Abstract
Background
Though many studies have been performed to elucidate the association between circulating vitamin D and prostate cancer, no conclusive result is available. We carried out a dose–response meta-analysis to quantitatively examine the association of circulating 25-hydroxyvitamin D (25[OH]D) concentration with prostate cancer.
Methods
Only prospective studies examining the associations of circulating 25[OH]D concentration with prostate cancer were eligible for the meta-analysis. A random-effect meta-analysis was done first, to calculate the summary relative risk (RR) and 95% confidence intervals (CIs) comparing the higher concentration with the lower concentration of 25[OH]D. A dose–response meta-analysis using random-effects model was then carried out to evaluate the nonlinearity and calculate the summary RR caused per 10 ng/mL increment.
Results
Nineteen prospective cohort or nested case–control studies were included. Higher 25[OH]D concentration was significantly correlated with elevated risk of prostate cancer (RR =1.15, 95% CI 1.06–1.24). No nonlinear relationship was found between 25[OH]D concentration and risk of prostate cancer (P=0.654). Dose–response meta-analysis showed that the summary RR caused per 10 ng/mL increment in circulating 25[OH]D concentration was 1.04 (95% CI 1.02–1.06). Subgroup analysis also found a modest dose–response relationship. Funnel plot and Egger’s test did not detect publication bias.
Conclusion
The findings suggest that highest 25[OH]D concentration is correlated with elevated risk of prostate cancer and a modest dose–response effect exists in this association; however, more studies are needed.
Introduction
Prostate cancer is the most common malignancy among men worldwide.Citation1 In addition, the incidence of prostate cancer has increased significantly in most Asian populations.Citation2 There has been a lot of progress in the therapeutic options including novel molecularly targeted therapeutics for prostate cancer patients in the past decade.Citation3,Citation4 Over the past decade, many clinical or experimental studies have provided many fundamental insights into the pathogenesis of prostate cancer.Citation5–Citation7 There are a number of risk factors for prostate cancer reported in published literatures, such as vasectomy and alcohol intake.Citation8–Citation10 However, there is still limited number of modifiable risk factors identified for prostate cancer and more studies are needed to identify some modifiable risk factors associated with prostate cancer.
The roles of vitamin D in human diseases have received increased attention, and it has been regarded as a vital hormone to maintain the normal functions of various organs or systems in the bodies.Citation11–Citation14 Vitamin D has some extraskeletal biological functions including inhibiting the progression of cancer cells.Citation15,Citation16 A previous study has found that vitamin D can exert a key role in decreasing cancer risk.Citation17 Meta-analyses of epidemiological studies have suggested that higher circulating 25-hydroxyvitamin D (25[OH]D) concentration is correlated with decreased risks of several common cancers, such as colorectal cancer and bladder cancer.Citation18,Citation19 Considering the preventive effect of vitamin D against cancer, many researchers also studied the association of circulating 25[OH]D concentration with prostate cancer.Citation20–Citation28 Some studies reported that higher serum 25[OH]D concentration modestly increased the risk of prostate cancer.Citation26,Citation29,Citation30 However, other studies did not find any correlation of vitamin D with prostate cancer.Citation25,Citation27–Citation29 These studies have obtained controversial results on the impact of circulating 25[OH]D on prostate cancer risk, and no conclusive result is available. Thus, we carried out a comprehensive literature search and performed a meta-analysis to examine the association of circulating 25[OH]D concentration with prostate cancer.
Methods
Search strategy and inclusion criteria
PubMed and Web of Science were searched for prospective studies, examining the correlation of circulating 25[OH] D concentration with prostate cancer, which were eligible for the meta-analysis. We carried out the literature search on December 20, 2016. We used combinations of the following keywords: (“vitamin D” or “25-hydroxyvitamin D” or “25[OH]D”) AND (“prostate cancer” or “prostate carcinoma”). The references from included articles were also checked to identify any additional studies.
