Abstract
Methicillin-resistant Staphylococcus aureus (MRSA) and Candidal prosthetic joint infections (PJIs) are very rare, and the optimal management for these patients is still unknown. A 54-year-old man with traumatic arthritis due to previous electric injury successfully retained the implant despite the successive infection with MRSA and Candida albicans after total knee arthroplasty (TKA). Continuous lavage with vancomycin was used to control MRSA infection and repeated local washout plus oral swallow with voriconazole tablet were administered to eradicate C. albicans. Additional three reported cases were identified by the criteria of selecting patients with concomitant and/or successive MRSA and Candidal PJIs. Different methods were applied with variable outcomes. Therefore, several risk factors such as intra-articular corticosteroid injection, high frequency of door openings in the operating room, excessive blood loss and allogeneic red blood cell transfusions should be avoided. Debridement, antibiotics and implant retention (DAIR) can be an alternative in dedicated patients to control acute MRSA and Candidal PJIs. Particularly, repeated intra-articular washout with susceptible drugs and a prolonged duration of oral antibiotics was essential for microbial control.
Introduction
Methicillin-resistant Staphylococcus aureus (MRSA) and Candidal prosthetic joint infections (PJIs) have presented a serious concern for surgeons and a disaster for patients.Citation1–Citation6 There is no guideline of the optimal treatment for these patients. Several treatment methods to control bacterial and fungal infections have been reported before, including antibiotics; debridement, antibiotics and implant retention (DAIR); resection arthroplasty (RA); one-stage revision (OSR); and two-stage revision (TSR); but the outcomes vary. DAIR has many advantages over RA and delay reimplantation in acute PJI: fewer operations, less expense, conservation of bone stock and better function.Citation7 Successful treatment of MRSA and Candidal PJI has been reported before,Citation2,Citation7–Citation15 but definitive information regarding therapy of the specific group of patients is limited. In this case report, we describe a single case and summarize previously reported cases to clarify the characteristics and explore successful treatment experience in patients with MRSA and Candidal PJI.
Patients and methods
Case report
On June 12, 2017, a 54-year-old man was diagnosed with extensive post-traumatic arthritis with apparent pain and disability in his left knee, which caused a limping gait and seriously affected his daily life. The patient experienced a serious electrical injury in March 2016 and received 4 operations including skin grafting and other multiple surgeries for left patellar dislocation. Neither history of autoimmune diseases, such as systemic lupus erythematosus or rheumatoid arthritis, nor hypertension, diabetes mellitus, or congestive heart failure was there. On examination, the patient was observed to have an 8-cm2 area of epidermal scar over the right knee and a 15-cm2 area of myocutaneous flap () over the medial aspect of the left knee just proximal to the joint line. There was a slight left knee joint effusion and patella baja, but no erythema or elevated skin temperature around the left knee joint. The left knee range of motion (ROM) was 0°–10°. Routine preoperative laboratory tests revealed that white blood cell (WBC) count, C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) were all within normal limits. Radiographs demonstrated severe degenerative arthritis of the left knee with patella baja and bony fusion of bilateral femoral condyle ().
Figure 1 (A) The wound of the patient was in a good condition at the third postoperative day; (B) a sterile needle aspiration was performed, with removal of 40 mL of dark bloody fluid at the ninth postoperative day; (C) the yellowish fluid with tofu-like tissue was observed around sinus tract in the knee joint.
