Abstract
Bilastine is a second generation antihistamine indicated for the treatment of seasonal or perennial allergic rhinoconjunctivitis and chronic urticaria with a daily dose of 20 mg, in adults and children over 12 years of age. The efficacy of bilastine has been shown to be similar to that of the comparator drugs for the control of the nasal and nonnasal symptoms of allergic rhinoconjunctivitis, while also showing a subjective improvement in the quality of life and in overall clinical impression. For chronic urticaria the symptoms (itching and the development of papules) lessens from the second day of treatment onwards, in a similar way to other antihistamines used as comparators. Bilastine should not be administered at meal times to avoid interference with the absorption process. It is not distributed to the central nervous system, is scarcely metabolized, and elimination is through the kidneys and feces, with a 14-hour elimination half-life. It has no effect on cytochrome P450. During clinical development, bilastine was shown to be a drug that is adequately tolerated, with a similar effect to placebo with regard to drowsiness and changes in heart rate. In relation to its use, headaches were the most frequent adverse effect to be reported. No cardiotoxic effects have been observed, and the therapeutic dose does not alter the state of alertness.
Bilastine
Bilastine is a recently marketed, orally administered antihistamine for the treatment of allergic rhinoconjunctivitis and chronic urticaria in adults and adolescents. The treatment of both disorders with antihistamines has been clearly established.Citation1 Second generation antihistamines have surpassed first generation ones due to their improved tolerance, primarily based on their lack of anticholinergic effects and lower risk of cardiotoxicity. However, second generation antihistamines are able to cross the blood-brain barrier and, therefore, can induce drowsiness. Likewise, many of these antihistamines are metabolized by cytochrome P450 enzymes, and thus can possibly interact with other drugs. As discussed below, these characteristics have been addressed and modified with bilastine. The aim of this paper is to assess the present situation of the aforementioned antihistamine for the treatment of chronic urticaria and allergic rhinoconjunctivitis, through an evaluation of the results published regarding the efficacy and safety of this new drug.
Pharmacodynamics
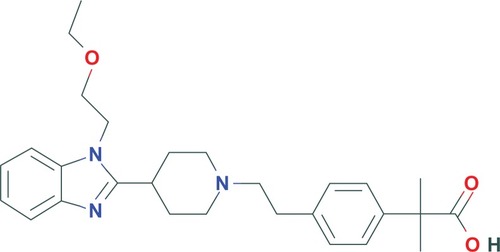
Bilastine, or 2-[4-[2-[4-[1-(2-ethoxyethyl) benzimidazol-2-yl] piperidin-1-yl] ethyl] phenyl]-2-methylpropionic acid (), is a molecule with a molecular weight of 463.6 Daltons. Based on bilastine’s structure, it is classified within the group of new antihistamines, as a second generation original molecule.
Bilastine is highly selective for histamine receptor (H1) binding, without inhibiting other receptors (muscarinic, adrenergic, serotonergic, other histamine receptors, brady-kinin, leukotriene d4, or calcium). It shows great affinity for the H1, comparable to other antihistamines, such as astemizole or diphenhydramine, and a three and five fold greater affinity than fexofenadine and cetirizine, respectively.Citation2 The inhibition of this receptor blocking is dose dependent. The antimuscarinic effect was specifically studied in preclinical trials and in healthy volunteers alike; this effect was not observed in the cellular models. In humans, no change was observed in pupillary reactivity following treatment with different dose levels of bilastine (20, 40, and 80 mg/day) over a 7-day period.Citation3 It can therefore be concluded that bilastine does not present undesirable anticholinergic effects associated with first generation antihistamines.
In different murine models, bilastine antagonizes the effect of histamine in a concentration-dependent manner (inhibiting increased capillary permeability, decreased microvascular extravasation, and decreased bronchospasm), with a similar potency to fexofenadine and greater than that of cetirizine. The antihistaminic effect of bilastine had a longer lasting duration than observed for cetirizine.Citation4
Inhibition of the release of histamine, interleukin (IL)-4, and tumor necrosis factor-α was observed in peripheral blood mast cells and granulocytes following bilastine treatment.Citation5
In a phase I study on healthy volunteers in which dose levels of 2.5 and 50 mg were evaluated, a reduction in histamine induced flare and wheal was observed, similar to that observed with cetirizine, although the higher dose levels for bilastine, (20 and 50 mg) meant faster results.Citation6
Pharmacokinetics
Bilastine is marketed as tablets and its pharmacokinetic properties were evaluated in preclinical and clinical trials, several of which were phase I trials (see ). The pharmacokinetic parameters of bilastine are shown in .
