Abstract
Background
Triamcinolone acetonide (TAC) is used frequently in the treatment of keloid scars, but has presented controversial results. In this study, we aim to evaluate the effectiveness of TAC compared with other common therapies used in keloid treatment.
Methods
MEDLINE, Embase, Web of Science and the Cochrane Library databases were searched until January 2018. Key data were extracted from eligible randomized controlled trials. Both pairwise and network meta-analyses were conducted for synthesizing data from eligible studies.
Results
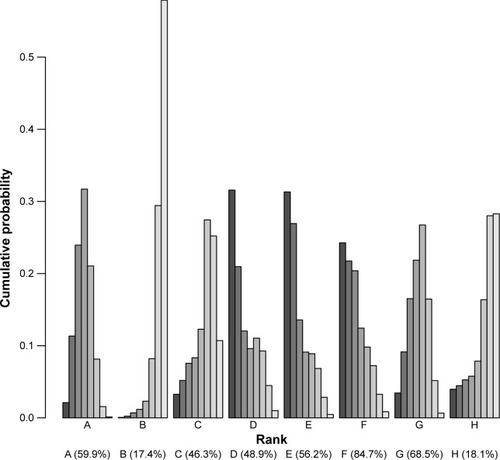
Ten randomized controlled trials were included in this meta-analysis. The relative risk of keloids associated with seven adjuvants was analyzed, including placebo, pulsed dye laser (PDL), 5-fluorouracil (5-FU), silicone, verapamil, TAC+5-FU and TAC+5-FU+PDL. Patients treated with the following adjuvants appeared to not have significantly reduced risk of keloid in relation to those treated with TAC: placebo (OR=1.86, 95% CI 1.12–2.61), PDL (OR=1.32, 95% CI 0.53–3.30), 5-FU (OR=1.13, 95% CI 0.48–2.68), silicone (OR=1.28, 95% CI 0.59–2.78), verapamil (OR=1.86, 95% CI 0.67–5.14), TAC+5-FU (OR=0.77, 95% CI 0.38–1.58) and TAC+5-FU+PDL (OR=0.80, 95% CI 0.16–4.03). The surface under the cumulative ranking curve values for each adjuvant were as follows: TAC, 59.9%; placebo, 17.4%; PDL, 46.3%; 5-FU, 48.9%; silicone, 56.2%; verapamil, 84.7%; TAC+5-FU, 68.5% and TAC+5-FU+PDL, 18.1%.
Conclusion
There were no differences between the efficacy of TAC and other common therapies in keloid treatment. TAC also acts as an effective alternative modality in the prevention and treatment of keloids. Incorporating adjuvants particularly verapamil appeared to be significantly associated with a decreased risk of keloids.
Introduction
Keloids are caused by the uncontrolled deposition of collagen and glycosaminoglycans around the wounds on the dermal dermis of the skin.Citation1 During wound healing, the balance between the anabolic and catabolic effects of collagen is destroyed due to various causes forming a pathological scar.Citation2,Citation3 Keloids are caused by the proliferation of fibrogenic cells and the formation of large extracellular matrix, and their development is characterized by excessive collagen synthesis and deposition.Citation4 Keloids can bring psychological and physical pain to patients from the aspect of appearance and body function. In severe cases, keloids even affect the self-confidence of patients leading to inferiority complex.Citation4 Therefore, in the burn trauma, plastic surgery and dermatology department, keloid is the focus of high clinical attention. Although drugs and treatments are currently available for keloids, there are enormous challenges in their prevention and treatment, and there is no satisfactory universal treatment for all keloids.Citation5
Corticosteroids are highly bioactive substances secreted by the adrenal cortex through tissue fluid and blood, which play a powerful role in physiological regulation.Citation6 Most of them are hormones, such as glucocorticosteroids and sex hormones. They have anti-inflammatory, anti-allergy, anti-drug, anti-nuclear fission and other effects, and their role is strong and lasting.Citation7–Citation9 Corticosteroids are most commonly used as injections in the early stage of the maturation phase, and intralesional injection of the corticosteroid triamcinolone acetonide (TAC) is one of the first-line treatment modalities for keloid treatment.Citation10
The use of TAC in keloid treatment is commonplace. Wong et al found TAC therapy reduces the incidence of keloids among patients.Citation11 However, the efficacy of TAC has not been compared with other common therapies efficacious in treating keloids. Therefore, to determine the efficacy and safety of TAC in keloid treatment compared to other common therapies, we conducted a network meta-analysis based on randomized controlled trials.
