Abstract
Chronic eosinophilic pneumonia (CEP) is an eosinophilic lung disease that is typically diagnosed by a triad of clinical symptoms including pulmonary symptoms, eosinophilia and characteristic radiographic abnormalities. It requires a high index of suspicion given its overlap with other eosinophilic conditions and lack of a specific diagnostic test. The diagnosis is made after careful consideration of other secondary causes of eosinophilia, such as infectious, drugs, or toxic etiologies. CEP generally responds rapidly to treatment, which primarily consists of corticosteroid therapy, but relapses are common. Novel therapies are being explored as more information is being discovered about the pathophysiology of eosinophilic disease processes. Close follow-up is important given the difficulty in weaning patients from glucocorticoids with many patients developing sequelae of chronic glucocorticoid therapy. Therefore, exploring alternative treatments is of upmost importance.
Introduction
Eosinophilic lung diseases are a group of diffuse parenchymal lung diseases characterized by prominent infiltration of eosinophils into the pulmonary interstitium and alveoli, with noted conservation of lung architecture.Citation1 This group of disorders can be classified by etiology, including secondary causes such as infectious, malignant, allergic, drug, and toxic etiologies.Citation2 Primary eosinophilic lung diseases can be further classified by isolated pulmonary involvement vs systemic involvement, and two major isolated pulmonary eosinophilic lung diseases include acute eosinophilic pneumonia (AEP) and chronic eosinophilic pneumonia (CEP), although these are much less common.
CEP was first recognized as a unique pulmonary entity in 1969, characterized by Carrington et al, when a series of female patients were described as presenting with symptoms of dyspnea, fever, and weight loss who demonstrated pulmonary opacities on chest X-ray and had noted eosinophilic infiltration on lung biopsy specimens.Citation3 The condition is thought to be idiopathic, with no known infectious or toxic etiology identified. CEP is a clinical diagnosis and has a presentation that is typically described as a triad of the following: pulmonary symptoms, abnormal chest imaging, and abnormal elevation of eosinophils in either serum or pulmonary tissue.Citation2
Epidemiology
Representing <3% of cases of various interstitial diseases, CEP is a rare disease.Citation4 It is, however, the most common of the eosinophilic pneumonias in nontropical areas where prevalence of parasitic infections is low.Citation4 Although it may present in every age group, it is thought to be rare in children, and does have a 2:1 female predominance and a mean age of 45 years at time of diagnosis.Citation4,Citation5 As many as two-thirds of patients have a prior history of asthma and approximately half a history of atopy, ranging from eczema to nasal polyposis, to urticaria.Citation4 Most patients are nonsmokers, and in fact it is thought that <10% diagnosed are smokers.Citation4,Citation5 This contrasts with acute eosinophilic pneumonia in which a smoking history is much more common.Citation4 Prior radiation for breast cancer has been reported to be a predisposing factor.Citation5
Pathophysiology
Eosinophils are multifunctional leukocytes implicated in innate and adaptive immunity. They mature in the bone marrow under stimulation from cytokines. In particular they are influenced by IL-5, IL-3, granulocyte-macrophage colony-stimulating factor, and transcription factors including delta-dbl-GATA-1, before circulating into the blood and subsequently into tissues.Citation6 Eosinophils have intracytoplasmic granules containing proteins, toxins, chemokines, and proinflammatory degranulation of eosinophils releases these toxic substances into the tissues, which contributes to the pathophysiology of eosinophilic disorders.Citation6 Eosinophils interact with multiple cellular pathways, including T helper lymphocytes, mast