Abstract
Background
The prevention of cardiac surgery-associated acute kidney injury (CSA-AKI) by statins remains controversial. Therefore, the present meta-analysis including randomized controlled trials (RCTs) was performed to assess the effect of perioperative statin on CSA-AKI.
Methods
Two reviewers independently searched for RCTs about perioperative statin for prevention of CSA-AKI. The primary endpoint was CSA-AKI. Relative risk was calculated between statin and placebo for preventing CSA-AKI using the random-effect model or fixed-effect model according to different heterogeneity.
Results
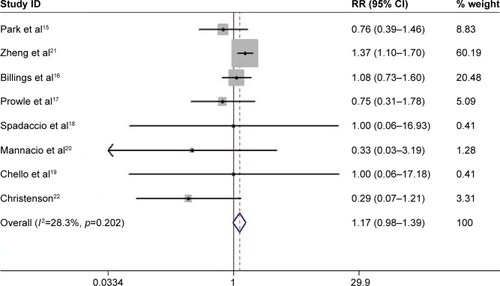
Eight RCTs met inclusion criteria, including five studies with atorvastatin, two with rosuvastatin, and one with simvastatin. There were 1,603 patients receiving statin treatment and 1,601 with placebo. Perioperative statin therapy did not reduce the incidence of CSA-AKI (relative risk =1.17, 95% CI: 0.98–1.39, p=0.076). Furthermore, perioperative statin increased the risk of CSA-AKI in the subgroup analysis with clear definition of CSA-AKI and those with JADAD score >3. Perioperative rosuvastatin produced slightly significantly higher risk of AKI than atorvastatin therapy (p=0.070). Statin intervention both pre and post surgery slightly increased the risk of CSA-AKI versus preoperative statin therapy alone (p=0.040).
Conclusions
Perioperative statin therapy might increase the risk of CSA-AKI after cardiac surgery.
Introduction
Acute kidney injury (AKI) is an important and common complication of cardiac surgery and has reported rates of 8.9%–45.8%.Citation1–Citation4 AKI independently increases the length and cost of hospitalizationCitation5 and is also associated with higher in-hospital mortality and poor long-term survival.Citation6,Citation7 Therefore, a number of studies have focused on preoperative therapies to prevent cardiac surgery-associated acute kidney injury (CSA-AKI).
Statins, with pleiotropic antiinflammatory and antioxidant effects, have been considered to reduce the risk of CSA-AKI, which is related to inflammation and oxidative stress.Citation8,Citation9 Wang et alCitation10 systematically reviewed 21 observational studies and 5 randomized controlled trials (RCTs) and found that preoperative statin treatment reduced the incidence of short- and long-term renal dysfunction after surgery. However, they mainly included observational studies, in which the results could be influenced by confounding factors. A recent meta-analysis of 10 RCTs showed that perioperative statin therapy did not reduce CSA-AKI, and in contrast, appeared to produce unfavorable outcomes.Citation11 However, this study combined the effects of dialysis and CSA-AKI, which could influence the conclusions. In addition, whether different statins type (atorvastatin or rosuvastatin) or administration time (pre and post operation) had different effects on CSA-AKI was not elucidated. Therefore, the present updated meta-analysis included studies of the incidence of CSA-AKI only and performed subgroup analyses to better demonstrate the efficacy of perioperative statins for the prevention of CSA-AKI.
Methods
Literature search
A systematic search of PubMed, ClinicalTrials.gov, Web of Science, Embase, Medline, and the Cochrane Library was performed from inception to July 2016 using the following terms: (surgery or cardiac surgery or coronary artery bypass surgery or heart surgery or valve surgery) AND (statin or atorvastatin or rosuvastatin or simvastatin) AND (renal failure or acute renal failure or acute kidney failure or ARF or renal insufficiency or acute renal insufficiency or acute kidney insufficiency or renal dysfunction or acute kidney injury or AKI or acute tubular necrosis or ATN). RCTs comparing statin versus placebo for preventing AKI after cardiac surgery were selected. Potentially suitable studies were also obtained from references of the included papers and meta-analyses. Furthermore, related abstracts with complete results sections from the American Heart Association, the American College of Cardiology, the European Society of Cardiology, and the American Society of Nephrology were included in this analysis. The language was restricted to English.