Only prospective cohort studies or nested case–control studies examining the associations of circulating 25[OH]D concentration with prostate cancer and reporting relative risks (RRs) of prostate cancer across at least three categories of circulating 25[OH]D levels were eligible for the meta-analysis. Case–control studies, cross-sectional studies, and retrospective cohort studies were excluded. Studies without RRs of prostate cancer across at least three categories of 25[OH]D concentrations were also excluded. Studies containing overlapping data were also excluded.
Data extraction and quality assessment
Two investigators extracted data independently, and any disagreement was resolved by consensus among all investigators. For each study, we extracted RRs of prostate cancer comparing the upper categories of circulating 25[OH]D concentration with the lowest category of circulating 25[OH]D level. For the dose–response meta-analysis, the number of cases and noncases, concentration level, and adjusted RR for each category and its 95% confidence interval (CI) were extracted. For the studies that did not provide the median or mean levels of serum 25[OH]D, we used the midpoint of each category as the alternative. For the open-ended category, the midpoint of this category was calculated by assuming that the interval was the same as that of the adjacent category. When the numbers of cases/noncases in each category were not available, the numbers were estimated by the methods proposed by Aune et al.Citation31 For studies that did not set the lowest category as reference, we used the method described by Hamling et alCitation32 to make a transformation. Furthermore, we gathered information on study design, country, sample size, matching factors, and time of follow-up or from blood collection to diagnosis. Studies with >300 prostate cancer cases were defined as studies with large sample size, while those with <300 prostate cancer cases were defined as studies with small sample size. The quality assessment was done by the recommendation from Newcastle–Ottawa Scale (NOS), which encompassed three perspectives including selection of participants, comparability, and outcome assessment, and studies scoring at least 6 stars were classified as high-quality studies.Citation33
Statistical analysis
The homogeneity among those included studies was estimated by the I2 statistic, and I2>50% represented high concentration of heterogeneity.Citation34 A random-effect meta-analysis was first done to calculate the summary RR and 95% CI comparing the higher concentration with the lower concentration of 25[OH]D.Citation35 The dose–response meta-analysis was performed using the method proposed by Greenland and LongneckerCitation36 and Orsini et al.Citation37 In order to explore the nonlinear dose–response curve, serum 25[OH]D concentrations were modeled using restricted cubic splines with three knots at fixed percentiles (0.10, 0.50, and 0.90) of the distribution. The P-value of nonlinearity was calculated by testing against the null hypothesis that the coefficient of the second spline was equal to 0. If the nonlinearity was not statistically significant, the linear dose–response outcomes were presented per 10 ng/mL (25 nmol/L) increment in serum 25[OH]D by random-effects model.Citation35–Citation37
Subgroup analysis was performed by sample size, publication year, study designs, and adjustment for calcium intake. Sensitivity analysis was carried out by excluding any single study by turns. Publication bias was evaluated by funnel plot and the Egger test.Citation38 The traditional meta-analysis was carried out using STATA (Version 12.0), and the dose–response meta-analysis was performed by R and its dosresmeta package.Citation39
Results
Characteristics of included studies
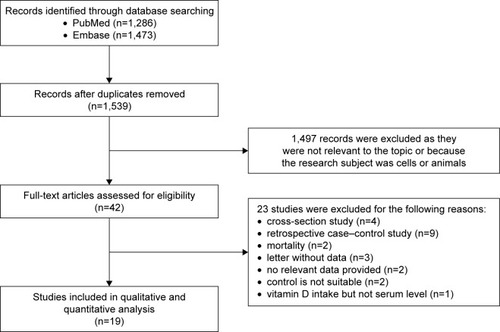
The study selection process is shown in . Though >1,530 articles were found, only 42 studies were possibly eligible and evaluated by checking the full texts.Citation16,Citation20–Citation30,Citation40–Citation69 Twenty-three studies were then excluded,Citation16,Citation40–Citation61 and the remaining 19 studies were considered eligible.Citation20–Citation30,Citation62–Citation69 There were three prospective cohort studies and 16 nested case–control studies (). Most studies were carried out in Europe and USA except one study from Japan (). The number of prostate cancer cases in those 19 studies varied obviously and ranged from 61 to 2,106 (). A total of 12,786 prostate cancer cases and 35,583 participants were included in those 19 studies. There were seven studies with <300 prostate cancer cases and 12 studies with >300 prostate cancer cases (). All 19 studies reported the adjusted RRs of prostate cancer across at least three categories of circulating 25[OH]D levels. According to the NOS criteria, all included studied scored >6 stars and thus had high quality.