cells and basophils, macrophages, and multiple ILs, but recruitment of the eosinophil to the lung is likely mostly directed by IL-5 and the eotaxin subfamily of chemokines.Citation6 In addition, recent work by Katoh et al suggests that IL-25 may perpetuate chronic eosinophilic inflammation of the lung.Citation7 Histopathological lesions are related to the toxicity of granule release of eosinophils and are largely reversible with treatment, although tissue damage and remodeling can be seen.Citation6 Osteopontin levels have been found to be elevated in bronchoalveolar lavage (BAL) fluid of patients with eosinophilic pneumonia including drug-induced eosinophilic pneumonia. They may play a role in the eosinophilic inflammation.Citation8
Clinical manifestations
While many of the eosinophilic lung diseases share overlapping symptoms and findings, the onset of CEP tends to be progressive or subacute, with symptoms developing over the course of several weeks to months before a diagnosis is made.Citation4 Presenting symptoms include dyspnea, which is present in 60%–90% of patients, and cough, which is present in about 90% of patients.Citation4 Other less common symptoms include rhinitis or sinusitis in about 20% of patients, and less commonly chest pain or hemoptysis in about 10%.Citation4 On examination, lung auscultation can reveal wheezing or crackles in as many as one-third of patients, but overt respiratory failure is exceptionally rare and more characteristic of AEP.Citation4
Despite CEP having isolated pulmonary involvement, patients experience systemic symptoms including fatigue, malaise, fever, night sweats, anorexia, and weight loss.Citation4 Occasionally extrathoracic manifestations such as arthralgias, nonspecific skin manifestations, pericardial effusion, or abnormal liver enzymes are detected.Citation4 It is important to conduct a careful evaluation for other systemic conditions such as eosinophilic granulomatosis with polyangiitis (EGPA), cryptogenic organizing pneumonia (COP), or idiopathic hypereosinophilic syndrome if other manifestations are prominent.Citation4
Asthma is often a part of the clinical picture in patients with CEP, and as many as 75% may have either preceding asthma or the concurrent development of asthma at some point during the course of the disease process.Citation4 The asthma that is concomitantly seen with CEP is often severe, and sometimes progressive despite treatment with corticosteroids.Citation4
Diagnostic evaluation
Laboratory
In addition to symptoms, blood eosinophilia is often a diagnostic criterion for CEP with mean values of eosinophils of 5,000–6,000/mm3, representing 20%–30% of leukocytes on differential.Citation4 In one literature review of 111 cases, eosinophilia was noted to be greater than 6% of leukocytes in 88% of cases.Citation6 ESR and CRP are often elevated with the inflammation. Total serum IgE is elevated in about half of cases, to levels of greater than 1,000 IU/mL in 15% of cases.Citation6 Less commonly circulating immune complexes and positive antinuclear antibodies have been reported, and urinary eosinophil-derived neurotoxin, which indicates eosinophil degranulation, can be markedly elevated.Citation6
Bronchoalveolar lavage
BAL reveals an abnormally elevated level of eosinophils, anywhere from 12% to 95%, with a mean of 58%.Citation5 In cases where CEP is suspected and serum eosinophilia is not significant, BAL may be helpful. This high level of eosinophils may also be helpful in differentiating CEP from COP, which shares many common features. CEP is usually associated with eosinophil counts higher than lymphocyte counts.Citation5
Pulmonary function testing