Table 1 Pharmacokinetic parameters of bilastine from single dose studiesCitation11,Citation18,Citation23,Citation27
Following oral administration, bilastine is rapidly absorbed, reaching a maximum concentration (Cmax) in 1 to 1.5 hours. Absorption is a first order process, observing dose linearity in the range from 2.5 to 220 mg, for single and multi doses alike, with no sign of accumulation after 14 days of treatment.Citation7,Citation8 This is a rapid process, with an absorption constant of 1.5 h−1.Citation7 The oral administration of 20 mg, considered to be the therapeutic dose, achieved a mean maximum concentration of 220 ng/ml 1.3 hours after administration, with subsequent biexponential elimination.
Bilastine has a bioavailability of 60%. It is a substrate of the membrane transporters present in the intestinal lumen cells, the P glycoprotein, and the organic anion-transporting polypeptide. The P glycoprotein is responsible for incomplete bioavailability, being an efflux protein, while the organic anion-transporting polypeptide facilitates absorption. Both transporters are present in the luminal membrane of the duodenum, indicating a powerful interaction between them, working in opposite directions. Both are liable to interactions when administered with fruit juices and some drugs.Citation9
Bioavailability is lessened when administered together with food rich in fats and with substances that modify the membrane transporter activity. In a study involving healthy volunteers, a 30% reduction in bioavailability was observed when administered together with a high fat meal and a 25% reduction when the meal had standard fat content.Citation10,Citation11 Grapefruit juice in particular caused a 30% reduction in bioavailability.Citation10,Citation11
Bilastine has a two compartment distribution, with an apparent volume of distribution of 1.29 l/kg. It binds to plasma proteins in a proportion from 84% to 90%. In studies on animals with radiolabeled bilastine, the drug was distributed in the gastrointestinal tract and liver, without reaching the brain.Citation12
Bilastine is scarcely metabolized, and is excreted unchanged through the kidneys and feces. A mass balance study showed that 33% of bilastine was excreted in the urine and 67% in the feces.Citation13 A study with hepatocyte cultures and liver microsomes demonstrated the non-existence of cytochrome P450 mediated metabolization, nor was any inhibiting or inducing effect observed with regard to this same enzyme.Citation7 This lack of inhibition is one of the characteristics that makes bilastine a compound of interest, according to the recommendations published by a group of experts on the treatment of allergic rhinitis;Citation1 96% of the dose administered was eliminated in 24 hours and at 72 hours it was completely eliminated from urine, with an elimination half-life of around 14 hours. Renal function impairment did not significantly alter the pharmacokinetics of bilastineCitation10 although an increase in the plasma concentrations of the antihistamine was observed. In general, this suggests that there is no need to modify the dose in the event of renal impairment, although this precaution should be taken into account in situations in which the concentrations of bilastine may be increased in the blood as a result of the concomitant administration of P glycoprotein inhibitors, as was observed with ketoconazole.Citation14
No evaluation of bilastine administration to patients with liver damage has been made, although given the fact that bilastine does not metabolize, no pharmacokinetic modification or the need for dosage adjustment whatsoever is to be expected for these patients.
No differences in bilastine pharmacokinetics have been observed with regard to sex or age, except for the highest maximum plasma concentration (Cmax) values in young women (18–35 years). In a population pharmacokinetics analysis, no influence of various covariables was observed (age, weight, height, sex, heart rate, serum albumin, creatinine, bilirubin, liver transaminases, alkaline phosphates, or urea).Citation7
Based on data from adults, it was estimated that the dose for children aged 2 to 12 years should be 10 mg per day and 5 mg per day for children under the age of two,Citation15 although studies are currently being conducted to confirm this dose.
The possibility of bilastine interacting with food and other drugs was previously evaluated. As mentioned, grapefruit juice reduces the bioavailability of bilastine.Citation10,Citation11 The mechanism responsible for this interaction could be the inhibition of the organic anion-transporting polypeptide membrane transporter, which facilitates the absorption of some drugs.Citation9 This effect on the transporter could be avoided by taking bilastine either one hour before or two hours after the intake of juices or food. Some substrate drugs or inhibitors of this transporter, such as ritonavir or rifampicin, could reduce bilastine plasma concentrations,Citation16 although this point has not been specifically evaluated.