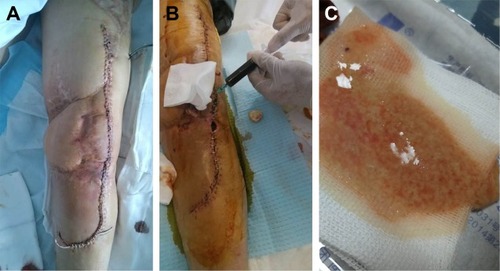
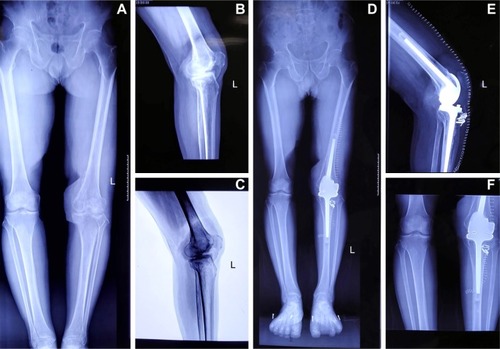
Figure 2 (A) The full length plain of lower extremity; (B) the lateral plain of the left knee; (C) the negative plain X-ray of the left knee. (A–C) Severe degenerative arthritis of the left knee with a low patella and bony fusion of femoral condyle. At 5 months of follow-up the radiographs show, (D) the full length plain of lower extremity; (E) the lateral plain of the left knee; (F) the positive film of the left knee. All the film of left knee revealed the prosthesis was well-fixed without loosening.
The patient underwent total knee arthroplasty (TKA) with a cemented, hinged implant (AK medical business; LINK® Endo-Model® Hinge Knee Prosthesis, Barkhausenweg/Hamburg, Germany) on June 24, 2017. Tibial tuberosity osteotomy (TTO) was performed to obtain proper surgical exposure, avoid damage to the extensor muscle and increase the mobility of the knee joint. Intravenous injection of 1 g Shiomarin (Latamoxef, Haikou/Hainan, China) was used as prophylactic antibiotic before the surgical incision. The operation has lasted for 4 h. The blood loss was 300 mL and fluid resuscitation was 2,000 mL. Three units of packed red blood cells and 200 mL of fresh frozen plasma were transfused intraoperatively. The patient had a benign postoperative course in the first few postoperative days with a knee ROM in 0°–90°. Latamoxef 1 g was intravenously administered twice a day until the 8th postoperative day when he complained about pain, moderate swelling without fever, chills, nausea and vomiting. Poor wound healing, persistently profuse drainage and wound dehiscence were observed in the surgical site 2 days later (the 10th postoperative day). A sterile needle aspiration was performed, with removal of 40 mL dark bloody fluid (). The pathogen of the fluid specimens was MRSA. Drug sensitivity test revealed the minimum inhibitory concentration (MIC) of Vancocin (vancomycin hydrochloride) for the organism was 1 μg/mL. At this time, The WBC count was 8.9 × 109/L (normal range, [3.5–9.5] × 109/L), CRP was 100.5 mg/L (normal range, [0–5] mg/L) and ESR was 44 mm/h (normal range, [0–15] mm/h). A diagnosis of acute PJI was made, and 0.5 g vancomycin hydrochloride was intravenously administered every 12 h. The patient was scheduled for urgent debridement and irrigation. Plain film radiographs did not reveal any evidence of loosening, lucency or osteolysis around the prosthetic components.
On July 15, 2017 the patient underwent surgery comprising: (1) complete debridement including the removal of all necrotic and fibrous tissue and proliferative inflammatory synovialis; (2) 3 samples of the suspected infected tissue were sent for microbiological evaluation; (3) polyethylene liner was exchanged; (4) washout with 100 mL of 3% hydrogen peroxide, soak in 300–400 mL 10% polyvinylpyrrolidone-iodine (PVP-I, Chengdu/Sichuan, China) for 15 min, exhaustive irrigation was carried out with 10 L saline mixed with gentamicin by using pulsed lavage and (5) indwelling the inlet and outlet tubes for further continuous lavage with saline and vancomycin hydrochloride (100 μg/mL). Intraoperative findings verified that all components were extremely well-fixed without loosening. Vancomycin hydrochloride was administered intravenously every 12 h combined with continuous lavage. The indwelling drains were removed on the 19th day (August 3, 2017), when the incision wound was dry, and CRP and ESR returned to normal levels, after the debridement and irrigation surgery. There was no tenderness or swelling around the joint, and the infection was regarded as resolved. The patient was ready to be discharged with oral compound Sulfamethoxazole (sinomine; Minhang/Shanghai, China), but he complained about the evaluated skin temperature, pain and increasing swelling in the left knee joint again 2 days later (August 5, 2017). A sinus tract communicating with knee joint was seen, around which was the yellowish fluid with tofu-like tissue (). It did not appear to be synovial