Spirometry in patients with CEP is normal in up to a third of the patients but can demonstrate either a restrictive or obstruction pattern.Citation4,Citation6 Obstruction is more likely to be seen in patients with underlying asthma.Citation4 Carbon monoxide transfer factor (diffusing capacity of the lung for carbon monoxide [DLCO]) is reduced in perhaps up to a quarter of patients, although one study demonstrated a reduction of <80% predicted in 52%. Arterial blood gases often demonstrate a mild to moderate hypoxemia as well.Citation5 It has been noted that impairment in pulmonary function can often be reversed with treatment, although a ventilatory obstructive defect may become permanent over time in some, especially those with a notable BAL eosinophilia at initial evaluation.Citation6
Imaging
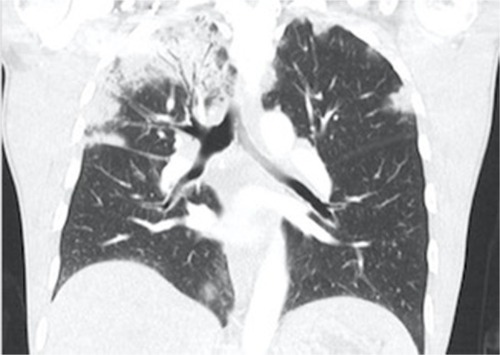
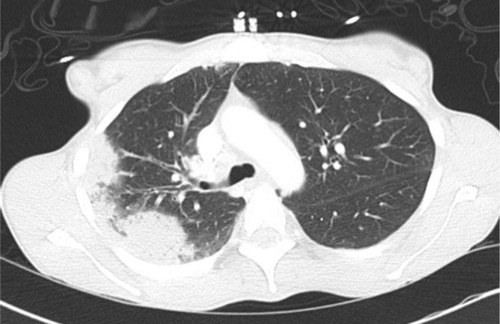
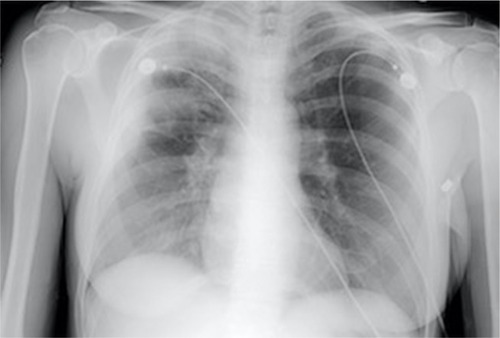
A hallmark feature for CEP seen on chest radiography is that of peripheral opacities, which are seen in almost all cases and are migratory in about a quarter of cases.Citation6 These opacities are representative of peripheral parenchymal infiltrates, which are alveolar in nature.Citation5 They can be unilateral but are most commonly bilateral. They can range in character from ground glass opacities to consolidation with air bronchograms.Citation5 There is a classic pattern of “photographic negative pulmonary edema” described which mimics the reversal of the shadows usually seen in acute pulmonary edema ().Citation6 This pattern, however, is only seen in about one quarter of patients and is not specific for the disease. It can also be a potential finding with cryptogenic organizing pneumonia, sarcoidosis, or drug-induced pneumonia.Citation5 Computing tomography can further characterize pulmonary findings and define CEP, with a higher sensitivity. Alveolar infiltrates are seen bilaterally in >97% of cases.Citation6 These opacities are predominately present peripherally in the upper lobes and can be both ground glass and/or consolidation ( and ). Less common findings include opacities of midzone distribution or centrilobular, and mediastinal lymphadenopathy.Citation5,Citation6 Very rare findings would include pleural effusions, cavitary lesions, and the reverse halo or atoll sign, and if present, may require reconsideration of the diagnosis.Citation6
Figure 1 Chest X ray of a patient with CEP demonstrating peripheral opacities.
Histopathology
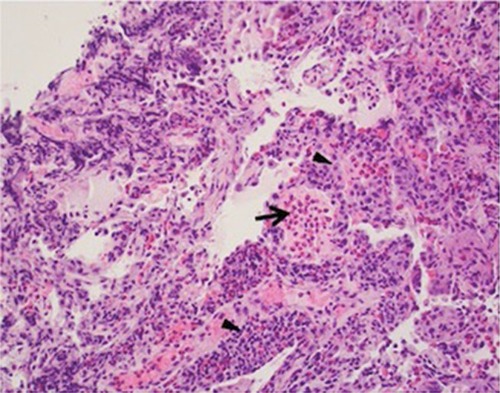
Given the utility of the aforementioned findings including eosinophilia, biopsy for pathologic confirmation is usually not necessary. If performed, tissue findings may include interstitial and alveolar inflammation with an eosinophilic predominance as well as foci of organizing pneumonia.Citation5 If pulmonary vasculature is observed on biopsy, eosinophilic infiltration may be observed; however, necrotizing or granulomatous vasculitis should not be present and may be suggestive of a more systemic eosinophilic process ().Citation5
Diagnosis
The diagnosis of CEP needs a heightened clinical suspicion as there are no absolute diagnostic criteria, and it has features, which overlap with various other pulmonary diseases. Although CEP was originally a histological diagnosis, it is now typically diagnosed based upon the combination of greater than 2 weeks of respiratory symptoms, BAL and/or blood eosinophilia (usually with BAL cell count differential >40% or serum eosinophils >1,000/mm3), pulmonary infiltrates with a peripheral predominance, and exclusion of other known causes of eosinophilic lung diseases ().Citation1,Citation9 Diagnostic uncertainty can arise in those already on steroids, which may reduce or mask eosinophilia.Citation1 If biopsy becomes necessary, open-lung biopsy is not practical in all patients but has the highest yield in terms of tissue diagnosis. Transbronchial lung biopsy can be limited by small biopsy samples or incorrect site of biopsy.Citation9
Table 1 CEP diagnostic criteria
Differential diagnosis
Given the overlapping nature of many eosinophilic lung diseases and systemic processes, it is important consider the broad array of etiologies upon initial evaluation and rule out secondary causes of eosinophilia, which are much more common and have different prognostic and treatment implications.