Study selection and quality assessment
Eligible articles were independently identified through their titles and abstracts by two reviewers. If unidentified, the full text of the paper was carefully reviewed for inclusion. Any controversies were discussed with a third reviewer. RCTs comparing perioperative statin versus placebo in preventing CSA-AKI were included. The following exclusion criteria were used: 1) duplicate papers, 2) non-RCTs, 3) preclinical (in vitro or animal) studies, and 4) studies lacking data about occurrence of CSA-AKI.
Methodological quality was evaluated using the JADAD scoring systemCitation12 by two independent reviewers and disagreements were resolved by a third one.
Study outcomes
The primary outcome was CSA-AKI. We accepted the definition of CSA-AKI in each included study, including the risk, injury, failure, loss, end-stage renal disease (RIFLE),Citation13 acute kidney injury network (AKIN),Citation14 and other criteria. The secondary outcome was major adverse clinical events (MACEs) including death or stroke or myocardial infarction.
Data extraction
The period of enrollment, first author, number of patients, mean age, proportion of females, baseline creatinine, statin dosing regimen, definition of CSA-AKI, incidence of CSA-AKI, and mortality in each group were extracted from enrolled papers.
Statistical analysis
The pooled relative risks (RR) with 95% CIs were calculated to assess the effect of perioperative statin in preventing CSA-AKI or MACEs after cardiac surgery. Funnel plots were displayed to subjectively assess publication bias. In addition, Egger’s and Begg’s tests were used to quantify the bias of publication. Heterogeneity was assessed by I-squared statistics. I-squared values <25% were regarded as low heterogeneity, 25%–50% as moderate, and >50% as severe. A fixed-effect model was performed in the absence of significant heterogeneity, defined as a p-value >0.10 and I2<50%. On the contrary, random-effects model was used (p-value ≤0.10 or I2≤50%). Several subgroup analyses were also conducted to identify potential differences in treatment across the trials. Subgroup analyses were performed according to different study protocols (definition of CSA-AKI, sample size, or population) and different types of statins (atorvastatin or rosuvastatin) or their administration time. A p-value <0.05 in a two-tailed test was considered statistically significant. Statistical analyses were performed using STATA version 12.0 (Stata Corp, College Station, TX, USA).
Results
Characteristics of identified studies
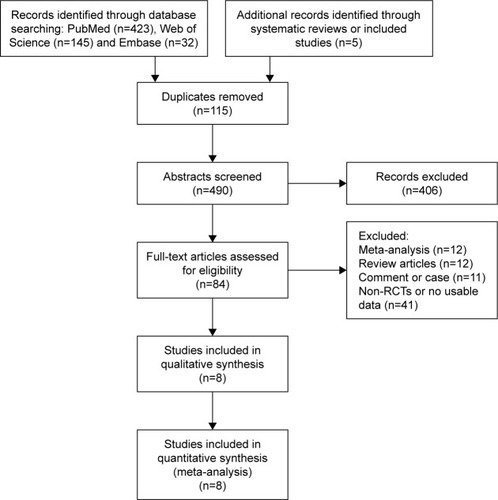
There were 605 articles screened according to the search strategy, and 84 relevant papers addressed the incidence of CSA-AKI. Finally, eight RCTs were included according to the inclusion and exclusion criteria (). The detailed characteristics are described in .