Table 1 Characteristics of included studies on the association between circulating vitamin D concentrations and prostate cancer
Meta-analysis
When performing meta-analysis of RRs comparing the higher concentration with the lower concentration of 25[OH]D, there was good homogeneity among those included studies (I2=0%). Higher 25[OH]D concentration was significantly correlated with elevated risk of prostate cancer (RR =1.15, 95% CI 1.06–1.24, P=0.001) (). The summary RR was not significantly changed in the sensitivity analysis. As shown in , in the subgroup analysis of studies with small sample size, with cohort study design, there was no significant correlation of circulating 25[OH]D concentration with prostate cancer. The adjustment for calcium supplementation did not change the positive association between the serum 25[OH]D and risk of prostate cancer.
Figure 2 Higher 25[OH]D concentration was significantly correlated with elevated risk of prostate cancer.
Abbreviations: 25[OH]D, 25-hydroxyvitamin D; CI, confidence interval; RR, relative risk.
![Figure 2 Higher 25[OH]D concentration was significantly correlated with elevated risk of prostate cancer.](/cms/asset/0c1c5781-d9e3-4d93-90b1-9f6a7b2d977a/dtcr_a_149325_f0002_c.jpg)
Table 2 The results of subgroup analysis between 25[OH]D concentration and risk of prostate cancer
For the dose–response meta-analysis, as shown in , there was no nonlinear relationship between circulating 25[OH]D concentration and the risk of prostate cancer (P=0.654). When performing meta-analysis of RRs of prostate cancer risk caused by per 10 ng/mL increment in circulating 25[OH]D level, there was also good homogeneity among those included studies (I2=0%). Linear dose–response meta-analysis showed the summary RR caused by per 10 ng/mL increment in circulating 25[OH]D concentration was 1.04 (95% CI 1.02–1.06, P<0.001) (). The summary RR was not significantly changed in the sensitivity analysis. As shown in , subgroup analysis using data from studies of large sample size also found a modest dose–response relationship (RR =1.04, 95% CI 1.02–1.06, P<0.001). However, subgroup analysis using data from studies of small sample size or cohort study design did not find an obvious dose–response relationship ().
Figure 3 Nonlinear dose–response relationship between 25[OH]D concentration and risk of prostate cancer.
![Figure 3 Nonlinear dose–response relationship between 25[OH]D concentration and risk of prostate cancer.](/cms/asset/8af8512b-cafd-4959-93f3-c6477b4ce363/dtcr_a_149325_f0003_b.jpg)
Figure 4 Linear dose–response relationship between circulating 25[OH]D concentration and prostate cancer.
Abbreviations: 25[OH]D, 25-hydroxyvitamin D; CI, confidence interval; RR, relative risk.
![Figure 4 Linear dose–response relationship between circulating 25[OH]D concentration and prostate cancer.](/cms/asset/2c1fcfbb-a381-4cb9-9f3c-46677134e9b3/dtcr_a_149325_f0004_c.jpg)
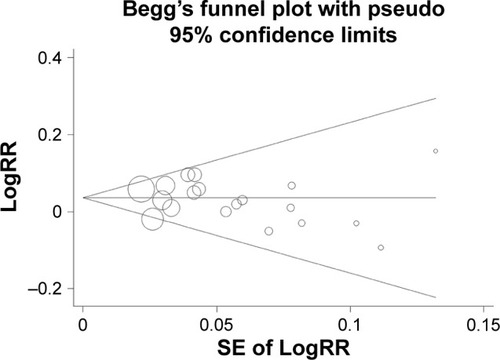
The funnel plot did not detect publication bias (). Besides, the P-value of Egger test was 0.48 and provided another evidence for the lack of publication bias.