Methods
Search strategy
Eligible studies were systematically searched in MEDLINE, Embase, Web of Science and the Cochrane Library databases until January 2018 with keywords including “Keloid” [MeSH] OR “Acne Keloid” [MeSH] OR “Hypertrophic” [MeSH] OR “Scar” [MeSH] AND “triamcinolone acetonide” [MeSH] OR “Corticosteroids” [MeSH] OR “steroids” [MeSH] AND “Randomized Controlled Trial” [MeSH].
Inclusion criteria
The studies that met the following inclusion criteria were included in the meta-analysis: (1) the study must have included keloid patients; (2) the relationship between TAC and keloid must have been studied; and (3) the study must be a randomized controlled trial.
Statistical analysis
We conducted a network meta-analysis (Bayesian approach) which included both direct and indirect evidence in the network. Adjuvants were ranked based on the surface under the cumulative ranking curve (SUCRA) values. One adjuvant is more preferable than the other if it has a larger SUCRA value. Small study effects or publication bias were visually inspected using the funnel plots.
Results
Literature search results
A total of 1,005 studies from MEDLINE, 1,127 studies from Embase and 1,074 studies from Web of Science were selected. After removing duplicates, 1,066 studies were identified. After reviewing their titles and abstracts, 1,029 citations were excluded. The remaining 37 citations were assessed in more detail for eligibility by reading the full text. Among them, one study was excluded due to no relevant outcome measure, 11 studies were excluded due to insufficient network connections and eight studies were excluded due to lack of detailed information. Finally, 10 studies were used for the final data synthesis.Citation12–Citation21 The flowchart of literature search is presented in , and the risk of bias of eight studies included in this meta-analysis is summarized in . The characteristics of the included studies are shown in , with the pattern of evidence within the network displayed in .
Figure 2 Risk of bias of the included randomized controlled trials (judgments about each risk-of-bias item for each included study: +, low risk; −, high risk; ?, unclear risk).
Figure 3 Network of randomized controlled trials comparing different adjuvant therapies for keloid treatment.
Abbreviations: TAC, triamcinolone acetonide; PDL, pulsed dye laser; 5-FU, 5-fluorouracil.
Table 1 Characteristics of included studies
Results of pairwise meta-analysis
displays the results produced by pairwise meta-analysis. Patients treated with the following seven adjuvants appeared to not have significantly reduced risk of keloids in relation to those treated with TAC: placebo (OR=1.86, 95% CI 1.12–2.61), pulsed dye laser (PDL; OR=1.32, 95% CI 0.53–3.30), 5-fluorouracil (5-FU; OR=1.13, 95% CI 0.48–2.68), silicone (OR=1.28, 95% CI 0.59–2.78), verapamil (OR=1.86, 95% CI 0.67–5.14), TAC+5-FU (OR=0.77, 95% CI 0.38–1.58) and TAC+5-FU+PDL (OR=0.80, 95% CI 0.16–4.03). Patients treated with the following adjuvants appeared to have a significantly reduced risk of keloids in relation to those treated with placebo: PDL (OR=0.34, 95% CI 0.27–0.43), 5-FU (OR=0.37, 95% CI 0.26–0.52), silicone (OR=0.40, 95% CI 0.29–0.54) and TAC+5-FU (OR=0.49, 95% CI 0.28–0.85). Moreover, there was no significant heterogeneity among studies for the abovementioned significant results (P-heterogeneity >0.05 and I2<50%).
Table 2 Summary ORs of TAC and heterogeneity for each direct comparison
Network meta-analysis
displays the results produced by network meta-analysis. Patients treated with the following seven adjuvants appeared to have a significantly reduced risk of keloids in relation to those treated with placebo: TAC (OR=0.08, 95% CI 0.00–0.18), PDL (OR=0.30, 95% CI 0.06–0.55), 5-FU (OR=0.32, 95% CI 0.05–0.78), silicone (OR=0.20, 95% CI 0.01–0.40), verapamil (OR=0.23, 95% CI 0.01–0.46), TAC+5-FU (OR=0.12, 95% CI 0.01–0.24) and TAC+5-FU+PDL (OR=0.46, 95% CI 0.27–0.77).
