Abstract
Background
While it is well known that teriparatide (TPTD) increases bone mineral density (BMD) in osteoporotic patients, it is unknown whether TPTD pretreatment affects BMD after denosumab (DMAb) therapy.
Methods
Fifty-seven patients in TPTD-pretreated group and 35 patients in DMAb-alone group had been further analyzed, all of whom were treated by DMAb for 1.5 years. Vitamin D (400 IU) and Ca (600 mg) supplementation was used in all patients. The BMD of lumbar 1–4 vertebrae (L-BMD), bilateral total hips (H-BMD), and bilateral femoral neck (FN-BMD) was quantified at first visit, and at 4, 8, 12, and 18 months after daily TPTD treatment following four times DMAb treatment.
Results
There were significant differences in L-BMD (p=0.004) and H-BMD (p=0.026) at baseline between TPTD-pretreated and DMAb-alone groups, although there was no significant difference in FN-BMD between the two groups. The increase of L-BMD by DMAb therapy was less in TPTD-pretreated group than in DMAb-alone group. There was no significant difference in H-BMD, although percent changes of H-BMD tended to be higher in the TPTD-pretreated group than those in the DMAb-alone group. Percent change in FN-BMD at 4 months (p=0.067) and 12 months (p=0.057) tended to be higher in TPTD-pretreated group than in DMAb-alone group. Percent change in FN-BMD at 18 months was significantly higher in TPTD-pretreated group (p=0.004) than in DMAb-alone group.
Conclusion
These findings suggest that the pretreatment of TPTD might have enhanced the increase of BMD in cortical bones treated by DMAb. Thus, it is favorable that TPTD can be used for osteoporotic patients who have high fracture risks with cortical bones.
Introduction
Osteoporosis (OP) is a systemic skeletal disease characterized by decreasing bone mass and microarchitectural deterioration of bone tissue that leads to an increased risk for bone fragility fracture. Pharmacologic treatments for OP include bisphosphonates (BPs), parathyroid hormone (PTH), and estrogen (in the form of menopausal hormone therapy) or selective estrogen receptor modulators (SERMs) for postmenopausal women. The ultimate goal of osteoporotic treatment is to prevent fracture, and to improve the activities of daily living and quality of life with accompanying fracture prevention. However, it is challenging to evaluate whether each drug can prevent fracture individually, and thus, bone mineral density (BMD) can be used to determine the efficacy of each drug.
Receptor activator of nuclear factor-κB ligand (RANKL) is a cytokine that plays an important role in bone resorption and remodeling through its effects on osteoclasts. Denosumab (DMAb), a fully humanized monoclonal antibody, binds and inhibits RANKL with high affinity and has a beneficial effect on bone remodeling.Citation1 The FREEDOM open-label extension study demonstrated that vertebral, nonvertebral, and hip fractures were decreased for up to 10 years after DMAb treatment and that BMD values increased linearly.Citation2 We have recently reported that DMAb could increase BMD even in BP-unresponsive cases.Citation3 Thus, it is considered one of the best therapeutic options for osteoporotic patients with respect to increase in BMD, improvement in bone turnover markers, and prevention of fractures.
Tsai et al have reported that the combined teriparatide (TPTD) (1- to 34-amino acid fragment of PTH) and DMAb therapy is useful to treat osteoporotic patients at high risk of fracture in the DATA study.Citation4 We also reported that the combination therapy of TPTD and DMAb could be useful for postmenopausal OP.Citation5 Leder et al have reported that the increase in BMD by DMAb after TPTD treatment is superior to that by TPTD alone. However, by TPTD treatment after DMAb therapy, lumbar BMD (L-BMD) decreased for the first 6 months, and then increased. In addition, total hip BMD (H-BMD) as well as femoral neck BMD (FN-BMD) decreased for 1 year, and then increased; however, percent changes of these were almost at baseline level after 2-year treatment of TPTD.Citation6 Thus, at the time of therapy, the order of application of DMAb and TPTD is very important.