No effect of bilastine has been observed on the pharmacokinetics of ketoconazole in healthy volunteers.Citation14 On the other hand, exposure to bilastine increased as a result of coadministration with ketoconazole, which doubled the exposure of the antihistamine, probably due to the inhibitor effect on the membrane transporters, such as P glycoprotein, in the intestinal lumen without modifying the hepatic clearance. Citation17
No interaction was observed with the central nervous system depressors, such as lorazepamCitation18 or alcohol.Citation19
Pharmacokinetics/pharmacodynamics
When jointly evaluating the phar macokinetic and pharmacodynamic data, it was concluded that bilastine is characterized by two compartment kinetics, with a rapid absorption phase and a maximum response 4 hours after administration. The pharmacodynamics of the disappearance of the wheal or flare was fitted to an indirect response model to define a concentration that produces a 50% inhibition (IC50) of 5.15 ng/ml and 1.25 ng/ml, respectively. With a daily administration of 20 mg of bilastine to adults, the plasma concentrations remain above these values for 20 and 24 hours, respectively, indicating that a once a day administration is adequate.Citation7 In a simulation performed for children aged from 2 to 12, the 10 mg dose was sufficient to maintain the plasma concentrations above this value for the entire administration interval.Citation15
Efficacy
Efficacy evaluation of bilastine from clinical investigation is shown in .
Table 2 Bilastine efficacy determined during clinical investigation
The onset of action and the duration of the antihistaminic effect are both decisive factors in selecting an antihistamine. Along these lines, an evaluation was made of the effects of bilastine on allergen-induced nasal and eye symptoms in 75 patients allergic to pollen. The patients were exposed to controlled concentrations of pollen using a standard allergy provocation method (Vienna Challenge Chamber).Citation20 The subjects underwent two provocation tests with pollen on two consecutive days, for 6 hours on the first day and for 4 hours on the second day. The nasal symptoms were assessed every 15 minutes. Four of these symptoms (rhinorrhea, nasal congestion, sneezing, and nasal itching) were considered to be the principal study variables. The treatments studied were 20 mg of bilastine, 10 mg of cetirizine, 120 mg of fexofenadine, or a placebo, administered 2 hours after the first provocation. In this study, onset of action was observed at around 1 hour for bilastine and cetirizine, with duration of action of at least 26 hours. The duration of action of fexofenadine was considered shorter, given the fact that it was less effective on the second day of the study.Citation20 A similar study compared the effect of 20 and 50 mg of bilastine to 10 mg of cetirizine with respect to the inhibition of cutaneous wheal and flare following a skin prick test at different times in relation to the drug administration. The response was similar for 20 mg of bilastine and 10 mg of cetirizine at the different measurement points, with the exception of the analysis obtained 1.5 hours following administration, which indicated that bilastine had a faster onset of action. The administration of 50 mg of bilastine prolonged the duration of the antihistaminic effect.Citation21 In a study on healthy volunteers, the results observed showed the existence of a certain dose dependency in regards to the inhibition of wheals following subcutaneously administered histamine.Citation3
Clinical studies
Allergic rhinoconjunctivitis (seasonal and perennial)
H1 antihistamines are first line drugs for the treatment of allergic rhinoconjunctivitis. Allergic rhinitis is one of the most common chronic diseases in the world, with an estimated prevalence of 10%–25%.Citation22 The most frequent symptoms of allergic rhinitis are nasal itching, sneezing fits, rhinorrhea, and nasal obstruction.Citation23 Patients with allergic rhinitis show a reduced quality of lifeCitation24 due to the inherent symptoms of rhinitis and the physiopathology, which can result in sleep disorders.Citation25
The nasal response to allergen stimulation provokes all the symptoms characteristic of allergic rhinitis. This response comprises two phases: the immediate response is observed 20 minutes after contact with the allergen and can continue for up to hours afterwards. This is primarily the result of mast cell degranulation. This phase is characterized by itching, sneezing, and rhinorrhea. Mast cells release chemotactic substances and cytokines that are able to attract other cells to the inflammatory focus, including eosinophils. This gives rise to the late phase of the allergic response (48 hours later), which comprises the cellular response. The principal response in this phase is nasal obstruction.Citation26
Histamine is one of the substances released by the mast cells and basophils. This amine is released in a few seconds in E immunoglobulin (IgE) mediated hypersensitivity reactions. A minute after the nasal allergic stimulation, the histamine released reaches its maximum concentration. Ten minutes after this peak, there is an abrupt drop in concentration.Citation27 Histamine induces increased chemotaxis of eosinophils, increased cytokine (IL-1β, IL-6, IL-4, and IL-5) release, increased vascular cell adhesion molecule (VCAM)-1 expression and activation of nuclear factor (NF)-κβ. Histamine is responsible for the characteristic symptomatology of allergic rhinitis, including nasal obstruction.
