Abstract
Cerebral palsy is an important health issue that has a strong socioeconomic impact. There is no cure for cerebral palsy, and therapeutic approaches only report small benefits for affected people. In this study we assessed the effects of growth hormone treatment (0.3 μg/kg/day) combined with physical rehabilitation in the recovery of gross motor function in children with growth hormone deficiency and cerebral palsy (four males and six females, mean age 5.63 ± 2.32 years) as compared with that observed in a similar population of cerebral palsy children (five males, five females, mean age 5.9 ± 2.18 years) without growth hormone deficiency treated only with physical rehabilitation for two months. The Gross Motor Function Measure (GMFM-88) and Modified Ashworth Scale were performed before commencing the treatment and after completion thereof. In children with cerebral palsy and growth hormone deficiency, Dimension A (P < 0.02), dimension B (P < 0.02), and dimension C (P < 0.02) of the GMFM-88, and the total score of the test (P < 0.01) significantly improved after the treatment; dimension D and dimension E did not increase, and four of five spastic patients showed a reduction in spasticity. However, in children with cerebral palsy and without growth hormone deficiency, only the total score of the test improved significantly after the treatment period. This indicates that growth hormone replacement therapy was responsible for the large differences observed between both groups in response to physical rehabilitation. We propose that the combined therapy involving growth hormone administration and physical rehabilitation may be a useful therapeutic approach in the recovery of gross motor function in children with growth hormone deficiency and cerebral palsy.
Introduction
Cerebral palsy describes a group of disorders of the development of movement and posture, causing activity limitation, that are attributed to nonprogressive disturbances that occurred in the developing fetal or infant brain. The motor disorders of cerebral palsy are often accompanied by disturbances of sensation, cognition, communication, perception, and/or behavior, and/or by a seizure disorder.Citation1 Cerebral palsy is the most common cause of physical disability in childhood.Citation2 The estimated prevalence of cerebral palsy in the general population is 2/1000,Citation3 and the cumulative incidence rate of cerebral palsy in the age group 5–7 years is 2.7/1000.Citation3 Major causes of cerebral palsy involve prematurity (40%–50% of cases), abnormal intrauterine development due to fetal-maternal infections, asphyxia during delivery, brain trauma during labor and delivery, and complications in the perinatal period.Citation4 Independent of causal factors responsible for the development of cerebral palsy, the disease has a strong socioeconomic impact.Citation5 Currently there is no cure for cerebral palsy, and the therapeutic approaches of physical therapy, occupational therapy, speech therapy, neuropsychology, pharmacology, and surgery achieve only partial benefits for affected individuals.Citation3
It has been shown that children with cerebral palsy often have poor linear growth during childhood, resulting in a diminished final adult height,Citation6 an issue that has received little attention so far.Citation6–Citation10 In the studies that have been reported, the investigators have demonstrated that children with cerebral palsy show cerebral palsy deficient growth hormone secretion, by using provocative tests for growth hormone, that insulin-like growth factor (IGF-1) and low growth hormone levels in children with cerebral palsy may explain their low height and that decreased plasma IGF-1 and IGF binding protein 3 (IGFBP3) levels are associated with osteopenia in children with cerebral palsy.Citation6–Citation10 We have previously shown that 70% of a population of 46 children with cerebral palsy lacked normal growth hormone secretion.Citation3 Among the phenomena that could cause impaired growth hormone secretion are the presence of an altered pool of neurotransmitters, psychosocial deprivation, or suboptimal nutritional status.Citation3
Neural plasticity and regeneration of the brain, in response to both neurologic injury and different neurological stimulations have been widely demonstrated in animal modelsCitation11–Citation14 and in clinical studies using imaging techniques,Citation15–Citation18 electro-physiologic assessments,Citation19,Citation20 and clinical tests.Citation21–Citation25 Moreover, it has been reported that the mechanisms of plasticity are increased in the developing central nervous system, so that children who suffer injury at this stage could achieve more complete recovery than adult patients.Citation26