TPTD is currently the only available drug to increase bone formation. It greatly increases L-BMD,Citation6,Citation7 and may be preferentially administered to osteoporotic patients, especially those with greatly diminished BMD and/or multiple vertebral fractures. Although TPTD prevents vertebral fractures and markedly increases L-BMD,Citation6,Citation7 the treatment period of TPTD is defined as less than 2 years in total. Thus, some suitable osteoporotic drugs should be used after the end of TPTD therapy. Generally, bone antiresorptive drugs are used after TPTD. Leder et al have reported that DMAb could improve BMD compared to BPs after the end of TPTD, comparing the increase of BMD between BPs and DMAb treatment.Citation6
Some authors have reported that TPTD induces new bone formation and improves cancellous bone microstructure and bone quality.Citation8–Citation10 Thus, we hypothesized that in lumbar spine, cancellous trabecular bones might be regenerated by TPTD, and DMAb might be able to enhance the increase of L-BMD in the TPTD-pretreated group than in the group treated with DMAb alone. In this study, we examined the increase of BMD in the cases treated with DMAb alone and those treated with DMAb after TPTD treatment.
Methods
Subjects and measurements
We have been performing TPTD treatment in cases with severe OP since TPTD was approved in November 2010 in Japan. Since the approval of DMAb in June 2013, we have been basically administering DMAb into BP-unresponsive patients, moderate osteoporotic patients, or patients after the end of TPTD. For this prospective study, we collected data of 101 primary osteoporotic patients except BP-unresponsive cases who underwent DMAb treatment between June 2013 and July 2015. We then classified the patients into the following two groups: 1) 62 severe osteoporotic cases who had taken TPTD for 2 years prior to DMAb treatment (TPTD-pretreated group) and 2) 39 moderate osteoporotic cases (DMAb-alone group). During the 1.5-year observational period, five cases dropped out in the TPTD-pretreated group while four cases dropped out in the DMAb-alone group. Finally, 57 cases in the TPTD-pretreated group and 35 cases in the DMAb-alone group were further analyzed, all of whom completed DMAb treatment for 1.5 years. Prior to selecting the subjects, male patients were excluded to avoid sex-related bias. No serious adverse events, such as hypocalcemia or fracture, occurred during the study.
The diagnosis of primary OP was made in accordance with the revised criteria established by the Japanese Society of Bone and Mineral Research.Citation11 All subjects received a single subcutaneous injection of 60 mg DMAb, and 400 IU vitamin D and 600 mg Ca supplementation was used in all the subjects treated with DMAb.
The L-BMD, H-BMD, and FN-BMD were quantified using a dual-energy X-ray absorption fan-beam bone densitometer (Lunar Prodigy; GE Healthcare, Little Chalfont, UK) at the baseline before DMAb treatment and at 4, 8, 12, and 18 months after DMAb treatment. Coefficients of variation of the BMD measurement at lumbar spine and hip were 0.6% and 0.5%, respectively. Least significant changes of these measurements were accordingly 1.6% and 1.5%, respectively.Citation12 Results are expressed as percent changes of each value in terms of mean ± SEM.
Data analysis
Age, L-BMD, H-BMD, and FN-BMD before DMAb treatment (baseline) between TPTD-pretreated and DMAb-alone groups were compared with an independent t-test. Mean percent changes in BMD from baseline at 4, 8, 12, and 18 months between TPTD-pretreated and DMAb-alone groups were also compared with an independent t-test. Repeated analysis of variance (ANOVA) with Bonferroni correction was used to assess the association between mean percent changes in BMD and period of the treatment in each treatment group. All p-values were two-sided, and values of p<0.05 were considered to indicate statistical significance. Data analyses were conducted with the Statistical Package for the Social Sciences (SPSS, version 19.0; IBM Inc., Armonk, NY, USA).
Ethical statement
This study was approved by the institutional ethics committee of Shinshu University School of Medicine and Showa-Inan General Hospital, and written informed consent was obtained from all patients. The methods were carried out in accordance with approved guidelines.
Results
There were significant differences in L-BMD (p=0.004) and H-BMD (p=0.026) at baseline between TPTD-pretreated and DMAb-alone groups, although no significant differences were observed in age and FN-BMD between the two groups ().
Table 1 Baseline characteristics of all subjects
The results for BMD are presented in and –. Mean percent change in FN-BMD at 18 months was significantly higher in TPTD-pretreated group (p=0.004) than in DMAb-alone group ( and ). Mean percent change in FN-BMD at 4 months (p=0.067) and 12 months (p=0.057) tended to be higher in TPTD-pretreated group than in DMAb-alone group, although there were no significant differences in other mean percent changes in skeletal BMD ( and –). The increase of L-BMD by DMAb therapy was less in TPTD-pretreated group than in DMAb-alone group ( and ). There was no significant difference in H-BMD, although percent changes of H-BMD tended to be higher in the TPTD-pretreated group than those in the DMAb-alone group ( and ).