Table 3 Network meta-analysis comparisons
The corresponding SUCRA values are presented in . The adjuvants were ranked based on SUCRA values as follows: TAC, 59.9%; placebo, 17.4%; PDL, 46.3%; 5-FU, 48.9%; silicone, 56.2%; verapamil, 84.7%; TAC+5-FU, 68.5%; and TAC+5-FU+PDL, 18.1%. Incorporating adjuvants particularly verapamil appeared to be significantly associated with a decreased risk of keloids.
Figure 4 SUCRA values, expressed as percentages, ranking the therapeutic effects and safety of treatments for keloids.
Abbreviations: SUCRA, surface under the cumulative ranking curve; TAC, triamcinolone acetonide; PDL, pulsed dye laser; 5-FU, 5-fluorouracil.
Publication bias
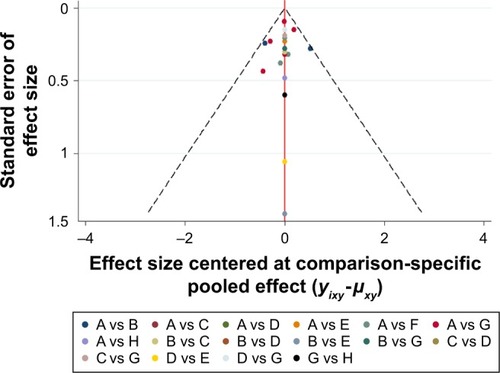
The result of the comparison-adjusted funnel plots did not reveal any evidence of apparent asymmetry (). No significant publication bias was observed.
Figure 5 Comparison-adjusted funnel plot for the network meta-analysis.
Abbreviations: TAC, triamcinolone acetonide; PDL, pulsed dye laser; 5-FU, 5-fluorouracil.
Discussion
Objective and reliable clinical evaluation methods and prevention and control measures for scars are still a hot issue. Most of the scar treatments have achieved good results in the past 20 years, but few have been supported by prospective surveys in the control group, and some of them even lack safety data. Many new therapies have shown early effects in small-sample trials, but these effects have not been confirmed with long-term follow-up and large samples. In recent years, the understanding of wound healing and scar formation has deepened. The accumulation of a great deal of clinical experience in scar treatment, and the research and application of new preparations and new treatment methods, especially the emerging technologies, have subverted some traditional treatment concepts and thus require the establishment of safe and effective standardized scar management protocols that can be used in routine clinical practice to guide clinical treatment.Citation22–Citation24 The mechanism of scar formation is not fully understood, but the relevant cognitive exploration in micro and macro aspects is ongoing. The whole process of scar formation involves not only micro cells (fibroblasts, myofibroblasts, mast cells, neutrophils, etc.) but also cytokines (transforming growth factor beta, tumor necrosis factor alpha, vascular endothelial growth factor, etc.), extracellular matrix components (collagen metabolism and arrangement of arrhythmia, the change in glycosaminoglycan interactions) and organization space structure (space regulation network of repair cells formed between the three dimensions).Citation25,Citation26 Surgical procedures are not the best choice for keloid management as the recurrence rate is high. As alternatives to the use of corticosteroids, chemotherapeutic agents, verapamil, silicone gel tablets and cryotherapy have become important, particularly in patients with high recurrence rates after surgery.Citation10 Khansa et al performed a meta-analysis and showed silicone gel, PDL, TAC and 5-FU had high efficacy in improving keloids.Citation27 However, which common therapy was the most effective in improving keloids was unknown.