Several hormones play a role in the recovery from brain injuries, acting either on neurogenesis and/or neural plasticity. Among these, the growth hormone-IGF-1 system seems to be very important for inducing adult neurogenesis and increasing brain plasticity.Citation3 Growth hormone and IGF-1 and their receptors are expressed locally in the brainCitation27–Citation36 and both hormones can cross the blood–brain barrier. Citation36 Thus, besides its role in several metabolic processes, it has been shown that the growth hormone- IGF-1 axis exerts multiple and important neurotrophic effects related to cell proliferation and survival, both in the central and peripheral nervous system.Citation3,Citation36 In fact, a number of studies have demonstrated that this endocrine axis induces strong proliferation of neural cell precursors in major neurogenic niches, under both physiologic and pathologic conditions.Citation35,Citation37–Citation39 Moreover, growth hormone expression is increased in the affected brain hemisphere after an ischemic injury.Citation40,Citation41 On the other hand, we demonstrated, for the first time, that growth hormone is an important factor for cell survival.Citation42 Thus, both growth hormone and IGF-1 display marked neuroprotectiveCitation43,Citation44 and antiapoptoticCitation45–Citation47 effects.
In a previous study we postulated that growth hormone replacement therapy might be useful in the treatment of children with cerebral palsy, not only for achieving a more normal height but also for correcting some of the neurologic disorders that these patients suffer.Citation3 This study was designed to test this hypothesis, and evaluated the effects of growth hormone treatment combined with physical rehabilitation in the recovery of gross motor function in children with growth hormone deficiency and cerebral palsy as compared with the effects obtained in a similar population of cerebral palsy children without growth hormone deficiency and treated only with physical rehabilitation. Despite the fact that exercise is a powerful stimulus for pituitary growth hormone secretion, cerebral palsy children without growth hormone deficiency subjected to intense physical therapy rehabilitation do not achieve significant improvements. We studied the effects of growth hormone therapy in children with cerebral palsy and growth hormone deficiency as compared with those obtained in children with cerebral palsy and without growth hormone deficiency subjected to the same physical rehabilitation. The rationale for this was to test whether or not exogenous growth hormone administration plays a role in neurogenesis and/or brain plasticity.
Method
Participants
The study was conducted in 20 children with cerebral palsy, comprising nine males and 11 females, aged 4.0–10.5 years, who attended the Medical Center Proyecto Foltra for physical and cognitive rehabilitation, selected from a population of 46 cerebral palsy children.Citation3 The reason for selecting 20 children from 46 was to achieve the best possible match between the groups with regard to age, gender, disabilities exhibited, and clinical history. The selection was made by the principal investigator, and none of the therapists knew whether or not the children selected had growth hormone deficiency. All patients were Level IV or V according to the Gross Motor Function Classification System.Citation48 Ten of these cerebral palsy children had been previously detected to have growth hormone deficiency,Citation3 and comprised the study group. The main characteristics of the patients in each group studied are shown in and .
Table 1 Main characteristics of control group patients
Table 2 Main characteristics of study group patients
Treatment of growth hormone deficiency
After obtaining informed consent from their parents, the children with cerebral palsy and growth hormone deficiency were given growth hormone (rhGH, Omnitrope®, Sandoz) 0.3 μg/kg/day subcutaneously for five days per week at 10.30 pm for two months). Growth hormone administration commenced when physical rehabilitation started.
Blood sample analyses was performed for routine hematology and biochemistry parameters. Baseline anterior pituitary hormone secretion and plasma levels of free T4 were measured by chemiluminescence assays. In addition, fasting plasma IGF-1 and IGFBP3 were measured by solid-phase, enzyme-labeled chemiluminescent immunometric assay (Inmulite 2000, Siemens). These analyses were carried out before commencing the study and at the end of the study.
