Abstract
Introduction
Lung metastasis of leiomyosarcoma that protrudes into the left atrium is an extremely rare condition. Severe complications may occur that prominently increase the mortality during the perioperative period. Currently, the anesthetic management reports are limited and there is no generally acknowledged algorithm available.
Case presentation
A 67-year-old man presented with cough and dyspnea for 10 days. Workup revealed bilateral pulmonary effusion. Transthoracic echocardiography showed a large mass in the left atrium. Urgent surgical resection under cardiopulmonary bypass was performed. We focused on oxygenation improvement and cardiac function management by applying protective ventilation with low positive end expiratory pressure, low dose inotropic agents, and other methods to maintain stable homeostasis. Results of biopsy established a diagnosis of metastatic leiomyosarcoma.
Conclusion
We reported a case of metastatic leiomyosarcoma presenting as a lung mass with left atrial extension and anesthetic management during surgical resection. Treating acute heart failure and refractory hypoxemia was the key focus perioperatively.
Introduction
Superficial leiomyosarcoma is a rare malignant tumor with an overall incidence of 0.04% among all cancers.Citation1 Lung metastasis of leiomyosarcoma that protrudes into the left atrium is extremely rare. Severe complications such as widespread emboli and circulation impairment may occur due to outflow obstruction. Anesthesia management of a patient under such circumstances is challenging and rarely reported, because the tumor may result in respiratory compromise and circulation collapse. In the present case, we described a patient who was preoperatively diagnosed as left atrial myxoma. He was scheduled for emergent left atrial mass resection under general anesthesia with cardiopulmonary bypass (CPB), and histopathological examination revealed features consistent with leiomyosarcoma. During the surgical procedure, the patient presented refractory hypoxemia, increased pulmonary arterial pressure and severely compromised cardiac function. Thus, we report the case focusing on the perioperative management during surgical resection of the tumor, including oxygenation improvement and circulation stabilization.
Case presentation
A 67-year-old man with a height of 183 cm and a weight of 105 kg was admitted for urgent resection of a left atrial mass. He presented with progressive heart failure that could not be alleviated by inotropic or vasodilating drugs and had been coughing for 10 days. The symptoms had aggravated, and he had been unable to keep supine position for the last 3 days. He denied any previous heart disease. He had a history of right elbow leiomyosarcoma and underwent resection surgery 1 year previously.
On physical examination, the patient showed orthopnea and a compulsive position of slight left tilting. Wet rales could be heard on bilateral lung base. III/6 diastolic rumble was heard through the apex of the heart, which attenuated with upright position.
The preoperative laboratory data were within normal limits, except for NT-proBNP 1,190 pg/L. The electrocardiogram showed sinus tachycardia and minor ST segment change. Chest X-ray demonstrated prominent pulmonary patches infiltration, suggesting possible pulmonary edema. A mass was noted on the left lung. Combined with the malignancy history of the patient, pulmonary metastasis was suspected, whereas primary pulmonary tumor could not be ruled out (). Transthoracic echocardiography (TTE) showed a mobile isoechoic to hyperechoic lumpy mass (54×27 mm, ) and medium pulmonary hypertension (67 mmHg), and the ejection fraction of the left ventricle was 65%.
Figure 1 Preoperative and postoperative chest X-ray of the patient.
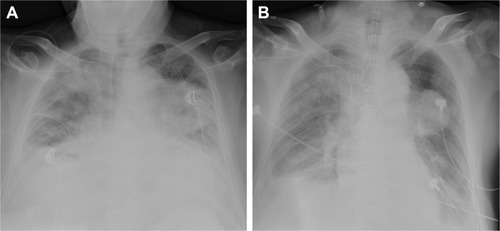
Figure 2 Hyperechoic lumpy mass detected on transthoracic echocardiography.
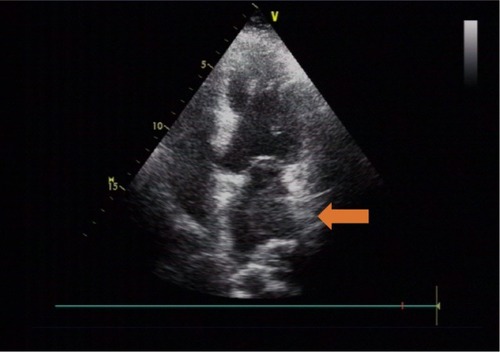
According to symptoms of left-sided heart failure, signs of mitral stenosis, and ultrasound imaging, myxoma was highly suspected. The TTE image indicated that the mass originated from the posterior wall of the left atrium (the typical myxoma is pedunculated with a stalk commonly attached to the fossa ovalis of interatrial septumCitation6). Differential diagnoses included idiopathic pulmonary arterial hypertension (IPAH), pulmonary embolism, pulmonary tumor thrombotic microangiopathy, and acute respiratory distress syndrome (ARDS). There is not enough evidence to support the differential diagnoses above.