Table 1 Study design of the included studies
A total of 3,204 operative subjects were enrolled, of whom 1,603 were randomized to receive statin and 1,601 placebo. Five studies used therapy with atorvastatin,Citation15–Citation19 two with rosuvastatin,Citation20,Citation21 and one with simvastatin.Citation22 Four studies involved coronary-artery bypass grafting (CABG) alone,Citation18–Citation20,Citation22 one study focused on elective valvular heart surgery,Citation15 and the other three addressed complicated cardiac surgery including CABG, valvular heart disease, and ascending aortic lesions.Citation16,Citation17,Citation21 The interventions in four studies were performed before the operation,Citation18–Citation20,Citation22 and in the other four they were performed pre and post surgery.Citation15–Citation17,Citation21
The definition of CSA-AKI varied in the included studies. In the study conducted by Christenson,Citation22 transient renal failure (serum urea >9 mmol/L and serum creatinine >125 mmol/L in a patient with normal preoperative values) was mentioned as a postoperative complication. Postoperative renal failure was defined as an increase in serum creatinine to >2.5 mg/dL in one study,Citation20 whereas Chello et alCitation19 and Spadaccio et alCitation18 just reported the incidence of renal insufficiency after CABG. Prowle et alCitation17 defined CSA-AKI according to the RIFLE consensus guidelines, whereas Park et al, Billings et al and Zheng et al all diagnosed CSA-AKI based on standards from the AKIN.Citation15,Citation16,Citation21
Study endpoints
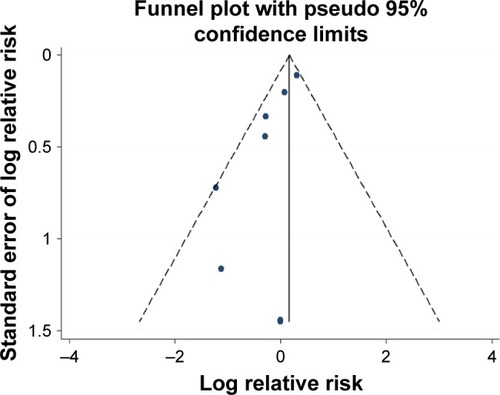
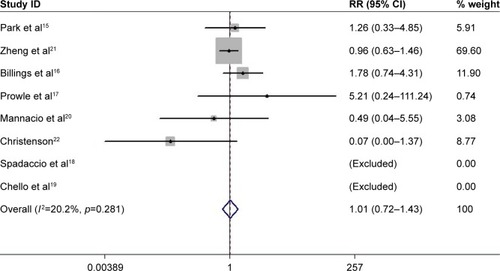
All the identified studies were evaluated by modified JADAD scores and had high technical quality. These studies were not symmetrically distributed in the funnel plots (). There was significant publication bias according to Egger’s test (bias =2.97, p=0.025). Moderate heterogeneity occurred in these studies (I-squared =28.3%; p=0.202). The fixed-effect model indicated that perioperative statin treatment did not significantly reduce incidence of CSA-AKI (RR =1.17, 95% CI: 0.98–1.39, p=0.076, ). In addition, statin therapy did not reduce the incidence of MACE (RR =1.01, 95% CI: 0.72–1.43, p=0.942, ), with low heterogeneity (I-squared =20.2%; p=0.281).
Subgroup analysis
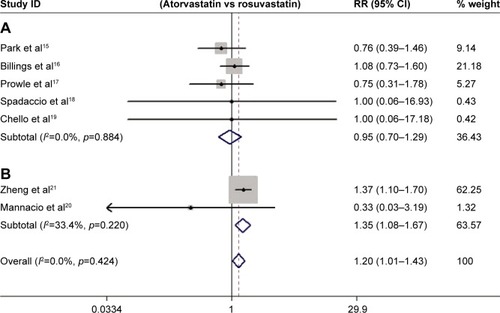
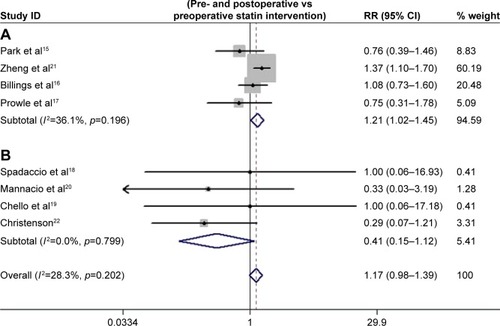
Four studies had a clear definition of CSA-AKI based on RIFLE or AKIN. The pooled estimate demonstrated that perioperative statin therapy significantly increased the risk of CSA-AKI (I-squared =36.1%, RR =1.21, 95% CI: 1.02–1.45, p=0.032). The analysis based on different sample size showed that statin therapy was associated with a higher incidence of CSA-AKI in studies with a sample size >500Citation15,Citation16,Citation21 (I-squared =5.6%, RR =1.29, 95% CI: 1.07–1.57, p=0.008), but no statistical difference in studies with a sample size <500 (I-squared =0%, RR =0.66, 95% CI: 0.41–1.04, p=0.074). Studies with a JADAD score >3 showed that perioperative statin was related to a higher risk of CSA-AKI (I-squared =0%, RR =1.20, 95% CI: 1.01–1.43, p=0.042).Citation15–Citation21 In addition, statin treatment did not significantly reduce the incidence of CSA-AKI in patients undergoing isolated CABG (I-squared =0%, RR =0.41, 95% CI: 0.15–1.12, p=0.081). Furthermore, perioperative rosuvastatin therapy produced slightly significantly higher risks of AKI than atorvastatin therapy (p=0.070, ). The effects of different intervention times were also evaluated, and the results showed that statin treatment both pre and post operation was associated with higher risk of CSA-AKI than preoperative statin therapy alone (p=0.040, ). Due to the greater statistical weight of the study performed by Zheng et al,Citation21 we also performed a subgroup analysis without this study, and the results showed that statin treatment did not significantly reduce the incidence of CSA-AKI (I-squared =0%, RR =0.74, 95% CI: 0.49–1.12, p=0.161).
