Consistent with a previous study,Citation25 our results confirm that the NUT combination favorably modifies the plasma lipid profile in terms of reduction of TC and LDL-C. In the present study, the most striking result is that, despite the lack of an effect on HDL-C plasma level, the NUT combination improves serum HDL-CEC, the main antiatherogenic property of these lipoproteins. Thus, the NUT combination appears to improve HDL functionality without altering plasma HDL-C concentrations. Only few studies have previously shown a positive effect of NUTs on HDL-CEC.Citation22,Citation34 Our NUT combination increased total HDL-CEC, the cholesterol efflux pathway recently identified as a CV risk biomarker and a promising target for the prevention and therapies of CV diseases, independently of plasma HDL-C concentrations.Citation4–Citation6
When we analyzed the individual pathways contributing to total efflux, ABCA1 and AD, we found that the increase of total CEC was mainly related to the improved process of ABCA1-mediated CEC. This cholesterol efflux pathway represents the main route by which macrophages release cholesterol to serum and oppose foam cell formation and atherosclerosis progression.Citation35 The improvement of the ABCA1-mediated CEC, without an effect on ABCG1-mediated CEC, suggests a shift of HDL particles distribution toward lipid-poor pre-β HDL specific for the ABCA1 pathway.Citation36,Citation37 Consistent with our results, the increase in pre-β HDL may occur independently of serum HDL-C.Citation36 In addition, when we measured apoA-I levels in apoB-depleted serum, we found that the NUT combination did not affect even apoA-I concentrations. Our finding can be explained by the fact that neither apoA-I nor HDL-C serum concentration values reflect the actual serum content of pre-β HDL, the specific HDL particles acting as acceptor for ABCA1.Citation27,Citation38 Moreover, free apoA-I active as ABCA1 acceptor constitutes only about 5% of the circulating apoA-I.Citation39
It may be speculated that the increase in pre-β HDL could be related to a NUT-induced production of these nascent particles that may involve the stimulation of hepatic ABCA1 transporter.Citation40 This NUT effect might be related to the presence of monacolin K, given the documented stimulating effect of statins on hepatic ABCA1 both in vitro and in vivo.Citation41,Citation42 However, the impact of statin therapy on HDL-CEC does not appear to be substantial, in agreement with the modest effect of statins on HDL-C concentration and on HDL subclasses distributionCitation43 and no data on CEC are available for berberine and M. alba. A possible effect by berberine and M. alba on HDL-CEC cannot be excluded, although no literature is available yet in this regard.
The other serum lipoprotein function that we evaluated, CLC, is the ability of serum to load macrophages with cholesterol. Treatment with the NUT combination reduced this pro-atherogenic potential of serum. Consistent with previous observations,Citation11 this effect did not correlate to the reduction of plasma LDL-C concentrations. Beyond LDL-C, also remnant cholesterol which includes VLDL, namely non-LDL and non-HDL-C,Citation31 contributes to macrophage cholesterol loading.Citation44 However, we did not observe any changes in remnants levels. Most probably, the observed reduced serum CLC is related to the combined effect of both decrease on LDL levels and the increase of HDL-CEC induced by the NUT combination treatment.
A recent study demonstrated that circulating PCSK9 was associated to carotid atherosclerosis independent of the effect on LDL-C plasma level.Citation15 More recently, we demonstrated that PCSK9 in macrophages directly inhibits the efflux of cholesterol specifically mediated by ABCA1.Citation17 This effect would counteract the beneficial increase in ABCA1-mediated CEC induced by our NUT combination. We therefore evaluated if our present NUT approach could modulate serum functionality not only in terms of CEC and CLC, but also of plasma PCSK9 concentrations.
In contrast to the results reported with monacolin K alone,Citation46 our results indicate that our NUT combination did not modify PCSK9 plasma levels. We may speculate that the effect of monacolin K, the statin produced by Monascus purpureus present in our NUT combination, toward an increase of plasma PCSK9,Citation46 may be counteracted by the presence of berberine, a known negative modulator of PCSK9 concentration in plasma.Citation45,Citation47 In addition, extracts from dried immature M. alba fruits have been recently shown to have an inhibitory activity on PCSK9.Citation48
This result indicates an additional potential benefit of a NUT combination compared to the statin single administration.
Limitations of the study
The small number of patients in this study, with a relatively wide range of age, limited the potential for detailed statistical analyses, but it provides a picture of a real-life population. In addition, our study population was large enough to meet the required statistical power to demonstrate the differences in both HDL-CEC and serum CLC. It is worth pointing out that the variation of the measured parameters was consistently observed iñ80% of the examined subjects. As this is the first study evaluating the effect of a NUT combination on lipoprotein functions, despite the modest effect, it provides an important direction for further confirmatory studies in a larger group of subjects.
Conclusion
Our results demonstrate that, despite the lack of effect on HDL plasma concentrations, our NUT combination may improve lipoprotein protective functions providing complementary beneficial effects for CV risk prevention, particularly useful in moderate dyslipidemic subjects.