Figure 1 Mean percent changes in BMD in the lumbar spine.
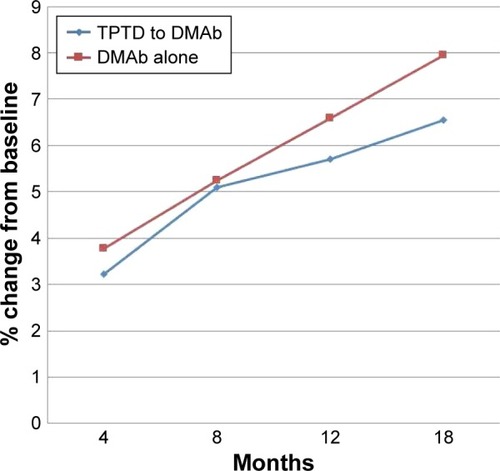
Figure 2 Mean percent changes in BMD in the total hips.
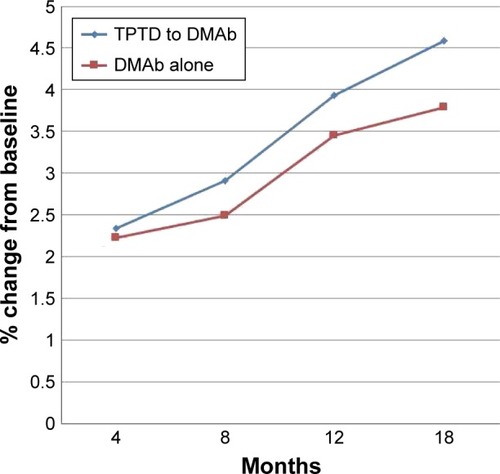
Figure 3 Mean percent changes in BMD in the femoral necks.
Abbreviations: BMD, bone mineral density; DMAb, denosumab; TPTD, teriparatide.
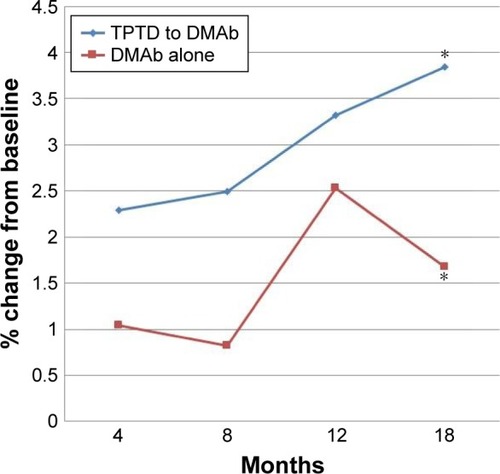
Table 2 Mean percent change in BMD from baseline at 4, 8, 12, and 18 months
Repeated ANOVA revealed that mean percent change in all skeletal BMDs was significantly associated with the period of the treatment in the TPTD-pretreated group (p<0.001). In the TPTD-pretreated group, mean percent change in L-BMD at 18 months was significantly higher than that at 4 months (p<0.001) and 8 months (p=0.001), and the change at 12 months was significantly higher than that at 4 months (p<0.001). Mean percent change in H-BMD at 18 months was significantly higher than that at 4 months (p<0.001), 8 months (p<0.001), and 12 months (p=0.026), and the change at 12 months was significantly higher than that at 4 months (p<0.001) and 8 months (p<0.001). Mean percent change in FN-BMD at 18 months was significantly higher than that at 4 months (p<0.001) and 8 months (p<0.001), and the change at 12 months was significantly higher than that at 4 months (p=0.001).
Repeated ANOVA revealed that mean percent change in L-BMD (p<0.001), H-BMD (p=0.003), and FN-BMD (p=0.015) was significantly associated with the period of the treatment in the DMAb-alone group. In the DMAb-alone group, mean percent change in L-BMD at 18 months was significantly higher than that at 4 months (p<0.001) and 8 months (p=0.001), and the change at 12 months was significantly higher than that at 4 months (p<0.001). Mean percent change in H-BMD at 18 months was significantly higher than that at 4 months (p=0.020) and 8 months (p=0.042), and the change at 12 months was significantly higher than that at 4 months (p=0.02) and 8 months (p=0.042).
