Abstract
Extracorporeal membrane oxygenation (ECMO) is considered a recognized lifesaving support for patients with cardiorespiratory failure. Acute kidney injury (AKI) and fluid overload are significant morbidity factors resulting in serious complications. The inline hemofilter system (IHS) and the continuous renal replacement therapy (CRRT) machine are different methods of renal replacement therapy for patients with ECMO. IHS is the alternate, safe dialysis modality of choice because it is user-friendly, inexpensive, and efficiently removes fluid overload and renal diffusive clearance. We report on a 20-day-old male neonate with multiple congenital cardiac defects who needed venoarterial ECMO and had AKI necessitating renal replacement therapy using IHS. The patient had stable electrolyte parameters, good ultrafiltration, and efficient diffusive clearance. He was decannulated from ECMO therapy after 9 days without any related complications. Therefore, neonatal IHS is a safe and efficient alternative approach to AKI.
Introduction
Extracorporeal membrane oxygenation (ECMO) is considered a recognized lifesaving support for patients with cardiorespiratory failure. The expected survival rate is ∼80% in infants compared to the survival rate of 20% in neonates.Citation1–Citation3 Acute kidney injury (AKI) and fluid overload are significant morbidity factors resulting in serious complications.Citation4–Citation7 Studies on pediatric and neonatal ECMO have used predominantly continuous renal replacement therapy (CRRT) using the machine, but none of the studies have compared the inline hemofilter system (IHS) in relation to fluid, renal diffusive clearance, technical difficulties, episodes of clotting, cost factors, CRRT circuits, management of alarms, and CRRT nursing issues.Citation8–Citation12 Moreover, the aforementioned issues have a significant impact on patient outcomes. However, IHS offers a relatively easier method to efficiently dialyze the patient without any related complications. Here, we describe a neonatal patient with significant improvement in fluid overload and renal diffusive clearance in relation to neonatal IHS.
Case presentation
A 20-day-old male neonate was referred from a private hospital with a case of hypoplastic aortic arch syndrome. He was born full term via normal vaginal delivery to a primiparous mother with a birth weight of 3.4 kg and a normal Apgar score. Antenatal examinations including an abdominal ultrasound were completely unremarkable. He had no neonatal intensive care admissions, and on routine clinical examination, a cardiac murmur was detected. A cardiac echocardiogram confirmed the diagnosis of the hypoplastic aortic arch syndrome. He was started on prostaglandin E1 infusion and referred to King Abdulaziz Medical City Hospital at 15 days of age for further management. On physical examination, the patient had mild central cyanosis, soft, systolic ejection murmur at the left sternal border and high-pitched systolic murmur at the lower left sternal border. His vital signs were completely normal. Initial laboratory tests revealed that blood urea nitrogen (BUN) was 7.84 mg/dL (normal range: 7–20 mg/dL) and serum creatinine was 0.452 mg/dL (0.2–0.7 mg/dL). Electrolytes and blood gas analyses were within normal limits. A complete blood count showed a hemoglobin level of 14.1 g/dL (normal range: 10–12 g/dL), normal leukocytes count, and a platelet count of 742 × 109/L (normal range: 150–400 × 109/L). There was no serological evidence of hepatitis B and C or viral infections. Blood and urine cultures were negative. Abdominal ultrasound and the chromosomal analysis including fluorescence in situ hybridization were normal. An electrocardiogram revealed the absence of Q waves in the inferior and lateral leads. An initial chest X-ray revealed mild cardiomegaly with the prominent bilateral pulmonary vasculature. Repeated cardiac echocardiogram showed a hypoplastic aortic arch, a small left ventricular outflow tract, an aortic annulus, large muscular ventricular septal defects with inlet extension, a restrictive patent foramen ovale, and a large patent ductus arteriosus.
The patient underwent surgical correction on day 20 of his life. Intraoperatively, a median sternotomy with aortic and bicaval cannulation was performed using 14 and 10 French catheters, respectively. A cardiopulmonary bypass with systemic cooling was instituted. The patent ductus arteriosus was divided, the ventricular septal defect was closed using a Gore-Tex patch, and an aortic arch reconstruction was performed using a pulmonary homograft patch. An atrial septectomy and a tricuspid valve commissuroplasty were performed. The Damus–Kaye–Stansel procedure was constructed in a side-to-side fashion and connected to the reconstructed aorta under circulatory arrest with antegrade cerebral perfusion. A 4 mm size Blalock–Thomas–Taussig shunt was constructed between the base of the innominate artery and the central pulmonary artery. A transesophageal echocardiogram was performed, which did not show any significant residual lesions. However, oxygen saturation declined, and the patient was placed on venoarterial ECMO (Quadrox-i pediatric; Maquet Getinge Group, Rastatt, Germany) to control the hemodynamics, allow the heart to rest, and the pulmonary vascular resistance to drop. The patient was transferred to the pediatric cardiac intensive care unit with an ECMO and ventilator support. The targeted ECMO blood flow was 70–100 mL/kg/minute with the initial flow started at ∼0.5 L/minute. The heparin dose was adjusted to maintain an activated clotting time between 180 and 220 seconds. Details of the ECMO and ventilation parameters during the hospital stay are illustrated in . In addition, the patient had severe hypotension and bleeding necessitating multiple courses of fresh frozen plasma, platelets, blood transfusions, and inotropes. Postoperatively, he was noted to be anuric and clinically had significant fluid overload and poor diffusive clearance. His chest X-ray showed significant fluid overload (). Renal replacement therapy was initiated using an inline hemofilter (Maquet hemofilter) directly into the ECMO circuit (). The priming volume of the hemofilter is 18 mL with a surface area of 0.25 m2 and designed using polypropylene fiber material. The hemofilter is incorporated after the pump and before the membrane oxygenator. After delivery through the hemofilter, the blood returned to the pre-pump limb of the circuit. Efficient ultrafiltration was balanced using a standard intravenous infusion pump connected to the effluent port of the hemofilter. The ultrafiltration was equal to the rate programmed into the infusion device. On follow-up, the patient had stable electrolyte parameters, balanced fluid removal, and efficient diffusive clearance (). He was decannulated from ECMO therapy after 9 days without any complications related to the IHS. Repeated chest X-rays showed significant improvement in relation to fluid overload ().
