Abstract
Objectives
Tophaceous gout seldom affects the axial skeleton. Symptoms vary according to the differential localization of urate deposits and the diagnosis is often delayed. Here, we report an unusual case of lumbar spinal stenosis caused by extradural tophaceous deposits.
Methods
We retrospectively reviewed a case of a patient with tophaceous gout of the lumbar spine and reviewed the relevant literature.
Results
A 62-year-old man with a 2-year history of lower back pain and a 3-month history of lower limb radiation pain and intermittent claudication was admitted. After laboratory and imaging investigations he underwent surgical decompression and stabilization. Histological analysis of the extracted specimen confirmed that it was gouty tophus. The patient’s symptoms improved progressively after the operation. He recovered very well with no complications.
Conclusion
The mechanism associated with axial gout is not yet clear. Obesity, inactivity, and previous degenerative disc disease may be the risk factors for spinal tophus. The clinical symptoms are diverse according to the differential localization of urate deposits. It is not easy to diagnose this disease radiographically by routine radiological examination. Analysis of a biopsy specimen is definitely the only way to confirm diagnosis. Surgical treatment should be considered in patients with spinal gout who are experiencing neurological deterioration.
Keywords:
Introduction
Although the incidence of gout in the recent years is getting higher in China due to changes in diet, tophaceous gout uncommonly affects the axial skeleton. Lumbar spinal stenosis secondary to epidural gouty tophi has been reported occasionally. We present a case of a patient with tophaceous gout of the lumbar spine and reviewed the relevant literature.
Case report
A 62-year-old man was admitted with a 2-year history of lower back pain. Radiating pain to the right lower limb and intermittent claudication occurred 3 months earlier. These symptoms progressed gradually until the time of admission. He had a 4-year history of gouty arthritis for which he received medical care occasionally. There was no history of spinal injury, fever, chills, weight loss, or night sweats. As the patient had no mental retardation, aggressive behavior, muscle spasms, or other symptoms of central nervous system developmental disorders, purine enzymopathy like deficiency of hypoxanthine-guanine phosphoribosyl transferase could be excluded. The family history was noncontributory, with all the family members being negative for gout.
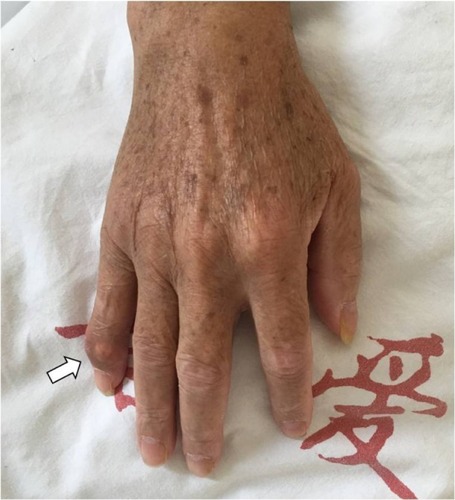
Physical examination revealed tenderness on the L2–L4 spinous process with decreased activity of lumbar vertebrae. Hypoesthesia was detected in both lower limbs at the L3–L5 dermatomes areas, with the right side being more severe. The muscle strength of the lower limb was normal. The right straight leg raising sign was positive. The Babinski sign was not detected. No joint swelling or tophi were found over the elbows, knees, ankles, and toes, except one gouty tophus, which developed on the right little finger 1 month earlier ().
Figure 1 A picture of the patient’s right hand.
Laboratory investigations revealed that the serum uric acid levels were 535 µmol/L (normal range: 208–428 µmol/L). Complete blood count and urinalysis on the day of admission were within normal limits. Erythrocyte sedimentation rate and C-reactive protein were also within the normal ranges. Blood biochemical analysis showed: Ca 2.33 mmol/L, inorganic P 1.27 mmol/L, Mg 0.83 mmol/L, K 3.8 mmol/L, Na 142 mmol/L, and alkaline phosphatase 83 U/L. All of these findings were within the normal range of our laboratory.
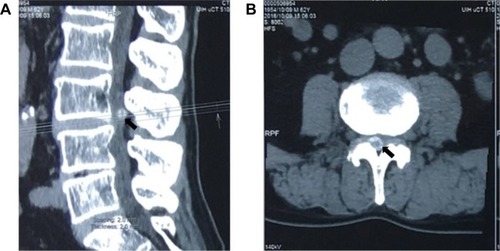
Imaging examinations of plain X-ray films showed no obvious abnormality of the lumbar spine except slight degenerative lumbar scoliosis (). Computed tomography (CT) () showed a partially calcified, round mass in the spinal canal at the level of L3/4 with a gas-like low-density nodule in the interior. The mass size was 1.1×1.1×0.7 cm. According to the axial tomography, the mass was situated in the right part of the spinal canal (). Spinal stenosis was found at the L3/4 accordingly.