Discussion
This meta-analysis demonstrated that perioperative statin therapy might in fact increase the risk of CSA-AKI, which was in accordance with the recent research by Putzu et al.Citation11 However, we also demonstrated that rosuvastatin showed a tendency to increase the risk of CSA-AKI more than atorvastatin. In addition, compared with preoperative statin therapy, statin therapy both pre- and postoperatively seemed to have a higher risk of CSA-AKI.
The relationship between statin therapy and CSA-AKI was first shown in an RCT conducted by ChristensonCitation22 and no significant differences in the occurrence of transient renal failure were found. However, in a later meta-analysis, preoperative statin treatment was reported to reduce both short-term and long-term postoperative renal dysfunction.Citation10 In addition, the incidence of CSA-AKI varied based on the different diagnostic criteria. The development of the RIFLECitation13 and AKINCitation14 criteria has standardized the definition of CSA-AKI. In a subgroup analysis of previous meta-analysis, statin therapy was associated with a lower incidence of CSA-AKI diagnosed using the AKIN or RIFLE criteria.Citation10 However, three new RCTs did not find a favorable effect of statins.Citation15,Citation16,Citation21
Recently, Li et alCitation23 included these three RCTs and reported that patients undergoing cardiac surgery might benefit from statins by reducing CSA-AKI and the mortality of the patients suffering from CSA-AKI. However, our results showed that perioperative statin therapy did not reduce CSA-AKI, and in contrast, appeared to produce an unfavorable influence. This finding was not in accordance with previous meta-analyses.Citation10,Citation23 Some differences could account for this phenomenon. In the meta-analysis conducted by Wang et al,Citation10 most of the included research was observational studies, in which confounding factors could impact the reported effects of statins on CSA-AKI. Also, the meta-analysis of Li et alCitation23 combined observational and randomized studies, which could increase heterogeneity. Although a recent meta-analysisCitation11 reported results similar to ours, they also included two studies with incident dialysis but not CSA-AKICitation24,Citation25 which were excluded in our analysis.
There are several possible explanations for our finding that statins had no protective effect against CAS-AKI. First, cardiac surgery was commonly accompanied by ischemic injury, and resultant liver and renal dysfunction.Citation26,Citation27 Statins, which are mainly metabolized by the liver and kidney, might aggravate the stress on these organs and increase the incidence of CSA-AKI. Second, although statins may exert favorable effects on contrast-induced AKI via antiinflammatory and antioxidant actions,Citation28,Citation29 this was not shown to have benefits on CSA-AKI. Different degrees of antiinflammatory actions could account for this discrepancy. Notable systemic oxidative stress and inflammation would be expected in patients undergoing cardiac surgery,Citation30 and this may not be inhibited significantly by statin therapy.Citation15 Lastly, Allou et alCitation31 reported that the positive effect of statin on CSA-AKI was seen only in patients with high cardiovascular risk. The four initial studiesCitation18–Citation20,Citation22 that included only patients undergoing CABG showed that statin therapy seemed to protect against CSA-AKI (RR =0.41, p=0.081). The later studiesCitation15–Citation17,Citation21 with relatively larger sample sizes did not focus on these population, so protective effects were not documented. Therefore, whether the effects of statins on CSA-AKI are influenced by operative types was unclear.