Author contributions
All authors have substantially contributed to the manuscript and approved the final article. FB conceived the study; VT, FR, and RI recruited patients; MPA, FZ, and SM performed the experiments and acquired data; and MPA, NF, and FZ analyzed and interpreted the data. The article was drafted by MPA, FZ, and NF and was critically revised by all authors. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
The authors acknowledge Akademy Pharma Srl (Milano, Italy) for supporting this study.
Disclosure
Franco Bernini received support for this study from Akademy Pharma Srl (Milano, Italy). The other authors report no conflicts of interest in this work.
References
- CatapanoALGrahamIDe BackerG2016 ESC/EAS guidelines for the management of dyslipidaemiasEur Heart J2016372999305827567407
- BarriosVEscobarCCiceroAFA nutraceutical approach (Armolipid Plus) to reduce total and LDL cholesterol in individuals with mild to moderate dyslipidemia: review of the clinical evidenceAtheroscler Suppl20172411527998714
- SahebkarASerbanMCGluba-BrzozkaALipid-modifying effects of nutraceuticals: an evidence-based approachNutrition2016321179119227324061
- AnastasiusMKockxMJessupWSullivanDRyeKAKritharidesLCholesterol efflux capacity: an introduction for cliniciansAm Heart J2016180546327659883
- KheraAVCuchelMde la Llera-MoyaMCholesterol efflux capacity, high-density lipoprotein function, and atherosclerosisN Engl J Med201136412713521226578
- RohatgiAKheraABerryJDHDL cholesterol efflux capacity and incident cardiovascular eventsN Engl J Med20143712383239325404125
- SaleheenDScottRJavadSAssociation of HDL cholesterol efflux capacity with incident coronary heart disease events: a prospective case-control studyLancet Diabetes Endocrinol2015350751326025389
- ModyPJoshiPHKheraAAyersCRRohatgiABeyond coronary calcification, family history, and C-reactive protein: cholesterol efflux capacity and cardiovascular risk predictionJ Am Coll Cardiol2016672480248727230043
- WeibelGLDrazul-SchraderDShiversDKImportance of evaluating cell cholesterol influx with efflux in determining the impact of human serum on cholesterol metabolism and atherosclerosisArterioscler Thromb Vasc Biol201434172524202308
- AdorniMPZimettiFPuntoniMCellular cholesterol efflux and cholesterol loading capacity of serum: effects of LDL-apheresisJ Lipid Res20125398498922414482
- RondaNGrecoDAdorniMPNewly identified antiathero-sclerotic activity of methotrexate and adalimumab: complementary effects on lipoprotein function and macrophage cholesterol metabolismArthritis Rheumatol20156751155116425605003
- NorataGDTavoriHPirilloAFazioSCatapanoALBiology of proprotein convertase subtilisin kexin 9: beyond low-density lipoprotein cholesterol loweringCardiovasc Res201611242944227496869
- FerriNMarchianoSTibollaGPCSK9 knock-out mice are protected from neointimal formation in response to perivascular carotid collar placementAtherosclerosis201625321422427477186
- LeanderKMalarstigAVan’t HooftFMCirculating proprotein convertase subtilisin/kexin type 9 (PCSK9) predicts future risk of cardiovascular events independently of established risk factorsCirculation20161331230123926896437
- XieWLiuJWangWAssociation between plasma PCSK9 levels and 10-year progression of carotid atherosclerosis beyond LDL-C: a cohort studyInt J Cardiol201621529329827128549
- RuscicaMFerriNFogacciFCirculating levels of proprotein convertase subtilisin/kexin type 9 and arterial stiffness in a large population sample: data from the Brisighella Heart StudyJ Am Heart Assoc Epub201753
- AdorniMPCipollariEFavariEInhibitory effect of PCSK9 on ABCA1 protein expression and cholesterol efflux in macrophagesAtherosclerosis20172561627940374
- FeigJEHewingBSmithJDHazenSLFisherEAHigh-density lipoprotein and atherosclerosis regression: evidence from preclinical and clinical studiesCirc Res201411420521324385513
- RizzoMBerneisKKoulourisSShould we measure routinely oxidised and atherogenic dense low-density lipoproteins in subjects with type 2 diabetes?Int J Clin Pract2010641632164220831734
- RichardCCouturePDesrochesSEffect of the Mediterranean diet with and without weight loss on surrogate markers of cholesterol homeostasis in men with the metabolic syndromeBr J Nutr201210770571121787450
- PuSRodriguez-PerezCRamprasathVRSegura-CarreteroAJonesPJDietary high oleic canola oil supplemented with docosahexaenoic acid attenuates plasma proprotein convertase subtilisin kexin type 9 (PCSK9) levels in participants with cardiovascular disease risk: a randomized control trialVascul Pharmacol201687606527374222
- ZhuYHuangXZhangYAnthocyanin supplementation improves HDL-associated paraoxonase 1 activity and enhances cholesterol efflux capacity in subjects with hypercholesterolemiaJ Clin Endocrinol Metab20149956156924285687