Discussion
Though the preventive roles of vitamin D have been found in several cancers, its role in the development of prostate cancer is still unclear. Those published studies did not report consistent findings. We therefore carried out a dose–response meta-analysis to quantitatively elucidate the impact of circulating 25[OH]D concentration on prostate cancer. A total of 19 prospective studies were finally eligible for the meta-analysis. We found that higher 25[OH]D concentration was significantly correlated with elevated risk of prostate cancer (RR =1.15, P=0.001; ). Dose–response meta-analysis showed the summary RR of prostate cancer caused by per 10 ng/mL increment was 1.04 (P<0.001; ). Therefore, the findings from the meta-analysis suggested that higher 25[OH]D concentration was correlated with elevated risk of prostate cancer and a modest dose–response effect existed in this association.
In human bodies, vitamin D is mainly synthesized in the skin after exposure to solar UV radiation and vitamin D can also be ingested from some foods.Citation70,Citation71 25[OH]D is the hydroxylated form of vitamin D, which is the mostly used biomarker of circulating vitamin D and widely used in clinical practice.Citation72 A large number of published studies have found that vitamin D can exert a key role in decreasing cancer risk.Citation17–Citation19,Citation73–Citation75 The antitumor effects of vitamin D have been well established in several cancers, such as colorectal cancer and bladder cancer.Citation18,Citation19 On the contrary, some studies found that vitamin D did not exert an antitumor effect in prostate cancer but even caused elevated risk of prostate cancer.Citation26,Citation29,Citation30 This present meta-analysis of 19 prospective studies provided epidemiological evidence for the tumor-promoting effect of vitamin D in prostate cancer though the effect was modest. However, no clear biological relationship has been found between high levels of vitamin D and an increased risk of prostate cancer. We can only speculate on the cause for the tumor-promoting effect of vitamin D in prostate cancer.Citation25 One reason might be that 25[OH]D may be a marker of other factors that related to the risk of prostate cancer. For example, insulin-like growth factor-I (IGF-I) has been related to prostate cancerCitation76–Citation78 and a relationship between 25[OH]D and insulin-like growth factor-1 has been reported.Citation79 Moreover, higher 25[OH]D might be associated with an increased detection rate of prostate cancer.Citation25 However, we cannot rule out this detection bias using the summary outcome from the included studies in our research. The findings in the meta-analysis may have important indications from the supplementation of vitamin D in men. The use of vitamin D in men with high risk of prostate cancer may be cautious considering the tumor-promoting effect of vitamin D in prostate cancer.
A major strength of this meta-analysis was the inclusion of a total of 19 prospective cohort studies or nested case–control studies. The large number of participants in the meta-analysis could help us quantitatively examine the association of circulating 25[OH]D concentration with prostate cancer and get a more credible finding. As shown in , all included studies used a prospective design and reported adjusted RRs of prostate cancer, which ensured the appropriate selection of participants, the correct assessment of outcomes. In addition, there were 12 studies with >300 prostate cancer cases, which could increase the statistical power and decrease the risk of possible bias caused by small sample size (). Another strength of this meta-analysis was the good homogeneity among those included studies (I2=0%), which suggested the lack of obvious heterogeneity in the meta-analysis. There was good homogeneity in both the meta-analysis of RRs comparing the higher concentration with the lower concentration of 25[OH]D and the meta-analysis of RRs of prostate cancer risk caused by per 10 ng/mL increment. There is no doubt that the homogeneity could strengthen the evidence for the tumor-promoting effect of vitamin D in prostate cancer found in the meta-analysis.