Rosuvastatin has more potent antiinflammatory effects than atorvastatin, which is thought to play an important role in AKI development.Citation32,Citation33 However, we found that rosuvastatin increased the risk of CSA-AKI more than atorvastatin. This was driven primarily by the results of Zheng et alCitation21 who reported AKI was more common with rosuvastatin. Previous research found that rosuvastatin might increase the risk of proteinuria due to its sulfur structure,Citation34 but not creatinine level or the incidence of kidney injury.Citation35 The way of excretion was another reason for our finding. Less than 1% of atorvastatin was excreted by kidney, so the renal toxic effect was not common.Citation36
Subgroup analysis of the different administration times indicated that statin intervention during the entire perioperative period slightly increased the risk of CSA-AKI relative to preoperative statin therapy alone. However, preoperative statin therapy was performed only in the initial studies,Citation18–Citation20,Citation22 which included patients with high cardiovascular risk. The inconsistent definitions of CSA-AKI and small sample sizes may affect the result. Unfortunately, the later studies did not continue this research design. Therefore, the value of statin administration times on the risk of CSA-AKI was not confirmed.
Limitations
Some limitations should be considered when applying the findings of this study. First, the inherent weakness of meta-analysis, in which publication bias and data from different or variable protocols are combined, might impact the results. Second, CSA-AKI might be considered a secondary endpoint in the included research studies, which could lead to miss some potentially relevant studies. However, we also included studies from previously published reviews. Third, the dose-dependent effects of statins on CSA-AKI are not fully understood because of the restrictions of the meta-analysis. Finally, although we performed subgroup analyses of different definitions of CSA-AKI, non-standardized definitions might influence the results. Therefore, the roles of statins (different types or administration times) in preventing CSA-AKI in patients with or without high cardiovascular risk remain to be clarified by further high-quality RCTs.
Conclusions
Perioperative statin therapy did not reduce the incidence of CSA-AKI, and in contrast, may exert unfavorable effects on renal function. Rosuvastatin showed a tendency of increasing risk of CSA-AKI compared with atorvastatin. In addition, compared with preoperative statin therapy, statin therapy throughout the perioperative period seemed to confer a higher risk of CSA-AKI.
Disclosure
The authors report no conflicts of interest in this work.
References
- ParolariAPesceLLPaciniDMonzino Research Group on Cardiac Surgery OutcomesRisk factors for perioperative acute kidney injury after adult cardiac surgery: Role of perioperative managementAnn Thorac Surg201293258459122269725
- KarkoutiKWijeysunderaDNYauTMAcute kidney injury after cardiac surgery: focus on modifiable risk factorsCirculation2009119449550219153273
- HaaseMBellomoRMatalanisGCalzavaccaPDragunDHaase-FielitzAA comparison of the RIFLE and Acute Kidney Injury Network classifications for cardiac surgery-associated acute kidney injury: A prospective cohort studyJ Thorac Cardiovasc Surg200913861370137619733864
- BagurRWebbJGNietlispachFAcute kidney injury following transcatheter aortic valve implantation: predictive factors, prognostic value, and comparison with surgical aortic valve replacementEur Heart J201031786587420037180
- ChertowGMBurdickEHonourMBonventreJVBatesDWAcute kidney injury, mortality, length of stay, and costs in hospitalized patientsJ Am Soc Nephrol200516113365337016177006
- LiSYChenJYYangWCChuangCLAcute kidney injury network classification predicts in-hospital and long-term mortality in patients undergoing elective coronary artery bypass grafting surgeryEur J Cardiothorac Surg201139332332820739188
- BihoracAYavasSSubbiahSLong-term risk of mortality and acute kidney injury during hospitalization after major surgeryAnn Surg2009249585185819387314
- ThieleRHIsbellJMRosnerMHAKI associated with cardiac surgeryClin J Am Soc Nephrol201510350051425376763
- Pinho-GomesACReillySBrandesRPCasadeiBTargeting inflammation and oxidative stress in atrial fibrillation: Role of 3-hydroxy-3-methylglutaryl-coenzyme a reductase inhibition with statinsAntioxid Redox Signal20142081268128523924190