This network meta-analysis attempted to evaluate the effectiveness of TAC compared with other common therapies used in keloid treatment. Our analysis suggests that verapamil is potentially more preferable than other adjuvants. Verapamil has been used as a coronary dilator since 1962. In the recent years, it has been used for the treatment of hypertension, angina, arrhythmia, cerebrovascular disease, finger vasospasm, abdominal pain, esophageal delaying, migraine and pulmonary hypertension and to prevent preterm birth. In 1997, Dong et al first reported the treatment of keloids with calcium channel blocker verapamil, which immediately attracted the attention of clinicians.Citation28 After 1997, attempts have been made to identify and treat keloid patients with verapamil. Verapamil has been shown to increase procollagenase synthesis in normal cultured fibroblasts. It also leads to the depolymerization of actin, cell conformation change and cell apoptosis, and ultimately leads to the reduction of fibrous tissue formation.Citation29 Boggio et al confirmed 50 µM verapamil was effective in wound healing, and it can also avoid the development of keloids and hypertrophic scars after plastic surgery.Citation30
As suggested by the SUCRA ranking scheme, TAC+5-FU was ranked behind verapamil. 5-FU is an anti-pyrimidine drug. It has to be enzymatically converted to 5-fluorodeoxyuridine nucleotides and exhibits antitumor activity.Citation31,Citation32 The action of this enzyme may also transfer one carbon unit of leucovorin to deoxyuridine nucleotide monophosphate for the synthesis of thymidine monoacid. At the same time, it also shows some inhibitory effect on the synthesis of RNA.Citation31 Shin et al found 5-FU was more effective than TAC in keloid treatment after surgical excision.Citation33
This meta-analysis also has some limitations. The test result of the corticosteroid therapy showed statistical heterogeneity was limited in randomized controlled trials, and limited evidence of a dose-dependent association between corticosteroids therapy and keloids, which provides limited confidence in the findings. Second, there is no record of keloid patients treated in a standardized manner, which leads to the difference between the trials; therefore, these results should be interpreted with caution. Third, study durations were short in these randomized controlled trials, and patients included in these trials may be different from real life. Fourth, these findings may not be generalizable to a specific group of patients because randomized controlled trials tend to exclude participants.
Our findings underscore the notion that any common therapy compared with TAC did not reduce keloid risk. Verapamil is potentially the most preferable adjuvant in keloid treatment. In the future, large-scale trials must be performed to validate the risk identified in the current meta-analysis.
Acknowledgments
This study was supported by the Sichuan Science and Technology Department Applied Basic Research Project (grant no. 2017JY0335) and (grant no. 2017JY0250).
Disclosure
The authors report no conflicts of interest in this work.
References
- YounaiSNichterLSWelliszTReinischJNimniMTuanTModulation of collagen synthesis by transforming growth factor-beta in keloid and hypertrophic scar fibroblastsAnn Plast Surg19943321481517979045
- RekhaAKeloids – a frustrating hurdle in wound healingInt Wound J20041214514816722888
- DiegelmannRFEvansMCWound healing: an overview of acute, fibrotic and delayed healingFront Biosci2004928328914766366
- OgawaRMechanobiology of scarringWound Repair Regen201119Suppl 1s2s921793962
- MajewskiSBoschFXDillnerJThe impact of a quadrivalent human papillomavirus (types 6, 11, 16, 18) virus-like particle vaccine in European women aged 16 to 24J Eur Acad Dermatol Venereol200923101147115519453788
- DateyKKPandyaVNCorticosteroidsMed Digest196129241254
- Fernandez-SerranoSDorcaJCorominesMCarratalàJGudiolFManresaFMolecular inflammatory responses measured in blood of patients with severe community-acquired pneumoniaClin Diagn Lab Immunol200310581382012965910
- Garcia-VidalCCalboEPascualVFerrerCQuintanaSGarauJEffects of systemic steroids in patients with severe community-acquired pneumoniaEur Respir J200730595195617690125