H1 antihistamines can act through different routes of action; they counteract the effects of histamine on the H1 receptors and they are also able to inhibit the release from mast cells and basophils, which contribute to the late phase of the allergic response.Citation28 Bilastine is a highly selective antihistamine, with moderate to high H1 receptor affinity. Furthermore, in vitro studies have shown anti-inflammatory activity by inhibiting the release of histamine, IL-4, and tumor necrosis factor (TNF)-α, and also by inducing the release of different stimulants.Citation5
The efficacy of bilastine in allergic rhinoconjunctivitis has been confirmed in several studies comparing it to either a placebo or to other antihistamines.
Bachert et al conducted a multicenter study to assess clinical efficacy through the rating of nasal and nonnasal symptoms at the start and after 14 days of treatment, and the safety of 20 mg of bilastine compared to 5 mg of desloratadine and placebo in patients with seasonal allergic rhinitis (n = 721). A significant decrease was observed in the nasal and nonnasal symptoms of patients treated with both drugs in relation to the placebo. Along these same lines, a reduction in the patient-rated discomfort score and an improvement in the investigator’s overall clinical impression were observed. However, no differences were found between bilastine and desloratadine.Citation29
A further study compared the administration of 20 mg of bilastine to 10 mg of cetirizine or placebo in patients with seasonal rhinitis, finding statistically significant differences in the improvement of the nasal symptoms in the group administered with bilastine (−43.8% after 7 days and −48.5% after 14 days) and cetirizine (−40.2% after 7 days, and −50.6% after 14 days) in relation to the placebo group (P < 0.001), yet without any differences between treatment groups.Citation30
Daily treatment with 2 mg of bilastine was effective for the treatment of perennial allergic rhinitis, a disease which affects a high percentage of the population (10%–15%). An assessment was made of the nasal symptoms (rhinorrhea, sneezing, congestion, itching) and eye symptoms (redness, tears) compared to the placebo; the results showed bilastine and cetirizine were similar in Europe and superior in Argentina, achieving an approximate 34% reduction in nasal symptoms after 28 days of treatment.Citation31
Bartra et al reviewed clinical trials conducted with bilastine in order to assess its effect on eye symptoms of allergic rhinoconjunctivitis (itching, redness, and tearing). The clinical test results showed bilastine to be as effective as the comparator drugs for controlling the eye symptoms in seasonal allergic rhinitis.Citation32
Likewise, an evaluation of the effects of bilastine on nasal obstruction in allergic rhinitis was performed. The efficacy of 20 mg of bilastine for controlling this symptom was tested and was determined to be along the same lines as that observed with active comparators (desloratadine 5 mg and cetirizine 10 mg).Citation23
The quality of life variable was also recently analyzed separately, given the fact that it has been studied as a secondary objective in three clinical studies on allergic rhinitis, by means of the Rhinoconjunctivitis Quality of Life Questionnaire (RQLQ) questionnaire on a total number of 2335 patients and in chronic urticaria through the Dermatology Life Quality Index (DLQI) questionnaire on a total of 525 patients, compared to levocetirizine and placebo.Citation33 The results are parallel to the efficacy results observed in the clinical trials.
Chronic urticaria
Chronic urticaria significantly affects patients’ quality of life. It is characterized by the appearance of pruritic erythematous papules. The papules have a duration of less than 24 hours in which they may appear daily or more than once a week for 6 weeks. In 50% of cases, this is associated with the appearance of angioedema.Citation34
The clinical guidelines for the treatment of chronic urticaria suggest increasing the normal dosage of the antihistamine by up to 4-fold, if the normal therapeutic dosing is not effective.Citation35 However, in the literature, there are no randomized, double blind trials that study whether there is any difference in efficacy between the therapeutic doses and higher doses for controlling chronic urticaria. In this respect, preliminary data with bilastine are available, such as the data obtained in a phase I double blind single dose clinical trial with four crossover periods on 21 volunteers administered with either placebo, 10 mg of cetirizine, or 2.5, 5, 10, or 50 mg of bilastine. An evaluation of the relationship between the dose of bilastine and the inhibition of the cutaneous response to histamine (measured by wheal and flare) was made. All bilastine doses inhibited the histamine-induced wheal between 1.5 and 12 hours and the flare between 4 and 24 hours following administration, showing a response that is similar or better than cetirizine. No clear dose dependent effect was observed, although it was seen that the 20 and 50 mg dose levels had a longer lasting effect.Citation13