Figure 2 Plain X-ray films of the lumbar spine showing no obvious abnormalities except slight degenerative lumbar scoliosis (anteroposterior view on the left and lateral view on the right).
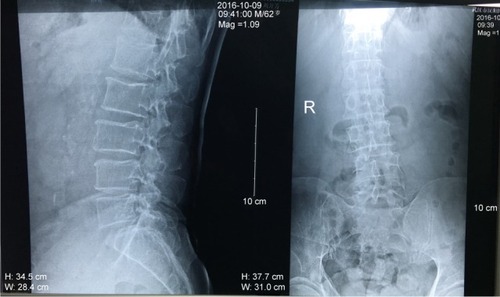
Figure 3 Computed tomography (CT) images of the lower spine.
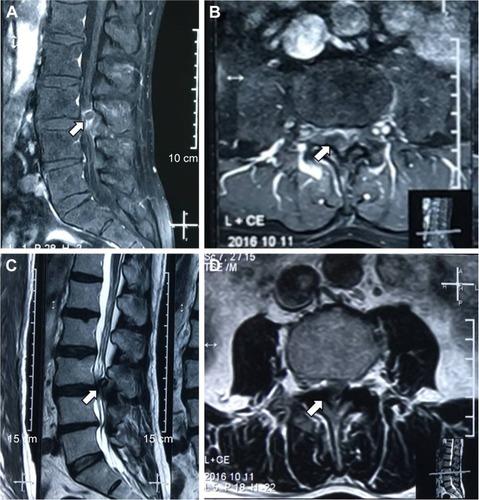
A lumbar spine magnetic resonance imaging (MRI) examination showed an abnormal round epidural collection at the L3/4 level, compromising the spinal canal and causing cauda equina compression (). This collection was of soft-tissue intensity with surrounded reactive high signal on the T1-weighted image sequence (). On the T2-weighted images, it appeared relatively hypointense with a high signal in the interior (). Lumbar disc herniations were also found at the level of L2/3 and L4/5.
Figure 4 Lumbar spine magnetic resonance imaging (MRI) images demonstrating an abnormal round epidural collection at the L3/4 level, compromising the spinal canal and causing cauda equina compression (indicated by the arrows).
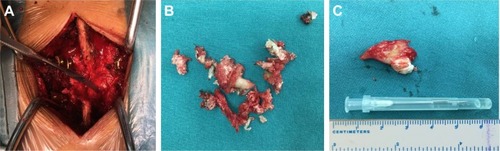
The patient underwent operation 1 week after admission. After stripping the paravertebral muscles, an amorphous white chalky material was found immediately eroding the facet joint from the L3 to the L4 levels. The L4 lamina was removed to permit adequate exploration of the lesion. The same white chalky material was found in the epidural space of the posterior and lateral spinal canal (). This material was partially encapsulated by fibrous tissue and grossly infiltrated the bone and soft tissue in several areas, which was removed completely with a currette (). Surgical stabilization with the screw and rod system was performed after surgical decompression.
Figure 5 Intraoperative pictures showing white chalky material deposited in the epidural space of the posterior and lateral spinal canal (A). This material was partially encapsulated by fibrous tissue and grossly infiltrated the bone and soft tissue in several areas, which was removed completely with a curette (B and C).
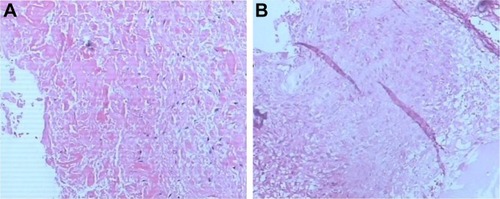
Samples of the amorphous material removed during the operation were fixed in 10% formalin for histological examination. Microscopic examination of the specimen showed granulomatous tissue with structureless coagulative necrosis and fibrinoid necrosis, while the acid-fast stain was negative ().
Figure 6 Microscopic examination of the specimen showed granulomatous tissue with structureless coagulative necrosis and fibrinoid necrosis (A and B).
The patient’s symptoms improved immediately after the operation. He recovered well without complications and was able to walk easily from the second day postoperation with the assistance of a thoracolumbar orthosis. A written informed consent has been signed by the patient to permit the publication of the case details and any accompanying images.