In the operating room, monitors consisting of noninvasive blood pressure (NIBP), five-lead electrocardiogram, and pulse oximetry (SpO2) were applied. Radial arterial line and central venous catheter in the right subclavian vein were inserted, and then, arterial blood pressure (ABP) and central venous pressure (CVP) were monitored. Vital signs of the patient were basically stable with continuous intravenous nitroglycerin at the rate of 0.5 μg/kg/min. ABP was 121/82 mmHg, and CVP was 8 cmH2O, while heart rate (HR) was 111 bpm. His SpO2 was 92%–93% (fraction of inspired oxygen [FiO2] 60%). Airway assessment indicated several risk factors: obesity, short neck, impaired neck mobility, and Mallampati classification grade III. When the patient was awake with topical anesthesia of the tongue and pharynx, partial glottis was observed (Cormack–Lehane 2b) with direct laryngoscope. Thus, we considered the mask ventilation was feasible and anesthesia induction was conducted. After preoxygenation with oxygen (10 L/min) for 5 min via a face mask, general anesthesia was induced using Renfushufen® (sufentanil) 1 μg/kg, etomidate 0.15 mg/kg, and rocuronium 0.6 mg/kg (ideal body weight). Orotracheal intubation was performed with video laryngoscope when the patient was kept in the 60° Semi-Fowler’s position to avoid circulation collapse. Because mitral valve occlusion by left atrial mass may result in low cardiac output. Volume-controlled ventilation was applied (tidal volume of 8 mL/kg, frequency adjusted to maintain partial pressure of carbon dioxide in artery [PaCO2] between 35 and 45 mmHg, and inspiratory/expiratory (I/E) ratio 1:2). The airway peak pressure was 28–30 cmH2O. After intubation, the SpO2 was only 90%–93% (FiO2 60%). Arterial gas analysis during surgery is shown in . Abnormalities of airway, breathing, drug effects, and equipment were not found; thus, pulmonary edema was considered as the main reason for hypoxia. FiO2 100% was provided to improve oxygenation. Respiratory rate was increased to enhance minute ventilation volume. Positive end expiratory pressure (PEEP) was supplied to prevent alveoli from collapsing to improve pulmonary compliance and gas exchange and to decrease extravascular lung water.Citation7 PEEP was titrated to 6 cmH2O to avoid high PEEP-related compromise preload. Then, SpO2 improved to 94%–95%. Vital signs and ventilation parameters during surgery are shown in . Anesthesia was maintained with sufentanil 20 μg/h and sevoflurane 2%–2.5% (concentration of sevoflurane was adjusted according to the patient’s increased ageCitation8), and rocuronium was given intermittently.
Table 1 Arterial blood gas analysis and ACT during surgery
Table 2 Vital signs and ventilation parameters during surgery
After median sternotomy, 400 U/kg heparin was administered. Arterial cannulation was performed via ascending aorta. Venous cannulation was inserted via the right atrial appendage into the superior and inferior vena cava. After CPB was established, oxygenation of the patient improved significantly with the SpO2 improved to 99%–100%. The patient was kept anesthetized with midazolam 0.1 mg/kg/h, sufentanil 0.15 μg/kg/h, and rocuronium 0.15 mg/kg/h intravenously under hypothermic CPB, and nasopharyngeal and rectal temperature were maintained at 25°C and 28°C, respectively. Activated clotting time (ACT) was performed intermittently, and the results are shown in .
Under direct view, the mass was large and occupied nearly the entire left atrium. The right atrium and atrial septum were incised, and the mass was found attached by a stalk to the opening of the left superior pulmonary vein. The mass in the left atrium was totally resected, and was 15×2 cm. The tumor in the left superior pulmonary vein could not be completely resected, and palliative resection of the tumor around the opening of the left superior pulmonary vein was performed to ease tumor-related symptoms. As a result, preload was improved while pulmonary hypertension remained a problem. After rewarming and deairing of the heart, the aortic cross-clamp was removed. The heart came back into sinus rhythm with 20J intracardiac defibrillation, and then, the CPB was discontinued gradually. Weaning was facilitated with norepinephrine 0.05 μg/kg/min and dopamine 5 μg/kg/min to achieve goal mean arterial BP.65 mmHg. Nitroglycerin 0.5–0.8 μg/kg/min was used to decrease systemic vascular resistance. A total of 800 mL of packed red blood cells were gradually infused to increase preload. Vital signs showed an ABP of 110/70 mmHg, an HR of 110 bpm, a CVP of 12 cmH2O, and an SpO2 of 93%–95%. Heparin was neutralized with protamine (1 mg/100 U heparin). The total CPB time was 100 min, and the aortic occlusion time was 85 min. The overall fluid balance during CPB was −2,700 mL. The estimated blood loss was 800 mL. Arterial blood gas analysis after CPB discontinuation is also showed in .