Audicana et al conducted a phase II study on 222 patients with chronic urticaria. The effect of bilastine (10, 20, and 30 mg) was evaluated against a placebo in the control group for the appearance of papules and itching. Statistically significant differences were observed in the three treatment groups administered bilastine compared to the placebo group, although no dose dependent effect was observed, as reported by Ferrer et al.Citation36
Bilastine was also assessed in a double blind controlled clinical trial with placebo and levocetirizine both administered for 28 days. A compound variable was evaluated by calculating the data for itching, number of wheals, and the maximum size of the wheals, based on patients’ diaries. The symptoms were reduced from the second day of treatment with 20 mg of bilastine, which was also the case for levocetirizine, showing better results than placebo.Citation37
Tolerance and safety
In the various studies conducted during the preclinical development of bilastine, no significant toxicity was demonstrated. The acute toxicity of bilastine was investigated through the oral and intravenous administration in mice and rats. Following the oral administration of 5000 and 2000 mg/kg in mice and rats respectively, no mortality was observed in either of the two species. However, following intravenous administration, the lethal dose for 50% of the animals was 33 mg/kg for mice and 45–75 mg/kg for rats.Citation38
In chronic toxicity studies of bilastine administered both orally and intravenously, no signs of toxicity were observed in any organ.Citation38 No effects on fertility, either on embryo-fetal toxicity or on teratogenicity, were observed in the studies conducted on mice and rabbits.Citation39
When studies were conducted on humans during the assessment of the efficacy of bilastine in the treatment of chronic urticaria and allergic rhinitis, tolerability was studied in relation to a placebo and other antihistamines (levocetirizine, desloratadine, and cetirizine), and most of the data correspond to the therapeutic dose of 10 mg a day; shows results of these clinical trials. Specific studies were also conducted to evaluate the incidence of problems commonly associated with the use of antihistamines, such as drowsiness and heart rate changes.
Table 3 Bilastine tolerability determined during clinical investigation
In general, bilastine was well tolerated and the majority of the adverse events described were either mild or moderate. The incidence of drug related adverse effects was 15%–30%, similar to observed with the placebo. There were no serious drug related adverse effects in any of the clinical trials. No significant changes were detected in vital signs (heart rate or blood pressure), in the electrocardiogram (ECG) parameters or in laboratory values (hematology and liver and kidney function).Citation29,Citation30,Citation37
The adverse events most commonly described by patients treated with bilastine and to some extent related to the drug, are: headache, drowsiness, dizziness, and fatigue.Citation29,Citation30,Citation37,Citation40 In a study in which the treatment was administered for 1-year, the most commonly described adverse effects were headache (9.6%) followed by nasopharyngitis (2.5%), while other less common bilastine related adverse effects included some changes in the ECG trace, which were not consistent over time.Citation31
No differences were observed in the tolerance of bilastine compared to cetirizine and desloratadine.Citation29,Citation37 One study described a statistically significant minor incidence in the appearance of drowsiness, (bilastine and cetirizine, 1.8% versus 7.5%) and fatigue, (bilastine and cetirizine, 0.4% versus 4.8%) with 20 mg a day of bilastine compared to cetirizine at normal dose levels.Citation30
Some second generation antihistamines have been associated with adverse cardiac effects, such as the prolongation of the QT interval and the development of torsades de pointes. These effects have been attributed to the direct blocking of the potassium channels. For this reason, and following the guidelines of International Conference on Harmonisation (ICH) E14, a study was conducted to evaluate the effect of the 20 mg therapeutic dose and that of a supratherapeutic dose of 100 mg on the QTc interval. This was a multidose randomized double blind crossover study with placebo and with 400 mg moxifloxacin, the latter an active comparator responsible for changes of this type. A fifth arm was included in which a 20 mg dose of bilastine in combination with the administration of 400 mg of ketoconazole. No effects with either 20 mg or 100 mg of bilastine were observed on the electrocardiogram trace, the QTc duration, or on the T wave morphology. The increase in the value of the ATc interval of 5 milliseconds or less confirms this lack of influence. No significant effects on the QTc interval were observed with any of the doses evaluated. The changes reported in the combination of bilastine with ketoconazole were related to the antifungal drug, given the fact that the plasma concentrations of bilastine in this coadministration were less than those observed with the supratherapeutic dose of 100 mg.Citation41,Citation42 These results are consistent with other investigations on bilastine and in clinical trials with patients in which no changes in the electrocardiogram or cardiac related adverse effects were observed.