As previously mentioned, CEP is an idiopathic condition characterized by a gradual onset and slower progression of pulmonary symptoms. Peripheral infiltrates are often seen on imaging along with peripheral or BAL eosinophilia. Conversely, acute eosinophilic pneumonia (AEP) is characterized by a more rapid onset of symptoms and can be seen with fever and diffuse infiltrates on imaging.Citation2,Citation10 There is a greater potential for a rapid and severe decompensation in respiratory status, sometimes over the course of hours or days, and sometimes ending with mechanical ventilation with an acute respiratory distress syndrome picture.Citation2 Similarly to CEP, the condition is idiopathic. BAL eosinophils ≥25% are often seen, and the symptoms usually resolve rapidly with steroid treatment.Citation2,Citation10 Interestingly enough, acute eosinophilic pneumonia has been reported to be associated with exposure to chemicals and medications and is frequently associated with cigarette smoke exposure unlike CEP.Citation2
Allergic bronchopulmonary aspergillosis is seen in some patients with asthma as well as cystic fibrosis. It often presents as difficult to control asthma with productive cough including mucus plugs.Citation10 Workup often reveals pulmonary infiltrates, central bronchiectasis, elevated IgE levels, and peripheral eosinophilia. The eosinophilia may occur during exacerbations or relapses.Citation10,Citation11
EGPA shares several common features with CEP. Known as a small vessel vasculitis, it is a multisystem disorder that primarily affects the pulmonary system but can involve the gastrointestinal, cardiac, renal, skin, and nervous systems.Citation2 It can progress through sequential phases over the course of years, starting with asthma and allergic rhinitis symptoms, followed by peripheral eosinophilia, which often exceeds 1,500/mm3. It finally morphs into a vasculitis phase leading to constitutional symptoms such as fever, weight loss, and fatigue.Citation2 Diagnostic criteria include the presence of asthma, eosinophilia, paranasal sinus abnormalities, pulmonary infiltrates, and mononeuropathy multiplex. Perinuclear antineutrophil cytoplasmic antibody positivity with specificity for myeloperoxidase occurs in 40% of patients. Vasculitis with eosinophilic infiltration and granulomas on biopsies of the skin, nerve, or muscle can be helpful.Citation2,Citation12,Citation13
Drug-induced eosinophilic pneumonia should be considered in patients who meet criteria for eosinophilic pneumonia with dyspnea, pulmonary infiltrates, eosinophilia, and especially in those who recently started a new medication.Citation14 Bartal et al shed further light on this with a large case series of 196 patients with drug-induced eosinophilic pneumonia. In that review, nearly every class of medication has been implicated. However, antibiotics, nonsteroidal anti-inflammatory drugs, and antiepileptic medications were most commonly associated.Citation14 Daptomycin, mesalamine, minocycline, sulfasalazine, and nitrofurantoin were the top five medications found to be associated with development of drug-induced eosinophilic pneumonia.Citation14
With the appropriate clinical situation, it may also be important to consider parasitic infections in patients with travel history, such as Ascaris species and Löffler’s syndrome, which is pulmonary eosinophilia in the context of parasitic larvae migrating into the lung.Citation15
Treatment
Corticosteroids
The mainstay of treatment is based on oral corticosteroid (OCS) therapy, with a goal of inducing remission as well as reducing the possibility of relapse.Citation4 Response to OCS is usually dramatic and fairly rapid, with both clinical improvement and resolution of infiltrates on imaging. Of note, response typically occurs within a few days, and if rapid clinical improvement is not seen, alternative diagnoses should be reconsidered.Citation16 Low-dose inhaled corticosteroids (ICS) have been used previously to assist with tapering and discontinuing OCS. ICS have therefore been proposed as monotherapy due to the suspected deposition of ICS in alveoli to prevent the development of the side effects from long-term usage of OCS. However, a study performed by Minakuchi et al suggests that ICS may not be an effective monotherapy.Citation17 Without treatment, spontaneous resolution occurs in <10% of patients with CEP, and there is a risk of progression to fibrosis.Citation17