Clinical assessment
To assess motor function in our patients, we used a widely recognized test, the Gross Motor Function Measure (GMFM-88).Citation49 The GMFM-88 is a scale constructed for evaluation of change in gross motor function in children with cerebral palsy. The GMFM-88 consists of 88 items grouped into five dimensions, ie, dimension A (lying and rolling, 17 items), dimension B (sitting, 20 items), dimension C (crawling and kneeling, 14 items), dimension D (standing, 13 items) and dimension E (walking, running, and jumping, 24 items). Scores for each dimension are expressed as a percentage of the maximum score for that dimension, adding the scores for all dimensions, and dividing by 5 to obtain the total score. The reliability, validity, and responsiveness of the GMFM-88 scores are documented for children with cerebral palsy.Citation50 We also used the Modified Ashworth ScaleCitation51 to measure spasticity in our spastic patients. The GMFM-88 and Modified Ashworth Scale were performed before commencing the treatment and after two months of treatment.
Intervention
All patients received physical rehabilitation, which was carried out for 45 minutes per day, five days per week, for two months. The therapy basically involved psychomotor and postural re-education based on neurodevelopmental reactions, phases, and processes (ie, neurodevelopmental therapy), and its contents were adapted to the specific needs of each patient, depending on the initial clinical assessment. In general, the treatment was aimed at normalizing muscle tone and improving normal postural reactions, postural control of head and trunk, balance, upper limb motor function, and mobility.
To avoid any kind of bias, each therapist was assigned to treat children having cerebral palsy with or without growth hormone deficiency, without knowing their growth hormone status. The Modified Ashworth scale of spasticity was analyzed by one of the physiotherapists who did not know the growth hormone status of the patient he was examining. Both groups of patients received the same type and duration of physical rehabilitation.
Data analysis
Pre- and post-treatment data from the GMFM-88 were compared using a nonparametric test for two related samples (Wilcoxon signed-rank test and Mann-Whitney U test). Data from the Modified Ashworth Scale were analyzed descriptively.
Results
In the study group, combined treatment using rhGH and physical rehabilitation led to significant improvements in dimension A (lying and rolling, P < 0.02), dimension B (sitting, P < 0.02), dimension C (crawling and kneeling, P < 0.02), and the total score of the GMFM-88 (P < 0.01); dimension D (standing) and dimension E (walking, running, and jumping) did not increase ( and ). Moreover, four of five spastic patients showed a reduction in their spasticity as measured by the Modified Ashworth Scale (). However, no significant changes were observed in any of these dimensions in the control group, although the total score significantly increased in these children ( and ), and their spasticity was not improved (data not shown).
Figure 1 Clinical data from the GMFM-88.
Notes: A) Control group. B) Study group; “A”, “B”, “C”, “D” and “E” in the horizontal axis are the different dimensions of the GMFM-88 and “TOTAL” is the total score of test. We show pre-treatment (white bars) and post-treatment (grey bars) means and standard errors for each specific assessment. Statistical significance was calculated from data obtained in the GMFM-88 before treatment and after 2 months of it (Wilcoxon signed-rank test) (*P < 0.05, **P < 0.02 and ***P < 0.01).
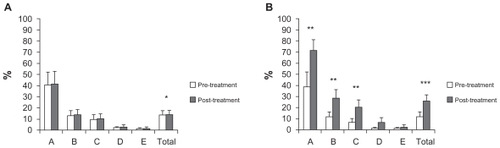
Table 3 Results obtained from the GMFM-88
Table 4 Results obtained from the modified ashworth scale in spastic patients from the study group
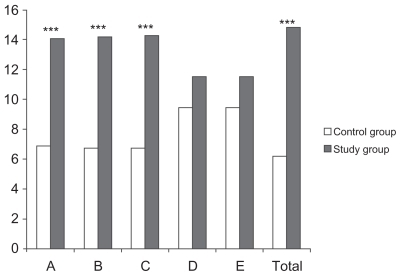
The differences between the improvements achieved at the end of the two-month treatment period for both groups are shown in and . Clearly, as these data show, children with cerebral palsy and growth hormone deficiency achieved significant improvements in dimensions A, B, and C, and in total score than the control children with cerebral palsy.