There were several limitations and the outcomes should be interpreted cautiously. First, some included studies did not consider the influence of other factors, such as vitamin D intake and sun exposure, on the association between circulating 25[OH]D concentration and prostate cancer, which might cause possible risk of bias. Therefore, more studies taking into account those factors are needed to provide a more definite assessment of the influence of circulating 25[OH]D concentration on prostate cancer risk. Second, the reagents used to detect circulating 25[OH]D concentration were various across those included studies, which could cause possible heterogeneity in the meta-analysis. However, there was good homogeneity among those included studies (I2=0%), which proved the little influence of different reagents used to detect circulating 25[OH]D concentration in the meta-analysis. Third, because all the included studies were done in developed countries and most studies were done in the Western countries (northern Europe and USA), the findings could not be generalized to other countries from different ethnicities. There was only one study with small sample size from Asian countries.Citation28 Participants in the studies that conducted in the USA were mostly white, and only one study with moderate sample size had multiple ethnics.Citation65 Therefore, more studies assessing the correlation of vitamin D with prostate cancer risk from other ethnicities and developing countries are needed. Finally, results of subgroups were based on a limited number of studies and we cannot rule out the possibility that insufficient statistical power may be present.
Conclusion
The findings from the meta-analysis suggest that higher 25[OH]D concentration is correlated with elevated risk of prostate cancer and a modest dose–response effect exists. Besides, these results need to be validated in further studies. The biological explanation for the positive correlation of vitamin D with prostate cancer risk is unclear, and further research is needed to address this issue.
Disclosure
The authors report no conflicts of interest in this work.
References
- AttardGParkerCEelesRAProstate cancerLancet201638710013708226074382
- ItoKProstate cancer in Asian menNat Rev Urol201411419721224595118
- RitchCRCooksonMSAdvances in the management of castration resistant prostate cancerBMJ2016355i440527754846
- YapTASmithADFerraldeschiRAl-LazikaniBWorkmanPde BonoJSDrug discovery in advanced prostate cancer: translating biology into therapyNat Rev Drug Discov2016151069971827444228
- SprattDEZumstegZSFengFYTomlinsSATranslational and clinical implications of the genetic landscape of prostate cancerNat Rev Clin Oncol2016131059761027245282
- LeeJKPhillipsJWSmithBAN-Myc drives neuroendocrine prostate cancer initiated from human prostate epithelial cellsCancer Cell201629453654727050099
- Cancer Genome Atlas Research NetworkThe molecular taxonomy of primary prostate cancerCell201516341011102526544944
- ZhaoJStockwellTRoemerAChikritzhsTIs alcohol consumption a risk factor for prostate cancer? A systematic review and meta-analysisBMC Cancer201616184527842506
- KellySPGraubardBIAndreottiGYounesNClearySDCookMBPrediagnostic body mass index trajectories in relation to prostate cancer incidence and mortality in the PLCO cancer screening trialJ Natl Cancer Inst20171093djw22527754927
- NayanMHamiltonRJMacdonaldEMCanadian Drug Safety and Effectiveness Research Network (CDSERN)Vasectomy and risk of prostate cancer: population based matched cohort studyBMJ2016355i554627811008
- ReynoldsJRayDAlexanderMYBruceIRole of vitamin D in endothelial function and endothelial repair in clinically stable systemic lupus erythematosusLancet2015385suppl 1S8326312905
- ChristakosSDhawanPVerstuyfAVerlindenLCarmelietGVitamin D: metabolism, molecular mechanism of action, and pleiotropic effectsPhysiol Rev201696136540826681795