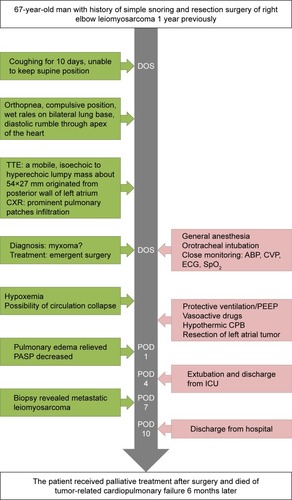
After CPB, the partial pressure of oxygen in artery (PaO2) was 68 mmHg with FiO2 100% and PEEP 6 cmH2O and was increased from that before tumor resection (48 mmHg). After excluding bronchospasm and acute lung injury, we assumed that this was due to unrelieved cardiogenic pulmonary edema and pulmonary hypertension, as well as residual tumor in the pulmonary vessels. Inotropic agents including epinephrine 0.05 μg/kg/min, dopamine 5 μg/kg/min, and nitroglycerin 0.5–0.8 μg/kg/min were continuously used to improve cardiac output and facilitate stable hemodynamics, so as to alleviate pulmonary edema. Hyperventilation was applied to decrease PaCO2 and prevent acidosis. Hypoxia pulmonary vasoconstriction may have been partially inhibited, and pulmonary artery systolic pressure was decreased from 67 to <35 mmHg (postoperative TTE). The patient was transferred to the intensive care unit with BP 110/71 mmHg, HR 110 bpm, and SpO2 95% during admission. Oxygenation was improved compared to the level before left atrial tumor resection (91 vs 48 mmHg), and HR was decreased (103 vs 111 bpm), indicating that pulmonary edema was partially relieved. Increasing FiO2, decreasing O2 consumption, and optimizing acid–base balance and hemoglobin, together with protective ventilation, were provided and resulted in oxygenation improvement. Vasoactive medications and mechanical ventilation were gradually weaned, and vital signs remained stable. Chest X-ray performed 3 h postoperatively revealed significantly relieved pulmonary edema (). The patient was extubated on postoperative day 4 while he was conscious, and the vital signs showed BP 121/72 mmHg, HR 72 bpm, and SpO2 95%–97% with FiO2 85%. He was discharged 10 days after the surgery with symptoms ameliorated and SpO2 97% with FiO2 21%. Histopathological examination of the resected lesion revealed features consistent with leiomyosarcoma. Further management of the lung metastasis of leiomyosarcoma and possible radiation therapy are needed.Citation9 Some authors suggest that multimodal therapy (surgical resection, radiation treatment, and chemotherapy) may result in reasonable survival improvement for patients.Citation10 Isambert et alCitation11 reported a mean survival of 18.2 months after incomplete resection and 11.2 months in nonresected patients. In our case, the patient received palliative treatment after surgery and died of tumor-related cardiopulmonary failure 6 months later. The timeline of history, interventions, and outcomes are summarized in .
Figure 3 Timeline of interventions and outcomes.
Consent for publication
Written informed consent was obtained from the patient’s next of kin for publication of this case report.
Discussion
Superficial leiomyosarcoma, a rare malignant lesion, constitutes 4.0%–6.5% of all soft tissue sarcomas,Citation1 including cutaneous and subcutaneous leiomyosarcoma. Subcutaneous leiomyosarcoma arises from vascular structures in the subcutaneous cellular tissue and has an increased risk of recurrence and metastasis.Citation12 Aneiros-Fernandez et alCitation12 concluded that subcutaneous subtype is likely to have a 18.28%–50% chance of recurrence and a 27.27%–66.66% chance to metastasize. The mortality rate of subcutaneous leiomyosarcoma is 9.09%–70%.Citation12 Pulmonary metastases may appear simultaneously with the diagnosis. Lung metastasis of subcutaneous leiomyosarcoma that protrudes in to left atrium is extremely rare. In fact, leiomyosarcoma accounts for <1% of tumors that metastasize to the heart.Citation2 Several cases of lung metastasis from leiomyosarcoma with atrial extension have been reported;Citation2–Citation5 two patients underwent surgical treatment and others received palliative therapy.