Discussion
Tophaceous gout is a common metabolic disorder in which deposition of monosodium urate crystals in the distal joints of the appendicular skeleton and soft tissues can occur.Citation1 The involvement of the axial spine is rare, with only 37 cases of gouty arthritis of the spine having been reported prior to 2001.Citation2 The number of cases only increased to 131 in 2015, and more than half of the cases reported (73 cases) were located in the lumbar spine.Citation3
There are several factors that may contribute to the development of gouty tophi, including the pH levels of the surrounding environment, low temperatures, trauma, and the presence of a nucleating agent within the synovial fluid, which can promote the crystallization of previously deposited monosodium urate (MSU).Citation4–Citation6
Gout is partly genetic, and many genome-wide studies have uncovered several genes associated with this disease. There are over 30 gene sequence variants commonly found to influence the serum uric acid concentrations, mostly in the SLC2A9, SLC22A12, and ABCG2 genes.Citation7,Citation8 ABCG2 plays a central role on extrarenal uric acid excretion. Severe ABCG2 dysfunction particularly increased the risk of early-onset gout.Citation9 Genetic analysis may be informative for the assessment of the disease prognosis in individuals with hyperuricemia or established gout, provide information for a personalized lifestyle advice, aid the selection and the dosing of urate-lowering therapy, and prevent serious medication adverse effects.Citation10 Whether axial gout is associated with differential gene expression is of interest. In our patient case, there was no family history of gout; however, we cannot exclude the possibility of genetic contribution since genetic tests were not performed. Identifying genetic risk factors for axial gout would be beneficial for the early diagnosis of the disease; therefore, more attention should be paid in this area in the future.
The exact mechanism of gouty tophi formation in the axial skeleton is not clear. Volkov et alCitation11 suggested that the poor vascularization within the area may be a contributing factor. The patient’s hyperlipidemic state, obese body habitus, and minimal physical activity can cause a small caliber vasculature in the axial skeleton, which can lead to inability to adequately filter the uric acid deposited in that area. If the patient had a previous degenerative disc disease (DDD), the acidic pH and the low oxygen microenvironment will promote uric acid deposition and tophi formation. Our patient also suffered from DDD, suggesting that his axial skeleton harbored the microenvironment required for uric acid formation.
According to the literature, spinal tophaceous gout can vary in symptoms as the urate deposits can be formed in different locations. Their appearance can mimic neoplasm or abscess, or they can cause cervical neck pain and spinal instability, frequently seen in degenerative process disorders.Citation11–Citation14 The most common symptom is back pain, accounting for 65.4%–68.5% of patients suffering from neurological deficits, including radiculopathy, loss of sensation, motor weakness, bowel/bladder dysfunction, and quadriparesis.Citation3 All segments of the spine could be affected by gout, and most of the gout tophi are located in the lumbar spine, followed by the cervical vertebrae.Citation3,Citation15 In our case, the main symptom of the patient was back pain, which in combination with intermittent claudication and paresthesia and other symptoms, mimicked the symptoms of lumbar spinal stenosis.
The features of spinal tophaceous gout on plane radiographs are nonspecific, and the abnormalities usually reported include spondylosis, spondylolisthesis, degenerative changes, and diffuse spinal hyperostosis.Citation16,Citation17 In other cases, the performance was normal,Citation18 like in the case reported in this study. The MRI features of spinal tophaceous gout are also nonspecific. The periarticular deposits containing low signal foci on all MRI image sequences could possibly indicate spinal tophaceous gout,Citation19 but this radiological finding cannot differentiate tophaceous gout from other disorders such as disc or vertebral infection, epidural abscess, and rheumatoid arthritis.Citation19 Kelly et alCitation20 suggested that gouty tophi could appear as a hypo-intense, homogenous mass associated with a joint on T1- and T2-weighted MRI, which enhances with gadolinium because of vascularized reactive tissue in the tophus. CT characteristics of axial gout are intraarticular and juxtaarticular erosions with sclerotic margins that are denser than the surrounding muscle.Citation12,Citation15,Citation21 Other CT findings include degenerative changes, lytic lesions, and spinal stenosis.Citation22–Citation25 We found that it was not easy to diagnose this disease radiographically. Are there any imaging methods with high specificity and accuracy to diagnose spinal tophaceous gout?