- LangerJPenna-MartinezMBonDHerrmannEWallaschMBadenhoopKInsufficient vitamin D response to solar radiation in German patients with type 2 diabetes or gestational diabetesHorm Metab Res201648850350827525476
- Maia-CecilianoTCBarreto-ViannaARBarbosa-da-SilvaSAguilaMBFariaTSMandarim-de-LacerdaCAMaternal vitamin D-restricted diet has consequences in the formation of pancreatic islet/insulin-signaling in the adult offspring of miceEndocrine2016541606927142413
- KellyJLSallesGGoldmanBLow serum vitamin D levels are associated with inferior survival in follicular lymphoma: a prospective evaluation in SWOG and LYSA studiesJ Clin Oncol201533131482149025823738
- NyameYAMurphyABBowenDKAssociations between serum vitamin D and adverse pathology in men undergoing radical prostatectomyJ Clin Oncol201634121345134926903577
- FeldmanDKrishnanAVSwamiSGiovannucciEFeldmanBJThe role of vitamin D in reducing cancer risk and progressionNat Rev Cancer201414534235724705652
- GarlandCFGorhamEDDose–response of serum 25-hydroxyvitamin D in association with risk of colorectal cancer: a meta-analysisJ Steroid Biochem Mol Biol20161681827993551
- ZhaoYChenCPanWComparative efficacy of vitamin D status in reducing the risk of bladder cancer: a systematic review and network meta-analysisNutrition201632551552326822497
- BraunMMHelzlsouerKJHollisBWComstockGWProstate cancer and prediagnostic levels of serum vitamin D metabolites (Maryland, United States)Cancer Causes Control1995632352397612803
- NomuraAMStemmermannGNLeeJSerum vitamin D metabolite levels and the subsequent development of prostate cancer (Hawaii, United States)Cancer Causes Control1998944254329794175
- BaronJABeachMWallaceKRisk of prostate cancer in a randomized clinical trial of calcium supplementationCancer Epidemiol Biomarkers Prev200514358658915767334
- BarnettCMNielsonCMShannonJSerum 25-OH vitamin D levels and risk of developing prostate cancer in older menCancer Causes Control20102181297130320383574
- BrandstedtJAlmquistMManjerJMalmJVitamin D, PTH, and calcium and the risk of prostate cancer: a prospective nested case–control studyCancer Causes Control20122381377138522706676
- MeyerHERobsahmTEBjorgeTBrustadMBlomhoffRVitamin D, season, and risk of prostate cancer: a nested case–control study within Norwegian health studiesAm J Clin Nutr201397114715423193007
- KristalARTillCSongXPlasma vitamin D and prostate cancer risk: results from the selenium and vitamin E cancer prevention trialCancer Epidemiol Biomarkers Prev20142381494150424732629
- SkaabyTHusemoenLLThuesenBHProspective population-based study of the association between serum 25-hydroxyvitamin-D levels and the incidence of specific types of cancerCancer Epidemiol Biomarkers Prev20142371220122924789846
- SawadaNInoueMIwasakiMPlasma 25-hydroxy vitamin D and subsequent prostate cancer risk in a nested case–control study in Japan: the JPHC studyEur J Clin Nutr201771113213627759068
- SchenkJMTillCATangenCMSerum 25-hydroxyvitamin D concentrations and risk of prostate cancer: results from the prostate cancer prevention trialCancer Epidemiol Biomarkers Prev20142381484149325085836
- AlbanesDMondulAMYuKSerum 25-hydroxy vitamin D and prostate cancer risk in a large nested case–control studyCancer Epidemiol Biomarkers Prev20112091850186021784952
- AuneDGreenwoodDCChanDSBody mass index, abdominal fatness and pancreatic cancer risk: a systematic review and non-linear dose–response meta-analysis of prospective studiesAnn Oncol201223484385221890910
- HamlingJLeePWeitkunatRAmbuhlMFacilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease categoryStat Med200827795497017676579
- WellsGASheaBO’ConnellDThe Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses2015 Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.aspAccessed September 28, 2017
- HigginsJPThompsonSGDeeksJJAltmanDGMeasuring inconsistency in meta-analysesBMJ2003327741455756012958120