In our case, according to the patient’s past medical history, perioperative symptoms, clinical and experimental examinations, and histological findings, we considered the possibility of him having atrial extension of lung metastasis from leiomyosarcoma. The pathophysiology changes of this case simulated pathophysiology of lung cancer invading the left atrium. Metastatic tumor in the pulmonary vein and left atrium may cause congestive heart failure due to blood flow obstruction and left ventricle filling defect. Passive backward transmission of the elevated atrial pressure led to insufficient preload of the left ventricle, increased pulmonary hydrostatic pressure, and then pulmonary edema. Giant myxoma shares similar mechanisms, which made it difficult to identify specific etiology preoperatively.
Anesthetic management remains a great challenge due to compromised cardiac function, increased pulmonary arterial pressure, pulmonary effusion, and refractory hypoxemia.Citation13 Hypoxemia was a constant problem in our case throughout the perioperative period. The reasons could be the left atrium pulmonary vein inducing left atrial pressure elevation and pulmonary hypertension,Citation13 and related pulmonary edema.Citation5 During the process, PEEP could be used to improve oxygenation and pulmonary compliance. The optimal PEEP to balance oxygenation, compliance, and ventilation-induced lung injury remains to be investigated. Increased PEEP in ARDS could protect alveoli and improve arterial oxygenation but reduces cardiac output and impairs tissue oxygen delivery.Citation14 Ladha et alCitation15 suggested a PEEP of 5 cmH2O and a plateau pressure of ≤16 cmH2O as protective mechanical ventilator settings during operation. In our case, relatively low PEEP level and 100% FiO2 were applied after intubation to increase oxygenation and minimize the effects on cardiac output. Acute lung injury (ALI) could not be fully excluded, and protective ventilation has been reported to associate with a lower rate of ALI,Citation16 so protective ventilation including low tidal volume of 6–8 mL/kg with PEEP and recruitment maneuver was applied during operation. Packed red blood cells were transfused to maintain hematocrit >0.25 and increase oxygen carrying capacity. During anesthesia induction, the patient was kept in a fixed position to avoid an occlusion effect of the tumor and following hemodynamic collapse, and a stable intraoperative hemodynamic status was maintained to improve oxygenation.
It is important to prevent systemic thromboembolic events during resection of the tumor. Dias et alCitation17 reported that thromboembolic events mainly occurred in the central nervous system (10.2%), coronary arteries (4.8%), and lower limbs (4.3%) and the histological type of tumors correlates with embolic events and mortality. Resection of cardiac tumor has been suggested to prevent emboli and sudden death.Citation18 Left pulmonary vein atrial inflow patch closure has been reported as a preventive and less invasive measure to reduce thromboembolic risk.Citation19 In our case, palliative resection of the tumor around the opening of the left superior pulmonary vein was performed instead of left pulmonary vein patch closure to increase preload and ameliorate symptoms of left heart failure.
In some cases, partial or lobar pneumonectomy together with atrial mass resection can be performed depending on the degree of cardiac involvement and the stage of the tumor.Citation20 Besides, complete resection of the atrial tumor is impossible in some cases and the mortality rate is high.Citation21 In our case, pneumonectomy was not conducted simultaneously because of advanced pulmonary edema, unclear boundary of pulmonary infiltration, risks of hemorrhage, and possible patient intolerance. Considering the patient’s malignancy history, and suspicious mass indicated by chest X-ray, a preoperative CT or MRI scan was recommended. They were not conducted because change of the patient’s position may have resulted in sudden mitral orifice obstruction, and preoperative TTE was conducted instead. Because the cardiac tumor resulted in unstable hemodynamics and the diagnosis was uncertain, transesophageal echocardiography (TEE) and pulmonary artery catheter were not used during the case. However, TEE has potential advantages and is recommended by the American Society of Echocardiography.Citation22 It could be the best way to assess the left atrium volume, transmitral flow, and the hemodynamic effects of surgical resection, and we recommend the use of TEE without contraindications.
Conclusion
Lung metastasis with left atrial extension of leiomyosarcoma is a rare and challenging scenario, during which refractory hypoxemia, pulmonary edema, severe compromised cardiac function, and possible embolic events are main difficulties for anesthetic management. In our case, we focused on oxygenation and cardiac function management through applying protective ventilation with low PEEP, increase in FiO2, decrease in oxygen consumption, optimization of acid–base balance and hemoglobin, low dose of inotropic agents, as well as surgical resection of the tumor.