Figure 2 Study group improvements compared with control group improvements
Growth hormone administration did not produce any secondary clinical effects. Plasma IGF-1 levels were not significantly different between the two groups of children at the end of the treatment period, and plasma cholesterol levels decreased significantly (P < 0.05) in children with cerebral palsy and growth hormone deficiency.
Discussion
Our results show that combined treatment using rhGH and physical rehabilitation leads to significant improvements in gross motor functions, including lying, rolling, sitting, crawling, and kneeling. All this tasks are contained in the normal ontogeny of human movement, and are important to be able to perform daily activities. However, other gross motor functioning, such as standing, walking, running, and jumping did not improve. This could be due to the brief duration of treatment and the subjects’ high level in the Gross Motor Function Classification System, such that they were not able to perform more complex motor tasks. Future studies with larger populations and a longer duration of treatment may be able to demonstrate differences in all motor functions tested.
Spasticity is defined as resistance to passive movement of the joints and is a key component of the so-called upper motor neuron syndrome.Citation52 Spasticity, in its broadest clinical sense, has been linked to various motoneuronal,Citation53 spinal,Citation54 and supraspinalCitation52,Citation55 pathophysiologic phenomena. Thus, we can assume that the reduction in post-treatment spasticity in four of five spastic patients could be related to the efficacy of the treatment used to achieve normalization of the balance of supraspinal inhibitory and excitatory signals,Citation52,Citation55 of the secondary structural and functional changes that occur at cellular level in the spinal cord itself below the level of the injury,Citation54 and/or of the voltage-dependent persistent intrinsic motoneuronal inflows.Citation53
The new physiotherapy concept, including whole body vibration, physiotherapy, resistance training, and treadmill training, without drug treatment for six months, had a significant and positive effect on bone mineral density, muscle force, and gross motor function in bilaterally spastic children with cerebral palsy.Citation56 However, a systematic review did not find a statistically significant effect of partial bodyweight- supported treadmill training in children with cerebral palsy, despite improvements in gross motor function. Citation57 In a review of randomized controlled trials on physical therapy interventions in children with cerebral palsy, it was shown that some methods (such as neurodevelopmental therapy) were moderately effective in improving motor function in the upper extremities, walking speed, and stride amplitude. However, conflicting evidence was found for the effect of strength training on gross motor function.Citation58 Similarly, a meta-analysis of randomized trials demonstrated that muscle strengthening had a small statistically significant, but not clinically meaningful, effect on gross motor function.Citation59
On the other hand, some studies have described the effects of growth hormone replacement in children with cerebral palsy. However, most of them have only reported growth data and have not assessed any possible functional recovery.Citation6,Citation7,Citation60
To our knowledge, this study is the first to demonstrate the positive effects of growth hormone administration in the recovery of some motor functions in children with cerebral palsy. Despite the fact that these were observed in children with cerebral palsy and growth hormone deficiency, because plasma IGF-1 values were similar in both groups at the end of the treatment period, it seems clear that exogenous administration of growth hormone was responsible for the effects observed. According to plasma IGF values, growth hormone status was similar in both groups at the end of the treatment period. In this regard, exercise is known to be a powerful stimulus for endogenous growth hormone release, and it has been demonstrated that inhibiting PI3-Akt signaling, one of the pathways by which growth hormone acts,Citation42 blocks exercise-mediated enhancement of adult neurogenesis and synaptic plasticity in rats.Citation61 This may explain the lack of positive effects obtained in children undergoing exhaustive daily physical work, perhaps because possible growth hormone deficiency has not been determined or treated. On the other hand, it has been recently demonstrated that exogenous growth hormone administration induces strong cellular proliferation in rodents with growth hormone deficiency.Citation62 The data observed in our study support our hypothesis about the effects of growth hormone administration.