Figure 1 Pictures of chest radiographs during ECMO therapy.
Abbreviation: ECMO, extracorporeal membrane oxygenation.
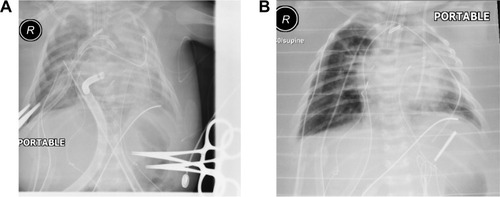
Figure 2 ECMO circuit with an inline hemofilter system.
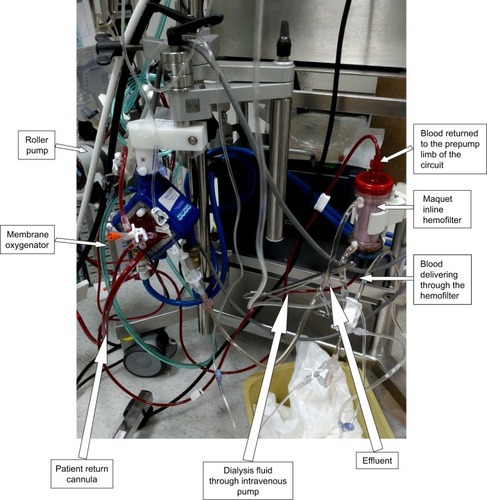
Table 1 Fluid, ECMO, and ventilation parameters
Table 2 Lab monitoring on pre- and postoperative days
Consent
Written informed consent for the publication of this case report and any accompanying images has been obtained from the patient’s father.
Discussion
We describe a neonatal ECMO patient with significant improvement in fluid overload and renal diffusive clearance in relation to IHS without any significant complications. Moreover, we performed neonatal venoarterial ECMO using IHS for 9 days and successfully decannulated the patient without any complications regarding clotting, technical aspects, and nursing issues. With regard to the technical aspects, combining ECMO with renal replacement therapy can be initiated in many ways. One method is by introducing the CRRT machine or a hemofilter into the ECMO circuit otherwise known as IHS.Citation13–Citation15 Renal replacement therapy can be conducted in a simple process in IHS, where blood for the CRRT circuit is accessed from and returned to the ECMO circuit. Moreover, the inlet and outlet of the CRRT circuit can be after or before the centrifugal pump and the membrane oxygenator depending on the medical center’s experience. Another common dialysis technique is using the CRRT machine, where blood is accessed from and returned to the ECMO circuit. To avoid such complications, blood flow should be constant and strictly maintained in the CRRT machine.Citation13,Citation14 In addition, incorporation of a CRRT venous line into the ECMO circuit especially before the centrifuge pump may potentially trigger low pressure venous alarms in the CRRT machine and may shut down the whole ECMO circuit. Low blood flow or clots in the filter can trigger the alarms in the CRRT machine and may eventually lead to hemolysis, disseminated intravascular coagulation, or activation of the clotting cascade.Citation14–Citation17
However, it is postulated that CRRT using the machine is the safest mode in relation to the clotting and rupture of membranes.Citation1,Citation2,Citation14 However, the priming volume of the extracorporeal circuit (ECV) for the neonate is only 18 mL in IHS, which eventually significantly assists the hemodynamic parameters. In contrast, CRRT with the machine using an ECV priming volume of 60 mL may result in detrimental consequences. It may also cause significant hemodynamic changes in the ECMO parameters especially in neonates who require multiple inotropes.