- AntunesGEvansSALordanJLFrewAJSystemic cytokine levels in community-acquired pneumonia and their association with disease severityEur Respir J200220499099512412694
- MustoeTACooterRDGoldMHInternational Advisory Panel on Scar ManagementInternational clinical recommendations on scar managementPlast Reconstr Surg2002110256057112142678
- WongTSLiJZChenSChanJYGaoWThe efficacy of triamcinolone acetonide in keloid treatment: a systematic review and meta-analysisFront Med2016371
- TanEChuaSLimJTopical silicone gel sheet versus intralesional injections of triamcinolone acetonide in the treatment of keloids – a patient-controlled comparative clinical trialJ Dermatol Treat1999104251254
- AsilianADaroughehAShariatiFNew combination of triamcinolone, 5-fluorouracil, and pulsed-dye laser for treatment of keloid and hypertrophic scarsDermatol Surg200632790791516875473
- ManuskiattiWFitzpatrickRETreatment response of keloidal and hypertrophic sternotomy scars: comparison among intralesional corticosteroid, 5-fluorouracil, and 585-nm flashlamp-pumped pulsed-dye laser treatmentsArch Dermatol200213891149115512224975
- SadeghiniaASadeghiniaSComparison of the efficacy of intralesional triamcinolone acetonide and 5-fluorouracil tattooing for the treatment of keloidsDermatol Surg201238110410922093096
- KhanMABashirMMKhanFAIntralesional triamcinolone alone and in combination with 5-fluorouracil for the treatment of keloid and hypertrophic scarsJ Pak Med Assoc20146491003100725823177
- MargaretSFXErnestKDhanrajPComparison of intralesional verapamil with intralesional triamcinolone in the treatment of hypertrophic scars and keloidsIndian J Dermatol Venereol Leprol200874434334818797054
- AhujaRBChatterjeePComparative efficacy of intralesional verapamil hydrochloride and triamcinolone acetonide in hypertrophic scars and keloidsBurns201440458358824182692
- DanielsenPLReaSMWoodFMVerapamil is less effective than triamcinolone for prevention of keloid scar recurrence after excision in a randomized controlled trialActa Derm Venereol201696677477826911400
- DaroughehAAsilianAShariatiFIntralesional triamcinolone alone or in combination with 5-fluorouracil for the treatment of keloid and hypertrophic scarsClin Exp Dermatol200934221922319018794
- HatamipourEMehrabiSHatamipourMGhafarian ShiraziHREffects of combined intralesional 5-fluorouracil and topical silicone in prevention of keloids: a double blind randomized clinical trial studyActa Med Iran201149312713021681697
- HuangCMurphyGFAkaishiSOgawaRKeloids and hypertrophic scars: update and future directionsPlast Reconstr Surg Glob Open201314e2525289219
- OgawaRThe most current algorithms for the treatment and prevention of hypertrophic scars and keloidsPlast Reconstr Surg2010125255756820124841
- KlingerMMarazziMVigoDTorreMFat injection for cases of severe burn outcomes: a new perspective of scar remodeling and reductionAesthetic Plast Surg200832346545918305985
- WangXSmithPPuLLKimYJKoFRobsonMCExogenous transforming growth factor beta(2) modulates collagen I and collagen III synthesis in proliferative scar xenografts in nude ratsJ Surg Res199987219420010600349
- OrienteAFedarkoNSPacochaSEHuangSKLichtensteinLMEssayanDMInterleukin-13 modulates collagen homeostasis in human skin and keloid fibroblastsJ Pharmacol Exp Ther2000292398899410688614
- KhansaIHarrisonBJanisJEEvidence-based scar management: how to improve results with technique and technologyPlast Reconstr Surg20161383 Suppl165S178S27556757
- DongHEarleMLJiangYLoutzenhiserKATriggleCRCardiovascular effects of CPU-23, a novel L-type calcium channel blocker with a unique molecular structureBr J Pharmacol19971227127112789421272
- BermanBMaderalARaphaelBKeloids and hypertrophic scars: pathophysiology, classification, and treatmentDermatol Surg201743Suppl 1S3S1827347634
- BoggioRFBoggioLFGalvaoBLMachado-SantelliGMTopical verapamil as a scar modulatorAesthetic Plast Surg201438596897525189298
- de WaardJWde ManBMWobbesTvan der LindenCJHendriksTInhibition of fibroblast collagen synthesis and proliferation by levamisole and 5-fluorouracilEur J Cancer19983411621679624252
- Al-AttarAMessSThomassenJMKauffmanCLDavisonSPKeloid pathogenesis and treatmentPlast Reconstr Surg2006117128630016404281
- ShinJYKimJSCould 5-fluorouracil or triamcinolone be an effective treatment option for keloid after surgical excision? A meta-analysisJ Oral Maxillofac Surg20167451055106026529198