The evaluation of neurological tolerance was conducted with specific studies to evaluate the appearance of drowsiness/sedation and the effect on attention when driving a vehicle following bilastine treatment. The sedating effect of first generation antihistamines is widely known, which in some cases has led these drugs to be used as sleeping pills.Citation43 This effect is related to the affinity for the P glycoprotein, which is responsible for the efflux of drugs and other substances from the central nervous system, and to drug lipophilicity; the greater the affinity and the lower the lipophilicity, the lower the blood-brain barrier crossing.
In a repeat dose crossover trial with healthy volunteers, three doses of bilastine (20, 40, and 80 mg) were compared to 25 mg of hydroxyzine and placebo following 7 days of treatment and with a washout period of 15 days between each scheduled administration. An evaluation was made of the effects on psychomotor function (motor activity, perception, attention, associative integration) and state of mind. Hydroxyzine caused an increase in the time to react, a decrease in the blinking rate, and a worsening of attention or motor activity. With the 20 and 40 mg doses of bilastine, no changes were observed in the parameters evaluated, although changes were observed with the highest dose (80 mg) when evaluating objective parameters. Subjectively, only the 20 mg dose was distinguishable from the placebo.Citation3
A clinical trial was conducted with 22 healthy volunteers, who were administered daily doses of bilastine (20 mg and 40 mg) for an 8-day period, and compared to a placebo and 50 mg a day of hydroxyzine (positive control) to evaluate the effect of the drug on driving. This was a crossover study with a 7-day interval between treatment periods. The position of the car was evaluated at a constant speed, recording the deviation of the car in relation to the standard deviation of lateral position. No changes were observed with the 20 and 40 mg doses of bilastine, providing results similar to the placebo, with evaluations made on the first and eighth day of treatment. With hydroxyzine, a significant increase was observed in the deviations for all measurements, although it was reduced in relation to the first administration, in a situation of pharmacokinetic balance, following the final dose.Citation44
In the overall analysis of the data on the development of sleepiness observed in the different studies conducted with bilastine, it was concluded that its appearance is similar to that observed with a placebo, and significantly less than that observed with cetirizine.Citation45
Further studies have been conducted to evaluate the effects of bilastine on the central nervous system in combination with other drugs such as lorazepam or substances such as alcohol, whose sedative effect is well known. None of these studies with 20 mg of bilastine produced such an effect, neither did they increase the effect produced by these known central nervous system depressants.Citation18,Citation19
Conclusion
Bilastine is a second generation antihistamine developed for the treatment of allergic rhinoconjunctivitis and chronic urticaria. It is a product that does not undergo hepatic metabolism nor does it modify the activity of the cytochrome P450 isoenzymes. It should not be administered at meal times, which may increase its bioavailability. Treatment with 20 mg, administered orally, is effective in controlling the symptoms and improving the quality of life of these patients. The studies conducted to date indicate that bilastine has an acceptable tolerability profile, given the fact that no cardiotoxic effects have been observed, and the therapeutic dose does not change a patient’s state of alertness.
Disclosure
The authors report no conflicts of interest in this work.
References
- BrozekJLBousquetJBaena-CagnaniCEGlobal Allergy and Asthma European Network; Grading of Recommendations Assessment, Development and Evaluation Working GroupAllergic rhinitis and its impact on asthma (ARIA) guidelines: 2010 revisionJ Allergy Clin Immunol2010126346647620816182
- CorcósteguiRLabeagaLInnerárityABerisaAOrjalesAPreclinical pharmacology of bilastine, a new selective histamine H1 receptor antagonist: receptor selectivity and in vitro antihistaminic activityDrugs R D20056637138416274260
- García-GeaCMartínez-ColomerJAntonijoanRMValienteRBarbanojMJComparison of peripheral and central effects of single and repeated oral dose administrations of bilastine, a new H1 antihistamine: a dose-range study in healthy volunteers with hydroxyzine and placebo as control treatmentsJ Clin Psychopharmacol200828667568519011437
- CorcósteguiRLabeagaLInnerárityABerisaAOrjalesAIn vivo pharmacological characterisation of bilastine, a potent and selective histamine H1 receptor antagonistDrugs R D20067421923116784247
- Alvarez-MonMSan AntonioELuceroMSanzELedoFDe la HeraABilastine, a novel antihistamine that preferentially inhibits histamine and interleukin-4 release from human mast cells and granulocytesAllergy200964Suppl 90555
- SologurenAValienteRCreanCMcLavertyDRelationship of dose to inhibition of wheal and flare for 5 doses of bilastine and 10 mg cetirizine. 36th Annual Meeting of the American College of Clinical Pharmacology; September 9–11, 2007; San Francisco, USAJ Clin Pharmacol20074791198 Abstract 69.