Moreover, using a CRRT machine in the ECMO circuit requires frequent alarm monitoring, circuit changes, and carries an additional burden for the CRRT nurses and significant costs for the parents. In contrast, IHS may provide an easy and inexpensive choice of modality and significantly reduces the cost burden for the parents. IHS can potentially remove not only the fluid but also the solute clearance as clearly demonstrated in our case. Diffusive clearance and fluid overload are well managed using countercurrent dialysis fluid through an intravenous infusion pump connected to the effluent port of the filter.Citation1,Citation14
Convective clearance can also be achieved by using replacement fluid directly through the ECMO circuit.Citation13,Citation14 Newer fiber characteristics of the ECMO hemofilter are considerably effective for removing fluids and toxins as demonstrated in our case. Although the maximum ultrafiltration rate is limited in both the intravenous infusion pumps and filter, in neonates, it is highly uncommon to take fluids >1 L/hour. Moreover, the frequency of clotting depends more on the blood flow and heparin dosage rather than the type of modality of dialysis. To our knowledge, no studies have correlated CRRT with IHS and with the machine, especially in relation to clotting, fluid overload, and clearance.
AKI in our patient was likely related to decreased perfusion to the kidneys secondary to poor oxygen saturation during surgery, which in turn may have resulted in acute tubular necrosis. Despite the good fluid challenges and boluses, our patient had significant oliguria and poor solute clearance and recovered only after a week. The pathophysiological process of AKI during ECMO therapy is not well understood; however, hormonal factors are significantly reduced in patients maintained with ECMO therapy, which can eventually lead to failure in renal homeostasis. In addition, inflammation induced by disease involving heart and lungs may have pronounced effects on the kidneys and can progress to AKI.Citation1,Citation4 Patients requiring CRRT especially from AKI have a minimal survival rate of 17%. In addition, CRRT is an additional burden and risk factor for ECMO therapy.Citation1,Citation2
Conclusion
For neonatal patients who have hemodynamic compromise and AKI, using renal replacement therapy with IHS may be more beneficial rather than using the machine; however, randomized studies should be conducted to compare the efficiency of different dialysis modalities particularly in relation to hemodynamics, cost burdens, clotting frequencies, and morbidity rates.
Acknowledgments
The authors would like to acknowledge our perfusionist Bader for the support and input in relation to the ECMO therapy.
Disclosure
The authors report no conflicts of interest in this work.
References
- AskenaziDJSelewskiDTPadenMLRenal replacement therapy in critically ill patients receiving extracorporeal membrane oxygenationClin J Am Soc Nephrol2012781328133622498496
- ChenHYuRGYinNNZhouJXCombination of extracorporeal membrane oxygenation and continuous renal replacement therapy in critically ill patients: a systematic reviewCrit Care201418667525482187
- WilliamsDCTuriJLHornikCPCircuit oxygenator contributes to extracorporeal membrane oxygenation-induced hemolysisASAIO J201561219019525419829
- SmithAHHardisonDCWordenCRFlemingGMTaylorMBAcute renal failure during extracorporeal support in the pediatric cardiac patientASAIO J200955441241619381083
- LinCYChenYCTsaiFCRIFLE classification is predictive of short-term prognosis in critically ill patients with acute renal failure supported by extracorporeal membrane oxygenationNephrol Dial Transplant200621102867287316799171
- YanXJiaSMengXAcute kidney injury in adult postcardiotomy patients with extracorporeal membrane oxygenation: evaluation of the RIFLE classification and the Acute Kidney Injury Network criteriaEur J Cardiothorac Surg201037233433819692267
- AskenaziDJAmbalavananNHamiltonKAcute kidney injury and renal replacement therapy independently predict mortality in neonatal and pediatric noncardiac patients on extracorporeal membrane oxygenationPediatr Crit Care Med2011121e1e620351617
- KecklerSJLaituriCAOstlieDJPeterSDStA review of venovenous and venoarterial extracorporeal membrane oxygenation in neonates and childrenEur J Pediatr Surg20102011419746333
- FordJWNeonatal ECMO: current controversies and trendsNeonatal Netw200625422923816913234
- KimESStolarCJECMO in the newbornAm J Perinatol200017734535612141521
- YeoASChongJHTanTHNgASRajaduraiVSChanYHNeonatal and paediatric extracorporeal membrane oxygenation (ECMO) in a single Asian tertiary centreAnn Acad Med Singapore201443735536125142471
- CavagnaroFKattanJGodoyLContinuous renal replacement therapy in neonates and young infants during extracorporeal membrane oxygenationInt J Artif Organs200730322022617417761
- SantiagoMJSanchezALopez-HerceJThe use of continuous renal replacement therapy in series with extracorporeal membrane oxygenationKidney Int200976121289129219794394
- SeczynskaBKrolikowskiWNowakIJankowskiMSzułdrzynskiKSzczeklikWContinuous renal replacement therapy during extracorporeal membrane oxygenation in patients treated in medical intensive care unit: technical considerationsTher Apher Dial201418652353425195931
- RicciZMorelliSVitaleVDi ChiaraLCruzDPicardoSManagement of fluid balance in continuous renal replacement therapy: technical evaluation in the pediatric settingInt J Artif Organs2007301089690117992650
- UrlesbergerBZobelGZenzWActivation of the clotting system during extracorporeal membrane oxygenation in term newborn infantsJ Pediatr199612922642688765625
- BetrusCRemenappRCharpieJEnhanced hemolysis in pediatric patients requiring extracorporeal membrane oxygenation and continuous renal replacement therapyAnn Thorac Cardiovasc Surg200713637838318292719