A new emerging modality for gout called dual-energy CT (DECT) scanning may be the answer. It was reported to have high sensitivity (91.9%) and specificity (85.4%)Citation26 in identifying gout tophi. DECT scanners capture images at two separate energy levels, compared to the standard single-energy CT, which allows substances of different chemical compositions to appear distinct based on their differential X-ray photon energy.Citation27 DECT scans can produce obvious color displays for urate deposits and help to identify subclinical gouty tophi. Furthermore, researchers can measure the tophi’s volume by using DECT.Citation28 DECT could also be considered during treatment evaluation. Lack of adherence to treatment should be considered when P-urate values vary significantly and when DECT scans over years persistently visualize MSU crystals.Citation29 However, DECT has lower sensitivity when restricted to individual crystal-proven gouty joints in nontophaceous disease or individual erosive lesions in tophaceous gout.Citation30 At the moment, the high cost of DECT and the lack of training of most radiologists in interpreting the images prevent its widespread use.Citation26
Gout is treated with colchicine, nonsteroidal anti-inflammatory drugs, or both, to stabilize the acute attacks, and by allopurinol for urate-lowering therapy.Citation31 The recommended first-line urate-lowering therapy is xanthine oxidase inhibitors (XOIs). Minimizing uric acid synthesis with XOIs is the preferred mechanism for lowering serum urate levels. Allopurinol and febuxostat are currently the only two US Food and Drug Administration-approved XOIs. If one fails to significantly lower uric acid levels or is poorly tolerated, then the other is considered an appropriate selection.Citation32 Uricosuric agents such as probenecid may be used in combination with an XOI or as monotherapy in refractory gout.Citation32,Citation33 New generation uricosurics, notably lesinurad and arhalofenate, in combination with XOIs, show a promising potential in achieving sufficient serum uric acid reduction.Citation34,Citation35
Surgical management should be considered and recommended in patients with neurological deterioration in spinal gout. Surgical management includes surgical decompression and surgical stabilization. According to the literature, the patients who underwent surgery had a favorable outcome.Citation36 Needle biopsy plays an important role in patients without neurological compromise, as it helps confirmation of diagnosis and the initiation of medical conservative therapy, preventing unnecessary surgical risks.Citation37 Regardless of surgical or conservative treatment, compliance with maintenance treatment of gout is mandatory for disease control. Our patient had lower back pain and a progressive lower limb radiation pain for 3 months with subacute deterioration resulting in gait impairment. Surgical decompression and surgical stabilization were necessary since neurological deterioration was identified.
Conclusion
Cases of patients with spinal gout are rarely presented in a clinical environment. Many doctors do not recognize the condition and make a delayed diagnosis, even in cases of patients with a long history of gout. The mechanism associated with axial gout is not yet clear; however, obesity, inactivity, and previous DDD may be the risk factors for spinal tophus. The clinical manifestations are diverse as the urate deposits can be formed in different locations. All segments of the spine could be affected by gout. The differential diagnosis of spinal gout includes spine tumor, epidural abscess, spondylodiscitis, rheumatoid arthritis, and spinal degenerative disease. It is not easy to diagnose this disease radiographically by the routine radiological techniques, but DECT may be a promising method, which needs further study. Examination of a biopsy specimen is undoubtedly the only way to confirm the diagnosis. Surgical management should be considered and recommended in patients with neurological deterioration in spinal gout. The long-term effect of treatment for spinal gout has not been reported, and thus, prospective research is necessary.
Disclosure
The authors report no conflicts of interest in this work.