Treatment dose and duration
There are no firm dose or duration guidelines. The overall goal is to maintain continued clinical improvement on the lowest possible dose of OCS in order to avoid relapse but also minimize steroid-related side effects. Initial treatment is typically oral prednisone dosed at 0.5–0.6 mg/kg/day with the dose tapering off by one-half after confirmation of both clinical and radiologic resolution.Citation5 A review of the current literature regarding treatment by Suzuki et al suggests that a starting dose of prednisolone around 30 mg would be adequate.Citation16 Treatment periods are variable from a few months to 12 months depending on the clinical response. Relapses are common, occurring in over half of patients.Citation4 Despite this generally accepted practice, only one study to date has prospectively evaluated the optimal treatment regimen with regard to relapse rate, in a randomized parallel-group study.Citation18 In that trial the study group received the OCS treatment at a daily dose of 0.5 mg/kg/day over a shorter 3-month period compared to the same dose in a longer 6-month period. In the 3-month group, the prednisolone was tapered by 20% every 2 weeks to completely off by 3 months, while in the 6-month group, the prednisolone was tapered off slower over 6 months. They were observed for 2 years after completion of treatment.Citation18 The relapse rates were similar between groups (52.1% vs 61.9%, P=0.56), suggesting that a shorter treatment period may be acceptable, with close monitoring for relapse.Citation18
Relapse
Although the long-term prognosis of the disease is good for many patients, relapse rates are high in this disease process particularly when trying to taper or discontinue OCS therapy. As many as half of patients have been noted to experience relapse, with some relapsing multiple times. Resumption of OCS therapy in relapsed cases is uniformly effective.Citation16 Predictors of relapse have not been rigorously evaluated but smoking status or underlying asthma are possible risk factors.Citation16,Citation19 These patients may require chronic steroid maintenance therapy, and efforts to mitigate side effects are important.Citation16 This has also led to efforts toward finding potential steroid-reducing therapies.
Biologic agents
Most of alternative therapies have been described in case reports with no robust evidence supporting their use in CEP. Omalizumab, a monoclonal antibody against IgE, was used in a patient with CEP after the second relapse in 2 years. This patient had an elevated IgE and responded to the omalizumab injections. The patient was deemed to be disease free after 15 months of therapy.Citation20 In a subsequent case report, Domingo et al described a successful use of omalizumab in a patient with CEP with prominent asthmatic features and dust mite allergen. They were able to taper the dose of omalizumab by 50% every 6 months.Citation21 Clinicians should be alert to the possibility of unmasking EGPA in these patients.Citation20
There are several other potential targets for CEP, most commonly against IL-5. Increased level of IL-5 with associated release of cytotoxic granular proteins from eosinophils is an important mechanism into the pathophysiology of CEP.Citation22
Mepolizumab and reslizumab prevent binding of circulating IL-5 to eosinophils. Benralizumab neutralizes IL-5 function by binding the alpha subunit of the IL-5 receptor and is also able to induce apoptosis of target cells via antibody-dependent cell-mediated cytotoxicity.Citation23,Citation24 In a large multicenter randomized controlled trial involving 621 patients, mepolizumab reduced the rate of asthma exacerbations in severe eosinophilic asthma.Citation25 It has also been demonstrated to reduce the frequency of asthma exacerbations and found useful as a potential steroid-sparing agent in hypereosinophilic syndromes.Citation26,Citation27 While anti-IL5 therapies have been demonstrated as efficacious in eosinophilic asthma, there are limited data on the use of these therapies in CEP.