Data sharing statement
All data and materials described in the article will be freely available to any scientist wishing to use them for noncommercial purposes. Raw data are not available, as all are in the patient’s electronic medical record.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper, and agree to be accountable for all aspects of the work.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No 81400869) and Key Research Foundation from Peking University Third Hospital.
Disclosure
The authors report no conflicts of interest in this work.
References
- WascherRALeeMYRecurrent cutaneous leiomyosarcomaCancer19927024904921617597
- LopezFFMangiAMylonakisEChenJLSchiffmanFJAtrial fibrillation and tumor emboli as manifestations of metastatic leiomyosarcoma to the heart and lungHeart Lung2000291474910636956
- CollinsNJBarlowMAWoodfordPAHayesPCIntracardiac extension of metastatic pulmonary leiomyosarcomaHeart Lung Circ200514212112216352268
- KhanAAhmadMOmranAArifiAAPulmonary metastatic leiomyosarcoma invading the left atrium through the pulmonary veinsJ Saudi Heart Assoc201224321321423960699
- PengYJHuengGGLeeHSAcute heart failure as manifestation of metastatic uterine leiomyosarcoma to the heart and lungHeart Lung2004331464914983139
- BurkeATavoraFThe 2015 WHO classification of tumors of the heart and pericardiumJ Thorac Oncol201611444145226725181
- WiesenJOrnsteinMTonelliARMenonVAshtonRWState of the evidence: mechanical ventilation with PEEP in patients with cardiogenic shockHeart201399241812181723539555
- NickallsRWMaplesonWWAge-related iso-MAC charts for isoflurane, sevoflurane and desflurane in manBr J Anaesth200391217017412878613
- SalemisNSRecurrent subcutaneous trunk leiomyosarcoma: management and review of the literatureJ Nat Sci Biol Med20134123824223633873
- BakaeenFGJaroszewskiDERiceDCOutcomes after surgical resection of cardiac sarcoma in the multimodality treatment eraJ Thorac Cardiovasc Surg200913761454146019464464
- IsambertNRay-CoquardIItalianoAPrimary cardiac sarcomas: a retrospective study of the French Sarcoma GroupEur J Cancer201450112813624135684
- Aneiros-FernandezJAntonio RetameroJHusein-ElahmedHOvalleFAneiros-CachazaJPrimary cutaneous and subcutaneous leiomyosarcomas: evolution and prognostic factorsEur J Dermatol201626191226678649
- PorteousMKFritzJSHypoxemia in a patient with pulmonary arterial hypertension: getting to the heart of the matterAnn Am Thorac Soc201411583684024936696
- ChikhaniMDasAHaqueMWangWBatesDGHardmanJGHigh PEEP in acute respiratory distress syndrome: quantitative evaluation between improved arterial oxygenation and decreased oxygen deliveryBr J Anaesth2016117565065827799180
- LadhaKVidal MeloMFMcLeanDJIntraoperative protective mechanical ventilation and risk of postoperative respiratory complications: hospital based registry studyBMJ2015351h364626174419
- NetoASHemmesSNTBarbasCSVIncidence of mortality and morbidity related to postoperative lung injury in patients who have undergone abdominal or thoracic surgery: a systematic review and meta-analysisLancet Respir Med20142121007101525466352
- DiasRRFernandesFRamiresFJMadyCAlbuquerqueCPJateneFBMortality and embolic potential of cardiac tumorsArq Bras Cardiol20141031131825029470
- CianciulliTFSaccheriMCLaxJALeft ventricular thrombus mimicking primary cardiac tumor in a patient with primary antiphospholipid syndrome and recurrent systemic embolismCardiol J200916656056319950093
- SantiseGRaffaGMPilatoMSurgical treatment of systemic embolization by cardiac metastasis of lung cancerAsian Cardiovasc Thorac Ann20142291103110524887850
- ShimizuJIkedaCAranoYAdvanced lung cancer invading the left atrium, treated with pneumonectomy combined with left atrium resection under cardiopulmonary bypassAnn Thorac Cardiovasc Surg201016428629021057449
- AndrushchukUOstrovskyYZharkovVSurgery for massive malignant tumors of the left atrium – one center’s experienceKardiochir Torakochirurgia Pol201613322923527785137
- SaricMArmourACArnaoutMSGuidelines for the Use of Echocardiography in the Evaluation of a Cardiac Source of EmbolismJ Am Soc Echocardiogr201629114226765302