- DerSimonianRLairdNMeta-analysis in clinical trialsControl Clin Trials1986731771883802833
- GreenlandSLongneckerMPMethods for trend estimation from summarized dose–response data, with applications to meta-analysisAm J Epidemiol199213511130113091626547
- OrsiniNLiRWolkAKhudyakovPSpiegelmanDMeta-analysis for linear and nonlinear dose–response relations: examples, an evaluation of approximations, and softwareAm J Epidemiol20121751667322135359
- EggerMDavey SmithGSchneiderMMinderCBias in meta-analysis detected by a simple, graphical testBMJ199731571096296349310563
- CrippaAOrsiniNMultivariate dose–response meta-analysis: the dosresmeta R packageJ Stat Softw201672Code Snippet 115
- AtoumMFAlKateebDAlHaj MahmoudSAThe Fok1 vitamin D receptor gene polymorphism and 25(OH) D serum levels and prostate cancer among Jordanian menAsian Pac J Cancer Prev20151662227223025824742
- ChooCSMamedovAChungMChooRKissADanjouxCVitamin D insufficiency is common in patients with nonmetastatic prostate cancerNutr Res2011311212621310302
- FangFKasperzykJLShuiIPrediagnostic plasma vitamin D metabolites and mortality among patients with prostate cancerPLoS One201164e1862521494639
- GalunskaBGerovaDKosevPAnakievskiDHinevASerum 25-hydroxy vitamin D levels in Bulgarian patients with prostate cancer: a pilot studyClin Lab2015613–432933525975000
- GilbertRMartinRMFraserWDPredictors of 25-hydroxyvitamin D and its association with risk factors for prostate cancer: evidence from the prostate testing for cancer and treatment studyCancer Causes Control201223457558822382867
- GilbertRMetcalfeCFraserWDAssociations of circulating 25-hydroxyvitamin D, 1,25-dihydroxyvitamin D, and vitamin D pathway genes with prostate-specific antigen progression in men with localized prostate cancer undergoing active monitoringEur J Cancer Prev201322212112522955340
- GrantWBA multicountry ecologic study of risk and risk reduction factors for prostate cancer mortalityEur Urol200445327127915036670
- GrantWBThe roles of ultraviolet-B irradiance, vitamin D, apolipoprotein E epsilon4, and diet in the risk of prostate cancerCancer Causes Control201122115715821052818
- HendricksonWKFlavinRKasperzykJLVitamin D receptor protein expression in tumor tissue and prostate cancer progressionJ Clin Oncol201129172378238521537045
- JacksonMDTulloch-ReidMKLindsayCMBoth serum 25-hydroxyvitamin D and calcium levels may increase the risk of incident prostate cancer in Caribbean men of African ancestryCancer Med20154692593525858172
- JohnEMSchwartzGGKooJVan Den BergDInglesSASun exposure, vitamin D receptor gene polymorphisms, and risk of advanced prostate cancerCancer Res200565125470547915958597
- MurphyABNyameYMartinIKVitamin D deficiency predicts prostate biopsy outcomesClin Cancer Res20142092289229924789033
- NelsonSMBataiKAhaghotuCAgurs-CollinsTKittlesRAAssociation between serum 25-hydroxy-vitamin D and aggressive prostate cancer in African American menNutrients20169112
- PallerCJKanaanYMBeyeneDARisk of prostate cancer in African-American men: evidence of mixed effects of dietary quercetin by serum vitamin D statusProstate201575131376138326047130
- PazdioraPSvobodovaSFuchsovaRVitamin D in colorectal, breast, prostate and lung cancer: a pilot studyAnticancer Res201131103619362121965787
- RocaEValcamonicoFAmorosoVSerum vitamin D and prostate cancer prognosis: the story continuesJ Clin Oncol2016343037093710
- SchwartzGGVitamin D in blood and risk of prostate cancer: lessons from the selenium and vitamin E cancer prevention trial and the prostate cancer prevention trialCancer Epidemiol Biomarkers Prev20142381447144925085835
- SteckSEArabLZhangHAssociation between plasma 25-hydroxyvitamin D, ancestry and aggressive prostate cancer among African Americans and European Americans in PCaPPLoS One2015104e012515125919866