A recent report described a patient with CEP who had responded to glucocorticoid therapy but failed a steroid taper and experienced significant steroid-induced side effects including weight gain, Cushingoid features, and muscle wasting. Eighteen months after initial presentation, mepolizumab was started at a dose of 100 mg every 4 weeks. After starting this therapy, her peripheral blood eosinophilia decreased, and her symptoms disappeared while tapering her glucocorticoids. However, she developed a mild anaphylactic reaction after 6 months of therapy. Therefore, reslizumab at a dose of 3 mg/kg was initiated every 4 weeks. After two additional months, her glucocorticoids were able to be discontinued.Citation28 Mepolizumab had also been used effectively in another patient with CEP and a 20-year history of asthma during his second relapse at 100 mg every month.Citation29 However, the duration of therapy with mepolizumab in such patients remains unclear.
Other potential targets for treatment of CEP include IL-25, IL-33, IL-4, and IL-13. IL-25 and IL-33 are primarily produced by airway epithelial cells, which induce the production of Th2-type cytokines including IL-5 and IL-13 on eosinophils.Citation7 Katoh et al examined the BAL fluid in 20 patients with AEP, 22 patients with CEP, 20 patients with idiopathic pulmonary fibrosis (IPF), and 20 patients with sarcoidosis. Patients with both acute and chronic eosinophilc asthma had higher IL-5 and eosinophil levels compared to patients with IPF and sarcoidosis. Interestingly, IL-25 levels were elevated in patients with CEP, but not AEP. IL-33 levels were not significantly different between the eosinophilic pneumonias compared to sarcoidosis and IPF.Citation7 The results of this study suggest that IL-25 may be a potential therapeutic target for CEP. Currently, antibodies to Il-25 are not commercially available.
Dupilumab is a human anti-IL-4 receptor α-monoclonal antibody that targets both IL-4 and IL-13 signaling and therefore TH2-type inflammation. Castro et al randomly assigned 1,902 patients to various doses of dupilumab vs placebo and demonstrated reduced frequency of asthma exacerbation and improvement in forced expiratory volume in 1 second.Citation30 Additionally, in the Liberty Asthma Venture trial, add-on dupilumab was able to significantly reduce the use of oral glucocorticoids in patients with severe asthma.Citation30 Dupilumab may also hold promise in selected relapsing cases of CEP, but it has not been used in these cases at this time.
Prognosis
The short-term prognosis for patients with CEP is generally favorable, given the remarkable and timely clinical improvement with corticosteroids. Around half of patients initially diagnosed with CEP have clinical improvement, without relapse or need for repeat treatment.Citation31
Among the other half of patients with relapse, repeat OCS dosing or even maintenance low-dose OCS long-term may be required.Citation31 In these patients, there is risk of development of steroid-related adverse effects, including hyperglycemia, diabetes mellitus, osteoporosis, psychosis, and infectious complications such as pulmonary nontuberculous mycobacterium.Citation16,Citation19
In terms of pulmonary function, the majority of patients with CEP have restriction or obstruction noted on spirometry.Citation16 Among those with abnormal pulmonary function tests, a majority of patients improve with treatment but as many as 37%–50% of the patients may have persistent defects after treatment.Citation16,Citation32
In summary, given the previously mentioned dramatic and quick response in a large percentage of patients, those who are diagnosed and treated in a timely manner may have a great clinical response. Conversely, given the incidence of relapse and the potential for steroid-induced side effects or fibrotic changes long term, further investigation of steroid-sparing therapies such as anti-IL5 antibodies are needed.
Disclosure
The authors report no conflicts of interest in this work.