- BadeauRJauhiainenMMetsoJEffect of isolated isoflavone supplementation on ABCA1-dependent cholesterol efflux potential in postmenopausal womenMenopause20071429329917224860
- MomtaziAABanachMPirroMKatsikiNSahebkarARegulation of PCSK9 by nutraceuticalsPharmacol Res201712015716928363723
- TrimarcoVIzzoRStabileEEffects of a new combination of nutraceuticals with Morus alba on lipid profile, insulin sensitivity and endothelial function in dyslipidemic subjects. A cross-over, randomized, double-blind trialHigh Blood Press Cardiovasc Prev20152214915425870124
- KhalilHMurrinCO’ReillyMTotal HDL cholesterol efflux capacity in healthy children – associations with adiposity and dietary intakes of mother and childNutr Metab Cardiovasc Dis201727707727919542
- FavariERondaNAdorniMPABCA1-dependent serum cholesterol efflux capacity inversely correlates with pulse wave velocity in healthy subjectsJ Lipid Res20135423824323103472
- PisciottaLFavariEMagnoloLCharacterization of three kindreds with familial combined hypolipidemia caused by loss-of-function mutations of ANGPTL3Circ Cardiovasc Genet20125425022062970
- MarkwellMAHaasSMBieberLLTolbertNEA modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samplesAnal Biochem19788720621098070
- RuscicaMFerriNMacchiCLiver fat accumulation is associated with circulating PCSK9Ann Med20164838439127222915
- VarboANordestgaardBGRemnant lipoproteinsCurr Opin Lipidol20172830030728548974
- ZimettiFWeibelGKDuongMRothblatGHMeasurement of cholesterol bidirectional flux between cells and lipoproteinsJ Lipid Res20064760561316327021
- YamamotoSNaritaIKotaniKThe macrophage and its related cholesterol efflux as a HDL function index in atherosclerosisClin Chim Acta201645711712227087419
- HernaezAFernandez-CastillejoSFarrasMOlive oil poly-phenols enhance high-density lipoprotein function in humans: a randomized controlled trialArterioscler Thromb Vasc Biol2014342115211925060792
- AdorniMPZimettiFBillheimerJTThe roles of different pathways in the release of cholesterol from macrophagesJ Lipid Res2007482453246217761631
- de la Llera-MoyaMDrazul-SchraderDAsztalosBFCuchelMRaderDJRothblatGHThe ability to promote efflux via ABCA1 determines the capacity of serum specimens with similar high-density lipoprotein cholesterol to remove cholesterol from macrophagesArterioscler Thromb Vasc Biol20103079680120075420
- FavariELeeMCalabresiLDepletion of pre-beta-high density lipoprotein by human chymase impairs ATP-binding cassette transporter A1- but not scavenger receptor class B type I-mediated lipid efflux to high density lipoproteinJ Biol Chem20042799930993614701812
- RondaNFavariEBorghiMOImpaired serum cholesterol efflux capacity in rheumatoid arthritis and systemic lupus erythematosusAnn Rheum Dis20147360961523562986
- DuongPTWeibelGLLund-KatzSRothblatGHPhillipsMCCharacterization and properties of pre beta-HDL particles formed by ABCA1-mediated cellular lipid efflux to apoA-IJ Lipid Res2008491006101418252847
- LeeJYParksJSATP-binding cassette transporter AI and its role in HDL formationCurr Opin Lipidol200516192515650559
- KobayashiMGoudaKChisakiIOchiaiMItagakiSIsekiKRegulation mechanism of ABCA1 expression by statins in hepatocytesEur J Pharmacol201166291421554872
- SongGLiuJZhaoZSimvastatin reduces atherogenesis and promotes the expression of hepatic genes associated with reverse cholesterol transport in apoE-knockout mice fed high-fat dietLipids Health Dis201110821241519
- GomaraschiMAdorniMPBanachMBerniniFFranceschiniGCalabresiLEffects of established hypolipidemic drugs on HDL concentration, subclass distribution, and functionHandb Exp Pharmacol201522459361525523003
- Bernelot MoensSJVerweijSLSchnitzlerJGRemnant cholesterol elicits arterial wall inflammation and a multilevel cellular immune response in humansArterioscler Thromb Vasc Biol20173796997528336558
- PirilloACatapanoALBerberine, a plant alkaloid with lipid- and glucose-lowering properties: from in vitro evidence to clinical studiesAtherosclerosis201524344946126520899
- SahebkarASimental-MendiaLEGuerrero-RomeroFGolledgeJWattsGFEffect of statin therapy on plasma proprotein convertase subtilisin kexin 9 (PCSK9) concentrations: a systematic review and meta-analysis of clinical trialsDiabetes Obes Metab2015171042105526183252
- CameronJRanheimTKulsethMALerenTPBergeKEBerberine decreases PCSK9 expression in HepG2 cellsAtherosclerosis200820126627318355829
- PelPChaeHSNhoekPKimYMChinYWChemical constituents with proprotein convertase subtilisin/kexin type 9 mRNA expression inhibitory activity from dried immature Morus alba fruitsJ Agric Food Chem2017655316532128649844