- JauregizarNde la FuenteLLuceroMLSologurenALealNRodríguezMPharmacokinetic-pharmacodynamic modelling of the antihistaminic (H1) effect of bilastineClin Pharmacokinet200948854355419705924
- CreanCRoupeKSologurenAValienteRThe pharmacokinetics of bilastine after single and 14 days once daily administration. 8th Congress of the European Association of Clinical Pharmacology and Therapeutics; August 29–September 1, 2007; Amsterdam, The NetherlandsBasic Clin Pharmacol Toxicol.2007101Suppl 1148 Abstract P252.
- BaileyDGFruit juice inhibition of uptake transport: a new type of food-drug interactionBr J Clin Pharmacol20107064565521039758
- Bilastine SPCTexto propuesto para la ficha técnica Bilastina 20 mg [Summary of Product Characteristics for Bilastine 20 mg.] Available from: http://www.aemps.gob.es/cima/especialidad.do?metodo=verFichaWordPdf&codigo=73027&formato=pdf&formulario=FICHAS&file=ficha.pdf. Accessed February 20, 2013. Spanish.
- CreanCValienteRSologurenAMcLavertyDEffect of grapefruit juice on the pharmacokinetics of bilastine. 36th Annual Meeting of the American College of Clinical Pharmacology; September 9–11, 2007; San Francisco, USAJ Clin Pharmacol20074791198 Abstract 71.
- MumfordRAllanLHoeyRThe disposition, metabolism and elimination in rats of bilastine, a potent, selective H1 receptor antagonist. 8th International ISSX Meeting; October 9–12, 2007; Sendai, JapanDrug Metab Rev.200739Suppl I200201 Abstract 282.
- SologurenALuceroMLValienteRValienteRCharlesHMairSJHuman mass balance with [14C]-bilastine following oral administration to healthy volunteers. 9th Congress of the European Association for Clinical Pharmacology and Therapeutics; July 12–15, 2009, Edinburgh, UKBasic Clin Pharmacol Toxicol.2009105Suppl 1106107 Abstract TP85.
- CreanCSologurenAValienteRMcLavertyDThe drug-drug interaction of ketoconazole on bilastine pharmacokinetics. 8th Congress of the European Association of Clinical Pharmacology and Therapeutics; August 29–September 1, 2007; Amsterdam, The NetherlandsBasic Clin Pharmacol Toxicol.2007101Suppl 1148149 Abstract P253.
- RodríguezMLuceroMLOrjalesAGonzaloALealNCalvoREstimation of bilastine dose in paediatrics. IX World Conference on Clinical Pharmacology Therapeutics; July 27–August 1, 2008; Quebec City, CanadaCan J Clin Pharmacol.2008153e680 Abstract 554.
- BousquetJAnsóteguiICanonicaGWEstablishing the place in therapy of bilastine in the treatment of allergic rhinitis according to ARIA: evidence reviewCurr Med Res Opin201228113113922149770
- TakanoMHasegawaRFukudaTYumotoRNagaiJMurakamiTInteraction with P-glycoprotein and transport of erythromycin, midazolam and ketoconazole in Caco-2 cellsEur J Pharmacol199835832892949822896
- BachertCKunaPZuberbierTBilastine in allergic rhinoconjunctivitis and urticariaAllergy201065Suppl 93S1S13
- García-GeaCClosSAntonijoanRMGichIValienteRBarbanojMJCrossover, randomised, double-blind, double-dummy, placebo and positive standard-controlled, unicenter clinical trial to assess the possible interaction on CNS effects between bilastine (20 mg and 80 mg) and alcohol (0.8 g/kg) after single simultaneous administration in healthy subjects. 20th Congress of the Spanish Clinical Pharmacology Society; October 29–November 2, 2006; Tenerife, SpainBasic Clin Pharmacol Toxicol.200699Suppl I30 Abstract EC13.