Thus, we conclude that combined therapy involving growth hormone replacement and physical rehabilitation is a useful therapeutic approach in the recovery of gross motor function in children with cerebral palsy and growth hormone deficiency. We are now carrying out larger and longer studies that will allow us to understand more precisely the effects of hormone treatment on children with cerebral palsy, aimed at improving both endocrine and neurologic disorders in children with cerebral palsy with or without growth hormone deficiency.
Acknowledgment
This study was supported by Foundation Foltra.
Disclosure
The authors report no conflicts of interest in this work.
References
- BaxMGoldsteinMRosenbaumPProposed definition and classification of cerebral palsyDev Med Child Neurol200547857157616108461
- Kerem GunelMRehabilitation of children with cerebral palsy from a physiotherapist’s perspectiveActa Orthop Traumatol Turc2009432173180 Turkish19448358
- DevesaJDevesaPReimundePGrowth hormone deficiency and cerebral palsyTher Clin Risk Manag2010641341820856687
- Krageloh-MannICansCCerebral palsy updateBrain Dev200931753754419386453
- KruseMMichelsenSIFlachsEMBronnum-HansenHMadsenMUldallPLifetime costs of cerebral palsyDev Med Child Neurol200951862262819416329
- ShimMLMoshangTJrOppenheimWLCohenPIs treatment with growth hormone effective in children with cerebral palsy?Dev Med Child Neurol200446856957115287249
- ConiglioSJStevensonRDGrowth hormone deficiency in two children with cerebral palsyDev Med Child Neurol19953711101310158566448
- ConiglioSJStevensonRDRogolADApparent growth hormone deficiency in children with cerebral palsyDev Med Child Neurol19963897978048810711
- AliOShimMFowlerECohenPOppenheimWSpinal bone mineral density, IGF-1 and IGFBP-3 in children with cerebral palsyHorm Res200768631632017912004
- KupermincMNGurkaMJHoulihanCMPuberty, statural growth, and growth hormone release in children with cerebral palsyJ Pediatr Rehabil Med20092213114120216931
- KleimJALussnigESchwarzERComeryTAGreenoughWTSynaptogenesis and fos expression in the motor cortex of the adult rat after motor skill learningJ Neurosci19961614452945358699262
- NudoRJMillikenGWJenkinsWMMerzenichMMUse-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeysJ Neurosci19961627858078551360
- JonesTAChuCJGrandeLAGregoryADMotor skills training enhances lesion-induced structural plasticity in the motor cortex of adult ratsJ Neurosci19991922101531016310559423
- BiernaskieJCorbettDEnriched rehabilitative training promotes improved forelimb motor function and enhanced dendritic growth after focal ischemic injuryJ Neurosci200121145272528011438602
- MarshallRSPereraGMLazarRMKrakauerJWConstantineRCDeLaPazRLEvolution of cortical activation during recovery from corticospinal tract infarctionStroke200031365666110700500
- NellesGJentzenWJueptnerMMullerSDienerHCArm training induced brain plasticity in stroke studied with serial positron emission tomographyNeuroimage2001136 Pt 11146115411352620
- CareyJRKimberleyTJLewisSMAnalysis of fMRI and finger tracking training in subjects with chronic strokeBrain2002125Pt 4773788 Growth hormone in cerebral palsy children11912111
- Johansen-BergHDawesHGuyCSmithSMWadeDTMatthewsPMCorrelation between motor improvements and altered fMRI activity after rehabilitative therapyBrain2002125Pt 122731274212429600
- LiepertJMiltnerWHBauderHMotor cortex plasticity during constraint-induced movement therapy in stroke patientsNeurosci Lett19982501589696052
- LiepertJUhdeIGrafSLeidnerOWeillerCMotor cortex plasticity during forced-use therapy in stroke patients: A preliminary studyJ Neurol2001248431532111374097