- StephanCLeinMMatalonJSerum vitamin D is not helpful for predicting prostate cancer aggressiveness compared with the prostate health indexJ Urol2016196370971426976204
- TrumpDLChadhaMKSungaAYVitamin D deficiency and insufficiency among patients with prostate cancerBJU Int2009104790991419426195
- TsengMBreslowRAGraubardBIZieglerRGDairy, calcium, and vitamin D intakes and prostate cancer risk in the National Health and Nutrition Examination Epidemiologic Follow-up Study cohortAm J Clin Nutr20058151147115415883441
- YaturuSZdunekSYoungbergBVitamin D levels in subjects with prostate cancer compared to age-matched controlsProstate Cancer2012201252420623304521
- AhnJPetersUAlbanesDProstate, Lung, Colorectal, and Ovarian Cancer Screening Trial Project TeamSerum vitamin D concentration and prostate cancer risk: a nested case–control studyJ Natl Cancer Inst20081001179680418505967
- Faupel-BadgerJMDiawLAlbanesDVirtamoJWoodsonKTangreaJALack of association between serum levels of 25-hydroxyvitamin D and the subsequent risk of prostate cancer in Finnish menCancer Epidemiol Biomarkers Prev200716122784278618086789
- JacobsETGiulianoARMartinezMEHollisBWReidMEMarshallJRPlasma levels of 25-hydroxyvitamin D, 125-dihydroxyvitamin D and the risk of prostate cancerJ Steroid Biochem Mol Biol200489–901–5533537
- ParkSYCooneyRVWilkensLRMurphySPHendersonBEKolonelLNPlasma 25-hydroxyvitamin D and prostate cancer risk: the multiethnic cohortEur J Cancer201046593293620064705
- PlatzEALeitzmannMFHollisBWWillettWCGiovannucciEPlasma 1,25-dihydroxy- and 25-hydroxyvitamin D and subsequent risk of prostate cancerCancer Causes Control200415325526515090720
- ShuiIMMucciLAKraftPVitamin D-related genetic variation, plasma vitamin D, and risk of lethal prostate cancer: a prospective nested case–control studyJ Natl Cancer Inst2012104969069922499501
- TravisRCCroweFLAllenNESerum vitamin D and risk of prostate cancer in a case–control analysis nested within the European Prospective Investigation into Cancer and Nutrition (EPIC)Am J Epidemiol2009169101223123219359375
- TuohimaaPTenkanenLAhonenMBoth high and low levels of blood vitamin D are associated with a higher prostate cancer risk: a longitudinal, nested case–control study in the Nordic countriesInt J Cancer2004108110410814618623
- Bandera MerchanBMorcilloSMartin-NunezGTinahonesFJMacias-GonzalezMThe role of vitamin D and VDR in carcinogenesis: through epidemiology and basic sciencesJ Steroid Biochem Mol Biol201616720321827913313
- TrummerCPandisMVerheyenNBeneficial effects of UV-radiation: vitamin D and beyondInt J Environ Res Public Health201613101028
- BataiKMurphyABNonnLKittlesRAVitamin D and immune response: implications for prostate cancer in African AmericansFront Immunol201675326941739
- IniestaRRPaciarottiIDavidsonI5-Hydroxyvitamin D concentration in paediatric cancer patients from Scotland: a prospective cohort studyBr J Nutr2016116111926193427974067
- ShanNLWahlerJLeeHJVitamin D compounds inhibit cancer stem-like cells and induce differentiation in triple negative breast cancerJ Steroid Biochem Mol Biol201617312212927923595
- LuanZMaYXinYQianJWangHPossible molecular mechanisms by which vitamin D prevents inflammatory bowel disease and colitis-associated colorectal cancerCurr Med Chem2016249911917
- ChanJMStampferMJGiovannucciEPlasma insulin-like growth factor-I and prostate cancer risk: a prospective studyScience199827953505635669438850
- ChanJMStampferMJMaJInsulin-like growth factor-I (IGF-I) and IGF binding protein-3 as predictors of advanced-stage prostate cancerJ Natl Cancer Inst200294141099110612122101
- StattinPBylundARinaldiSPlasma insulin-like growth factor-I, insulin-like growth factor-binding proteins, and prostate cancer risk: a prospective studyJ Natl Cancer Inst200092231910191711106682
- HypponenEBoucherBJBerryDJPowerC25-hydroxyvitamin D, IGF-1, and metabolic syndrome at 45 years of age: a cross-sectional study in the 1958 British Birth CohortDiabetes200857229830518003755