Discussion
This study showed that contrary to our expectations, the increase of L-BMD caused by DMAb in the TPTD group tended to be less than that in the DMAb-alone group. On the other hand, the FN-BMD, which mostly involves cortical bone, was more significantly increased in the TPTD group than in the DMAb group.
TPTD is a widely used bone anabolic drug for OP which greatly increases BMD; especially, it increases the L-BMD since the spine consists of enriched cancellous bone. However, the increase of BMD in cortical bone by TPTD is approximately similar to that by other agents, while the BMD in the distal radius is decreased by TPTD.Citation6
Eastell et al have reported that based on aging, the imbalance in bone formation and resorption has effects on trabecular bone (loss of connectivity) and cortical bone (cortical thinning and porosity).Citation13 Bone turnover markers generally predict bone loss and fragility fracture. Shigdel et al have recently reported that the increased bone turnover marker levels are associated with higher cortical porosity, thinner cortices, larger bone size, and higher odds for fracture.Citation14 In addition, Ahmed et al have described that cortical porosity at the proximal femur is a quantifiable risk factor for fracture independent of BMD and fracture risk assessment (FRAX) tool.Citation15 Zebaze et al have reported that daily dosing of TPTD, particularly higher dose, but not weekly dosing, increased cortical porosity suggesting the increase of hip fracture risk.Citation16 Thus, the increase of hip fracture risk is concerned at the time of daily TPTD use.
Our findings suggest that the increase of H-BMD (which mainly involves cortical bone) was greater in the TPTD-pretreated group than that in the DMAb group. Further, the increase of FN-BMD, which entirely involves cortical bones, was significantly greater in the TPTD-pretreated group than that in the DMAb group. These results suggest that the pretreatment of TPTD might have enhanced the increase of BMD in cortical bones treated by DMAb.
The increase of cortical BMD in the TPTD-pretreated group was much greater than that in the treatment-naïve osteoporotic group. We considered that the cortical porosity caused by TPTD might have been related to our findings. It is speculated that cortical porosity may have increased the areas of bone surface where bone formation was caused by the inhibitory effects of bone metabolism by DMAb. As a result, the increase of BMD in the cortical bone was greater than that in the cancellous bone by TPTD pretreatment.
Against our hypothesis that DMAb might be able to enhance the increase of L-BMD in the TPTD-pretreated group than in the DMAb-alone group, this study showed that the increase of L-BMD in the treatment-naïve OP group was greater than that in the TPTD-pretreated group. The reason for these findings is unknown.
We have also reported that TPTD increased up to 13.0% of L-BMD at 12 months after treatment in Japanese primary osteoporotic patients.Citation17 Thus, the amount of L-BMD increase by DMAb might not have been different between the groups. In osteoporotic treatment, TPTD, DMAb, BPs, and SERMs significantly improve L-BMD compared to H-BMD.Citation18 Among them, TPTD mainly and greatly increases L-BMD.Citation5,Citation6 Although the TPTD administration period is limited, the increase of L-BMD is generally much greater compared to other osteoporotic drugs. In addition, TPTD is superior with respect to vertebral fracture prevention. Further, this study showed that the increase of L-BMD by DMAb was high even after TPTD treatment. Thus, before administration of DMAb, TPTD can be considered as an effective treatment option for cases with very low L-BMD.
On the other hand, DMAb likely increases H-BMD.Citation4,Citation19 Eriksen et al have reviewed the effects of TPTD treatment on hip OP and concluded that TPTD showed positive effect in patients with hip OP.Citation20 Further, in this study, H-BMD, especially FN-BMD, was greatly increased by DMAb after pretreatment with TPTD. Thus, even in the cases with low H-BMD, DMAb could be a good option to be used after TPTD treatment. When the physicians aim to improve H-BMD, before DMAb administration, it might be better to administer TPTD in those patients at relatively low risk of hip fracture.
Generally, bone antiresorptive drugs do not likely improve cancellous bone microstructure. Fahrleitner-Pammer et al have recently reported that cancellous bone microstructure improved with TPTD therapy irrespective of prior antiresorptive use.Citation11 Thus, theoretically, it is desirable to administer TPTD first, and then administer bone antiresorptive drugs, such as DMAb, for OP to improve the microarchitecture of bones, which increases not only H-BMD but also L-BMD.