- BehrmanALBowdenMGNairPMNeuroplasticity after spinal cord injury and training: An emerging paradigm shift in rehabilitation and walking recoveryPhys Ther200686101406142517012645
- DalyJJRuffRLConstruction of efficacious gait and upper limb functional interventions based on brain plasticity evidence and model- based measures for stroke patientsScientific World Journal200772031204518167618
- DunlopSAActivity-dependent plasticity: Implications for recovery after spinal cord injuryTrends Neurosci200831841041818602172
- LanghornePCouparFPollockAMotor recovery after stroke: A systematic reviewLancet Neurol20098874175419608100
- RensinkMSchuurmansMLindemanEHafsteinsdottirTTask- oriented training in rehabilitation after stroke: Systematic reviewJ Adv Nurs200965473775419228241
- JohnstonMVPlasticity in the developing brain: Implications for rehabilitationDev Disabil Res Rev20091529410119489084
- GossardFDihlFPelletierGDuboisPMMorelGIn situ hybridization to rat brain and pituitary gland of growth hormone cDNANeurosci Lett19877932512563658217
- AraujoDMLapchakPACollierBChabotJGQuirionRInsulin-like growth factor-1 (somatomedin-C) receptors in the rat brain: Distribution and interaction with the hippocampal cholinergic systemBrain Res1989484121301382713673
- WertherGAAbateMHoggALocalization of insulin-like growth factor-I mRNA in rat brain by in situ hybridization – relationship to IGF-I receptorsMol Endocrinol1990457737782177145
- LaiZNEmtnerMRoosPNybergFCharacterization of putative growth hormone receptors in human choroid plexusBrain Res199154622222262070259
- LobiePEGarcia-AragonJLincolnDTBarnardRWilcoxJNWatersMJLocalization and ontogeny of growth hormone receptor gene expression in the central nervous systemBrain Res Dev Brain Res1993742225233
- AguadoFRodrigoJCacicedoLMellstromBDistribution of insulin-like growth factor-I receptor mRNA in rat brain. Regulation in the hypothalamo-neurohypophysial systemJ Mol Endocrinol19931122312398297478
- D’ErcoleAJYePCalikogluASGutierrez-OspinaGThe role of the insulin-like growth factors in the central nervous systemMol Neurobiol19961332272558989772
- ChungYHShinCMJooKMKimMJChaCIRegion-specific alterations in insulin-like growth factor receptor type I in the cerebral cortex and hippocampus of aged ratsBrain Res2002946230731312137935
- AbergMAAbergNDPalmerTDIGF-I has a direct proliferative effect in adult hippocampal progenitor cellsMol Cell Neurosci2003241234014550766
- AbergNDBryweKGIsgaardJAspects of growth hormone and insulin-like growth factor-I related to neuroprotection, regeneration, and functional plasticity in the adult brainScientific World Journal2006186538016432628
- AbergMAAbergNDHedbackerHOscarssonJErikssonPSPeripheral infusion of IGF-I selectively induces neurogenesis in the adult rat hippocampusJ Neurosci20002082896290310751442
- ChristophidisLJGorbaTGustavssonMGrowth hormone receptor immunoreactivity is increased in the subventricular zone of juvenile rat brain after focal ischemia: A potential role for growth hormone in injury-induced neurogenesisGrowth Horm IGF Res200919649750619524466
- AbergNDJohanssonIAbergMAPeripheral administration of GH induces cell proliferation in the brain of adult hypophysectomized ratsJ Endocrinol2009201114115019171566
- BeilharzEJRussoVCButlerGCo-ordinated and cellular specific induction of the components of the IGF/IGFBP axis in the rat brain following hypoxic-ischemic injuryBrain Res Mol Brain Res19985921191349729323
- GustafsonKHagbergHBengtssonBABrantsingCIsgaardJPossible protective role of growth hormone in hypoxia-ischemia in neonatal ratsPediatr Res199945331832310088648