- WangJGuCGaoMYuWYuYPreoperative statin therapy and renal outcomes after cardiac surgery: a meta-analysis and meta-regression of 59,771 patientsCan J Cardiol20153181051106026081692
- PutzuACapelliBBellettiAPerioperative statin therapy in cardiac surgery: A meta-analysis of randomized controlled trialsCrit Care201620139527919293
- JadadARMooreRACarrollDAssessing the quality of reports of randomized clinical trials: Is blinding necessary?Control Clin Trials19961711128721797
- BellomoRRoncoCKellumJAMehtaRLPalevskyPAcute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) GroupCrit Care200484R204R21215312219
- MehtaRLKellumJAShahSVAcute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injuryCrit Care2007112R3117331245
- ParkJHShimJKSongJWSohSKwakYLEffect of atorvastatin on the incidence of acute kidney injury following valvular heart surgery: a randomized, placebo-controlled trialIntensive Care Med20164291398140727120082
- BillingsFTHendricksPASchildcroutJSHigh-dose perioperative atorvastatin and acute kidney injury following cardiac surgery: a randomized clinical trialJAMA2016315987788826906014
- ProwleJRCalzavaccaPLicariEPilot double-blind, randomized controlled trial of short-term atorvastatin for prevention of acute kidney injury after cardiac surgeryNephrology (Carlton)201217321522422117606
- SpadaccioCPollariFCasacalendaAAtorvastatin increases the number of endothelial progenitor cells after cardiac surgery: a randomized control studyJ Cardiovasc Pharmacol2010551303819834333
- ChelloMPattiGCanduraDEffects of atorvastatin on systemic inflammatory response after coronary bypass surgeryCrit Care Med200634366066716505650
- MannacioVAIorioDDe AmicisVDi LelloFMusumeciFEffect of rosuvastatin pretreatment on myocardial damage after coronary surgery: a randomized trialJ Thorac Cardiovasc Surg200813661541154819114204
- ZhengZJayaramRJiangLPerioperative rosuvastatin in cardiac surgeryN Engl J Med20163741744175327144849
- ChristensonJTPreoperative lipid-control with simvastatin reduces the risk of postoperative thrombocytosis and thrombotic complications following CABGEur J Cardiothorac Surg199915439439910371111
- LiMZouHXuGThe prevention of statins against AKI and mortality following cardiac surgery: a meta-analysisInt J Cardiol201622226026627497107
- BaranCDurduSDalvaKEffects of preoperative short term use of atorvastatin on endothelial progenitor cells after coronary surgery: a randomized, controlled trialStem Cell Rev20128396397122076751
- CarrascalYArnoldRJDe la FuenteLEfficacy of atorvastatin in prevention of atrial fibrillation after heart valve surgery in the PROFACE trial (PROphylaxis of postoperative atrial Fibrillation After Cardiac surgEry)J Arrhythm201632319119727354864
- RamanJSKochiKMorimatsuHBuxtonBBellomoRSevere ischemic early liver injury after cardiac surgeryAnn Thorac Surg20027451601160612440615
- O’NealJBShawADBillingsFTAcute kidney injury following cardiac surgery: current understanding and future directionsCrit Care201620118727373799
- MarenziGCosentinoNWerbaJPTedescoCCVegliaFBartorelliALA meta-analysis of randomized controlled trials on statins for the prevention of contrast-induced acute kidney injury in patients with and without acute coronary syndromesInt J Cardiol2015183475325662053
- LiuYHLiuYDuanCYStatins for the prevention of contrast-induced nephropathy after coronary angiography/percutaneous interventions: a meta-analysis of randomized controlled trialsJ Cardiovasc Pharmacol Ther201520218119225193735
- CremerJMartinMRedlHSystemic inflammatory response syndrome after cardiac operationsAnn Thorac Surg1996616171417208651772
- AllouNAugustinPDufourGPreoperative statin treatment is associated with reduced postoperative mortality after isolated cardiac valve surgery in high-risk patientsJ Cardiothorac Vasc Anesth201024692192620638866
- QuHYXiaoYWJiangGHWangZYZhangYZhangMEffect of atorvastatin versus rosuvastatin on levels of serum lipids, inflammatory markers and adiponectin in patients with hypercholesterolemiaPharm Res200926495896419082693
- RidkerPMDanielsonEFonsecaFAJUPITER Study GroupRosuvastatin to prevent vascular events in men and women with elevated C-reactive proteinN Engl J Med2008359212195220718997196
- SavareseGMusellaFVolpeMPaneniFPerrone-FilardiPEffects of atorvastatin and rosuvastatin on renal function: a meta-analysisInt J Cardiol201316762482248922633671
- TothPPDayspringTDDrug safety evaluation of rosuvastatinExpert Opin Drug Saf201110696998621999163
- LennernäsHClinical pharmacokinetics of atorvastatinClin Pharmacokinet200342131141116014531725