References
- CottinVCordierJFEosinophilic lung diseasesImmunol Allergy Clin North Am201232455758623102066
- RoseDMHrncirDEPrimary eosinophilic lung diseasesAllergy Asthma Proc2013341192523406932
- CarringtonCBAddingtonWWGoffAMChronic eosinophilic pneumoniaN Engl J Med1969280157877985773637
- CottinVDiseases Elung. Eosinophilic lung diseasesClin Chest Med201637353555627514599
- MarchandECordierJFIdiopathic chronic eosinophilic pneumoniaOrphanet J Rare Dis2006111116722612
- StollerJames KEosinophilic lung diseasesBroaddusVCMurray and Nadel’s Textbook of Respiratory Medicine6th ed Chapter 68Elsevier/Saunders201612211228
- KatohSIkedaMMatsumotoNPossible role of IL-25 in eosinophilic lung inflammation in patients with chronic eosinophilic pneumoniaLung2017195670771228875265
- UenoTMiyazakiEAndoMNurekiSIKumamotoTOsteopontin levels are elevated in patients with eosinophilic pneumoniaRespirology20101571111112120796249
- MatsuseHShimodaTFukushimaCDiagnostic problems in chronic eosinophilic pneumoniaJ Int Med Res19972541962019283992
- AkuthotaPWellerPFEosinophilic pneumoniasClin Microbiol Rev201225464966023034324
- StevensDAMossRBKurupVPAllergic bronchopulmonary aspergillosis in cystic fibrosis-state of the art: cystic fibrosis foundation consensus ConferenceClin Infect Dis200337Supl 3S225S26412975753
- ChurgAPulmonary angiitis and granulomatosis revisitedHum Pathol198314108688836352458
- GuillevinLCohenPGayraudMChurg-Strauss syndrome. Clinical study and long-term follow-up of 96 patientsMedicine199978126379990352
- BartalCSagyIBarskiLDrug-induced eosinophilic pneumoniaMedicine2018974e968829369189
- WoolnoughKWardlawAJEosinophilia in pulmonary disordersImmunol Allergy Clin North Am201535347749226209896
- SuzukiYSudaTLong-term management and persistent impairment of pulmonary function in chronic eosinophilic pneumonia: a review of the previous literatureAllergol Int201867333434029395966
- MinakuchiMNiimiAMatsumotoHAmitaniRMishimaMChronic eosinophilic pneumonia: treatment with inhaled corticosteroidsRespiration200370436236614512670
- OyamaYFujisawaTHashimotoDEfficacy of short-term prednisolone treatment in patients with chronic eosinophilic pneumoniaEur Respir J2015451624.e3125614171
- IshiguroTTakayanagiNUozumiRThe long-term clinical course of chronic eosinophilic pneumoniaIntern Med201655172373237727580536
- KayaHGümüşSUçarEOmalizumab as a steroid-sparing agent in chronic eosinophilic pneumoniaChest2012142251351622871762
- DomingoCPomaresXCan omalizumab be effective in chronic eosinophilic pneumonia?Chest20131431274
- MukherjeeMSehmiRNairPAnti-IL5 therapy for asthma and beyondWorld Allergy Organ J20147111524383710
- LimHFNairPEfficacy and safety of reslizumab in patients with moderate to severe eosinophilic asthmaExpert Rev Respir Med20159213514225578680
- VarricchiGBagnascoDBorrielloFHefflerECanonicaGWInterleukin-5 pathway inhibition in the treatment of eosinophilic respiratory disorders: evidence and unmet needsCurr Opin Allergy Clin Immunol201616218620026859368
- PavordIDKornSHowarthPMepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trialLancet2012380984265165922901886
- LiuYZhangSLiDWJiangSJEfficacy of anti-interleukin-5 therapy with mepolizumab in patients with asthma: a meta-analysis of randomized placebo-controlled trialsPLoS One201383e5987223544105
- GarrettJKJamesonSCThomsonBAnti-interleukin-5 (mepolizumab) therapy for hypereosinophilic syndromesJ Allergy Clin Immunol2004113111511914699394
- SarkisEPatelSBurnsKBatarsehHMadorMJAnti-interleukin (IL)-5 as a steroid-sparing agent in chronic eosinophilic pneumoniaJ Asthma201815
- ToMKonoYYamawakiSA case of chronic eosinophilic pneumonia successfully treated with mepolizumabJ Allergy Clin Immunol Pract20186517461748.e130201098
- CastroMCorrenJPavordIDDupilumab efficacy and safety in moderate-to-severe uncontrolled asthmaN Engl J Med2018378262486249629782217
- NaughtonMFahyJFitzgeraldMXChronic eosinophilic pneumonia: a long-term follow-up of 12 patientsChest199310311621658031327
- DurieuJWallaertBTonnelABLong-term follow-up of pulmonary function in chronic eosinophilic pneumonia. Groupe d’Etude en Pathologie Interstitielle de la Société de Pathologie Thoracique Du NordEur Respir J19971022862919042622