- CostoyaJAFinidoriJMoutoussamySSenarisRDevesaJArceVMActivation of growth hormone receptor delivers an antiapoptotic signal: Evidence for a role of akt in this pathwayEndocrinology1999140125937594310579361
- ScheepensAWilliamsCEBreierBHGuanJGluckmanPDA role for the somatotropic axis in neural development, injury and diseaseJ Pediatr Endocrinol Metab.200013Suppl 61483149111202225
- ScheepensASirimanneESBreierBHClarkRGGluckmanPDWilliamsCEGrowth hormone as a neuronal rescue factor during recovery from CNS injuryNeuroscience2001104367768711440801
- ShinDHLeeEKimJWProtective effect of growth hormone on neuronal apoptosis after hypoxia-ischemia in the neonatal rat brainNeurosci Lett20043541646814698483
- GuanJWilliamsCGunningMMallardCGluckmanPThe effects of IGF-1 treatment after hypoxic-ischemic brain injury in adult ratsJ Cereb Blood Flow Metab19931346096168314914
- GuanJBennetLGeorgeSInsulin-like growth factor-1 reduces postischemic white matter injury in fetal sheepJ Cereb Blood Flow Metab200121549350211333359
- PalisanoRRosenbaumPWalterSRussellDWoodEGaluppiBDevelopment and reliability of a system to classify gross motor function in children with cerebral palsyDev Med Child Neurol19973942142239183258
- RussellDJAveryLMRosenbaumPLRainaPSWalterSDPalisanoRJImproved scaling of the gross motor function measure for children with cerebral palsy: Evidence of reliability and validityPhys Ther200080987388510960935
- PalisanoRJHannaSERosenbaumPLValidation of a model of gross motor function for children with cerebral palsyPhys Ther2000801097498511002433
- BohannonRWSmithMBInterrater reliability of a modified Ashworth scale of muscle spasticityPhys Ther19876722062073809245
- SheeanGThe pathophysiology of spasticityEur J Neurol.20029Suppl 13911918643
- GorassiniMAKnashMEHarveyPJBennettDJYangJFRole of motoneurons in the generation of muscle spasms after spinal cord injuryBrain2004127Pt 102247225815342360
- NielsenJBCroneCHultbornHThe spinal pathophysiology of spasticity – from a basic science point of viewActa Physiol (Oxf)2007189217118017250567
- DietzVSupraspinal pathways and the development of muscle- tone dysregulationDev Med Child Neurol1999411070871510587049
- StarkCNikopoulou-SmyrniPStabreyASemlerOSchoenauEEffect of a new physiotherapy concept on bone mineral density, muscle force and gross motor function in children with bilateral cerebral palsyJ Musculoskelet Neuronal Interact201010215115820516632
- MutluAKrosschellKSpiraDGTreadmill training with partial body- weight support in children with cerebral palsy: A systematic reviewDev Med Child Neurol200951426827519207302
- AnttilaHAutti-RamoISuorantaJMakelaMMalmivaaraAEffectiveness of physical therapy interventions for children with cerebral palsy: A systematic reviewBMC Pediatr200881418435840
- ScianniAButlerJMAdaLTeixeira-SalmelaLFMuscle strengthening is not effective in children and adolescents with cerebral palsy: A systematic reviewAust J Physiother2009552818719463078
- AliOShimMFowlerEGrowth hormone therapy improves bone mineral density in children with cerebral palsy: A preliminary pilot studyJ Clin Endocrinol Metab200792393293717179200
- Bruel-JungermanEVeyracADufourFHorwoodJLarocheSDavisSInhibition of PI3-Akt signaling blocks exercise-mediated enhancement of adult neurogenesis and synaptic plasticity in the dentate gyrusPloS One2009411e790119936256
- David AbergNLindJIsgaardJGeorg KuhnHPeripheral growth hormone induces cell proliferation in the intact adult rat brainGrowth Horm IGF Res201020326426920106687