- HorakFZieglmayerPZieglmayerRLemellPThe effects of bilastine compared with cetirizine, fexofenadine, and placebo on allergen-induced nasal and ocular symptoms in patients exposed to aeroallergen in the Vienna Challenge ChamberInflamm Res201059539139819943178
- CruchMKComparative inhibition by bilastine and cetirizine of histamine-induced wheal and flare response in humansInflamm Res201160121107111221874559
- BousquetJKhaltaevNCruzAAAllergic Rhinitis and its Impact on Asthma (ARIA) 2008 updateAllergy20086386816018331513
- DávilaISastreJMullolJEffect of bilastine upon nasal obstructionJ Investig Allergol Clin Immunol201121Suppl 328
- JuniperEFRohrbaughTMeltzerEOA questionnaire to measure quality of life in adults with nocturnal allergic rhinoconjunctivitisJ Allergy Clin Immunol2003111348449012642826
- WoodsLCraigTJThe importance of rhinitis on sleep, daytime somnolence, productivity and fatigueCurr Opin Pulm Med200612639039617053486
- GelfandEWInflammatory mediators in allergic rhinitisJ Allergy Clin Immunol2004114Suppl 5S135S13815536444
- WangDSmitzJWaterschootSClementPAn approach to the understanding of the nasal early-phase reaction induced by nasal allergen challengeAllergy19975221621679105520
- SchroederJTSchleimerRPLichtensteinLMKreutnerWInhibition of cytokine generation and mediator release by human basophils treated with desloratadineClin Exp Allergy20013191369137711591186
- BachertCKunaPSanquerFBilastine International Workin GroupComparison of the efficacy and safety of bilastine 20 mg vs desloratadine 5 mg in seasonal allergic rhinitis patientsAllergy200964115816519132976
- KunaPBachertCNowackiZBilastine International Working GroupEfficacy and safety of bilastine 20 mg compared with cetirizine 10 mg and placebo for the symptomatic treatment of seasonal allergic rhinitis: a randomized, double-blind, parallel-group studyClin Exp Allergy20093991338134719438584
- SastreJMullolJValeroAValienteRBilastine Study GroupEfficacy and safety of bilastine 20 mg compared with cetirizine 10 mg and placebo in the treatment of perennial allergic rhinitisCurr Med Res Opin201228112113022077106
- BartraJMullolJMontoroJEffect of bilastine upon the ocular symptoms of allergic rhinoconjunctivitisJ Investig Allergol Clin Immunol201121Suppl 32433
- JáureguiIBartraJdel CuvilloABilastine and quality of lifeJ Investig Allergol Clin Immunol201121Suppl 31623
- KaplanAPChronic urticaria: pathogenesis and treatmentJ Allergy Clin Immunol2004114346547415356542
- ZuberbierTAseroRBindslev-JensenCDermatology Section of the European Academy of Allergology and Clinical Immunology; Global Allergy and Asthma European Network; European Dermatology Forum; World Allergy OrganizationEAACI/GA(2)LEN/EDF/WAO guideline: management of urticariaAllergy200964101427144319772513
- FerrerMSastreJJáureguiIEffect of antihistamine up-dosing in chronic urticariaJ Investig Allergol Clin Immunol201121Suppl 33439
- ZuberbierTOantaABogackaEBilastine International Working GroupComparison of the efficacy and safety of bilastine 20 mg vs levocetirizine 5 mg for the treatment of chronic idiopathic urticaria: a multi-centre, double-blind, randomized, placebo-controlled studyAllergy201065451652819860762
- ArtecheJKLedoFCasadesusABilastine. Preclinical toxicology review. XII International Congress of Toxicology; July 19–23, 2010; Barcelona, SpainToxicol Lett.2010196SupplS256 Abstract P302-026.
- SommerEWeberKLuceroMLArtecheJKOrjalesAOncogenicity risk assessment of bilastine, a novel antihistamine compound. 45th Congress of the European Societies of Toxicology (Eurotox); October 5–8, 2008; Rothes, GreeceToxicol Lett.2008180SupplS156 Abstract P20.
- CarterNJBilastine: in allergic rhinitis and urticariaDrugs20127291257126922686617
- GraffCStruijkJJKantersJKAndersenMPToftETylBEffects of bilastine on T-wave morphology and the QTc interval. A randomized, double-blind, placebo-controlled, thorough QTc studyClin Drug Investig2012325339351
- TylBKabbajMAzzamSLack of significant effect of bilastine administered at therapeutic and supratherapeutic doses and concomitantly with ketoconazole on ventricular repolarization: results of a thorough QT study (TQTS) with QT-concentration analysisJ Clin Pharmacol201252689390321642470
- RichardsonGSRoehrsTARosenthalLKoshorekGRothTTolerance to daytime sedative effects of H1 antihistaminesJ Clin Psychopharmacol200222551151512352276
- ConenSTheunissenELVan OersACValienteRRamaekersJGAcute and subchronic effects of bilastine (20 and 40 mg) and hydroxyzine (50 mg) on actual driving performance in healthy volunteersJ Psychopharmacol201125111517153320855350
- MontoroJMullolJDaáilaIBilastine and the central nervous systemJ Investig Allergol Clin Immunol201121Suppl 3915