One limitation of this study is the difference of pretreatment background of the two groups due to clinical setting. Other limitations include small sample size, short follow-up period, and lack of evaluation of fracture prevention during observation. Future studies are required to confirm our findings.
Disclosure
The authors report no conflicts of interest in this work.
References
- McClungMRLewieckiEMCohenSBAMG 162 Bone Loss Study GroupDenosumab in postmenopausal women with low bone mineral densityN Engl J Med2006354882183116495394
- BoneHGWagmanRBBrandiML10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extensionLancet Diabetes Endocrinol2017551352328546097
- KamimuraMNakamuraYIkegamiSUchiyamaSKatoHTaguchiASignificant improvement of bone mineral density and bone turnover markers by denosumab therapy in bisphosphonate-unresponsive patientsOsteoporos Int201728555956627650642
- TsaiJNUihleinAVLeeHTeriparatide and denosumab, alone or combined, in women with postmenopausal osteoporosis: the DATA study randomised trialLancet20133829886505623683600
- NakamuraYSuzukiTKamimuraMTwo-year clinical outcome of denosumab treatment alone and in combination with teriparatide in Japanese treatment-naive postmenopausal osteoporotic womenBone Res201751605528690911
- LederBZTsaiJNJiangLALeeHImportance of prompt antiresorptive therapy in postmenopausal women discontinuing teriparatide or denosumab: the Denosumab and Teriparatide Follow-up Study (DATA-Follow-up)Bone201798545828286299
- NeerRMArnaudCDZanchettaJREffect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosisN Engl J Med2001344191434144111346808
- ArlotMMeunierPJBoivinGDifferential effects of teriparatide and alendronate on bone remodeling in postmenopausal women assessed by histomorphometric parametersJ Bone Miner Res20052071244125315940379
- Fahrleitner-PammerABurrDDobnigHImprovement of cancellous bone microstructure in patients on teriparatide following alendronate pretreatmentBone201689162427185100
- YagiMOhneHKonomiTTeriparatide improves volumetric bone mineral density and fine bone structure in the UIV+1 vertebra, and reduces bone failure type PJK after surgery for adult spinal deformityOsteoporos Int201627123495350227341809
- OrimoHNakamuraTHosoiTJapanese 2011 guidelines for prevention and treatment of osteoporosis – executive summaryArch Osteoporos2012732023203733
- IkegamiSKamimuraMUchiyamaSNakamuraYMukaiyamaKKatoHClinical implications of hip flexion in the measurement of spinal bone mineral densityJ Clin Densitom20161927027626778480
- EastellRO’NeillTWHofbauerLCPostmenopausal osteoporosisNat Rev Dis Primers201621606927681935
- ShigdelROsimaMAhmedLABone turnover markers are associated with higher cortical porosity, thinner cortices, and larger size of the proximal femur and non-vertebral fracturesBone2015811626112819
- AhmedLAShigdelRJoakimsenRMMeasurement of cortical porosity of the proximal femur improves identification of women with nonvertebral fragility fracturesOsteoporos Int20152682137214625876879
- ZebazeRTakao-KawabataRPengYIncreased cortical porosity is associated with daily, not weekly, administration of equivalent doses of teriparatideBone201799808428323145
- KamimuraMNakamuraYIkegamiSUchiyamaSKatoHBisphosphonate Pre-Treatment Diminishes the Therapeutic Benefits of Teriparatide in Japanese Osteoporotic PatientsTohoku J Exp Med2016239172427150954
- MinneHAudranMSimõesMEEUROFORS Study GroupBone density after teriparatide in patients with or without prior antiresorptive treatment: one-year results from the EUROFORS studyCurr Med Res Opin200824113117312818838053
- NakamuraYSuzukiTKamimuraMIkegamiSUchiyamaSKatoHAlfacalcidol increases the therapeutic efficacy of ibandronate on bone mineral density in Japanese women with primary osteoporosisTohoku J Exp Med2017241431932628458336
- EriksenEFKeavenyTMGallagherERKregeJHLiterature review: the effects of teriparatide therapy at the hip in patients with osteoporosisBone20146724625625053463