References
- MallinsonPIReaganACCoupalTMunkPLOuelletteHNicolaouSThe distribution of urate deposition within the extremities in gout: a review of 148 dual-energy CT casesSkeletal Radiol201443327728124337414
- BarrettKMillerMLWilsonJTTophaceous gout of the spine mimicking epidural infection: case report and review of the literatureNeurosurgery200148511701172 discussion 1172–117311334288
- ToproverMKrasnokutskySPillingerMHGout in the spine: imaging, diagnosis outcomesCurr Rheumatol Rep2015177026490179
- DalbethNHaskardDOMechanisms of inflammation in goutRheumatology (Oxford)2005441090109615956094
- PankhaniaACPatankarTDu PlessisDNeck pain: an unusual presentation of a common diseaseBr J Radiol20067953753916714761
- MekelburgKRahimiARGouty arthritis of the spine: clinical presentation and effective treatmentsGeriatrics200055717410771704
- StiburkovaBMiyataHZavadaJNovel dysfunctional variant in ABCG2 as a cause of severe tophaceous gout: biochemical, molecular genetics and functional analysisRheumatology (Oxford)20165519119426428519
- GeorgeRLKeenanRTGenetics of hyperuricemia and gout: implications for the present and futureCurr Rheumatol Rep201315230923307580
- MatsuoHIchidaKTakadaTCommon dysfunctional variants in ABCG2 are a major cause of early-onset goutSci Rep20133201423774753
- DalbethNStampLKMerrimanTRThe genetics of gout: towards personalised medicine?BMC Med201715110828566086
- VolkovARhoineyDLClaybrooksRTophaceous gout of the lumbar spine: case report and review of the literatureTurk Neurosurg201525695495826617149
- BeierCPHartmannAWoertgenCBrawanskiARothoerlRDA large, erosive intraspinal and paravertebral gout tophus. Case reportJ Neurosurg Spine20053648548716381213
- LiuTLiuHZhuTThoracic spinal cord compression by extradural tophus: a case report and review of the literatureSpinal Cord Ser Cases2015115015
- WillnerNMonoranuCMStetterCErnestusRIWestermaierTGout tophus on an intradural fascicle: a case descriptionEur Spine J201625suppl 1162166
- KonatalapalliRMDemarcoPJJelinekJSGout in the axial skeletonJ Rheumatol200936360961319208604
- ChangICSurgical versus pharmacologic treatment of intraspinal goutClin Orthop Relat Res2005433106110
- TsaiCHChenYJHsuHCChenHTBacteremia coexisting with tophaceous gout of the spine mimicking spondylodiscitis: a case reportSpine (Phila Pa 1976)2009342E106E10919139655
- UdayakumarDKtelehTAlfataSBaliTJosephASpinal gout mimicking paraspinal abscess: a case reportJ Radiol Case Rep2010461520
- HsuCYShihTTHuangKMChenPQSheuJJLiYWTophaceous gout of the spine: MR imaging featuresClin Radiol2002571091992512413917
- KellyJLimCKamelMKeohaneCO’SullivanMTopacheous gout as a rare cause of spinal stenosis in the lumbar region. Case reportJ Neurosurg Spine2005221521715739537
- ZhengZFShiHLXingYLiDJiaJYLinSThoracic cord compression due to ligamentum flavum gouty tophus: a case report and literature reviewSpinal Cord2015531288188626078231
- YamamotoMTabeyaTMasakiYTophaceous gout in the cervical spineIntern Med20125132532822293812
- FraserJFAnandVKSchwartzTHEndoscopic biopsy sampling of tophaceous gout of the odontoid process. Case report and review of the literatureJ Neurosurg Spine200771616417633489
- PopovichTCarpenterJSRaiATCarsonLVWilliamsHJMaranoGDSpinal cord compression by tophaceous gout with fluorodeoxyglucose-positron-emission tomographic/MR fusion imagingAJNR Am J Neuroradiol20062761201120316775264
- YoonJWParkKBParkHTophaceous gout of the spine causing neural compressionKorean J Spine201310318518824757485
- HuHJLiaoMYXuLYClinical utility of dual-energy CT for gout diagnosisClin Imaging201539588088525725947
- NicolaouSLiangTMurphyDTKorzanJROuelletteHMunkPDual-energy CT: a promising new technique for assessment of the musculoskeletal systemAJR Am J Roentgenol20121995 supplS78S8623097171
- ChoiHKAl-ArfajAMEftekhariADual energy computed tomography in tophaceous goutAnn Rheum Dis2009681609161219066180
- ChristensenHDShetaHMMorillonMBHansenIMTophaceous gout in an anorectic patient visualized by dual energy computed tomography (DECT)Am J Case Rep20161749449827418121
- BaerANKuranoTThakurUJDual-energy computed tomog-raphy has limited sensitivity for non-tophaceous gout: a comparison study with tophaceous goutBMC Musculoskelet Disord2016179126891750
- RichettePBardinTGoutLancet201037531832819692116
- KhannaDFitzgeraldJDKhannaPP2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemiaArthritis Care Res (Hoboken)2012641431144623024028
- RichettePDohertyMPascualE2016 updated EULAR evidence-based recommendations for the management of goutAnn Rheum Dis2017761294227457514
- PascartTRichettePCurrent and future therapies for goutExpert Opin Pharmacother201718121201121128689430
- TerkeltaubREmerging uricosurics for goutExpert Rev Clin Pharmacol20171024724927937050
- ElgafyHLiuXHerronJSpinal gout: a review with case illustrationWorld J Orthop201671176677527900275
- HouLCHsuARVeeravaguABoakyeMSpinal gout in a renal transplant patient: a case report and literature reviewSurg Neurol20076716573 discussion 7317210304