in nature so it was collected for pathogen and drug sensitivity test, which was subsequently identified as Candida albicans. The result was initially regarded as a contamination but repeated punctures confirmed the infection of C. albicans. The MIC of fluconazole for C. albicans was 1 μg/mL. At that point, the WBC count was 2.8 × 109/L, the CRP was 4.3 mg/L and the ESR was 35 mm/h. The patient refused surgical treatment, therefore vancomycin hydrochloride was stopped and he was treated with fluconazole (Datong/Sanxi, China) intravenously (100 mg/d) and daily aspirations, local washout with 100 mL fluconazole (1 mg/mL), which was 1,000 times higher than MIC and finally intra-articular injection of 10 mL fluconazole (1 mg/mL). Eighteen days later (August 24, 2017), the susceptibility of fluconazole changed from “S (susceptible)” to “I (intermediate)”. Thereafter we replaced fluconazole with voriconazole (Chengdu/Sichuan, China) based on the susceptibility test. The MIC of voriconazole for C. albicans was 0.06 μg/mL. Antibiotic treatment regimens were as follows: oral voriconazole (200 mg/d) was administered; local washout with voriconazole (2 mg/mL) every other day for the first 3 weeks and then adjusted to every 5 days for the next 3 weeks.
On October 20, 2017, arthrocentesis was performed to examine the effect of the treatment method so far. The results of fungal stains, gram stains and bacterial cultures were all negative. The patient had no systematic discomfort and no local erythema, no local heat, no resting pain, no discharge sinus in the infected joint. The patient was discharged with oral voriconazole (200 mg/d) for 6 months. After 5 months of follow-up, the film of the left knee revealed the prosthesis was well-fixed () and laboratory findings were normal. Currently, the patient is on a continued 6-month oral antifungal treatment.
Literature review
Using the key words “fungal”, “candida”, “Methicillin-resistant Staphylococcus aureus”, “MRSA”, “prosthetic joint infection” and “arthroplasty”, we searched CINAHL, MEDLINE (National Library of Medicine, Bethesda, MD) and Web of Science, Cochrane systematic review databases for cases with PJI associated to MRSA and Candida. A secondary search of references cited in the articles related to our topics was performed. Four cases diagnosed as concomitant and/or successive MRSA and Candidal PJI, whose data on demographic characteristics, treatment, outcome and follow-up were identified in the English literature. summarizes demographic characteristics, treatment, outcome of patients with MRSA and Candida PJI. The patients had an average age of 59.75 years (range: 31–88 years), and they had an average body mass index (BMI) of 26.96 kg/m2. The involved joint was the hip in 3 (75%) and the knee in 1 (25%). A sinus tract was present in 2 patients (patients 1 and 4). Three patients had one or more underlying systemic diseases, including COPD in 2 patients, chronic renal insufficiency in 1 patient, diabetes mellitus in 2 patients and renal cell carcinoma in 1 patient. The case in our hospital did not have systemic illness, but had a complicated history of multiple electric injury wounds to the extremity with the infected joint. Two patients with a previous history of bacterial infection prior to C. albicans were identified, one was MRSA and Serratia marcescens infection (patient 1) and the other was MRSA infection (patient 4). Concomitant infection with MRSA and Candida was found in 2 patients, 1 was C. parapsilosis and MRSA infection and the other was C. albicans, MRSA infection. The delay between index surgery and diagnosis varied from 8 days to 4 months and 26 months; it was unavailable in 1 patient. Four patients developed the infection after an average of 2.75 surgeries (range: 1–4). Patient 1 received intravenous injection with antibiotics including flucytosine combined with amphotericin B, but without oral antibiotics. Patients 2 and 3 underwent fluconazole combined with vancomycin hydrochloride intravenously each and oral fluconazole. Our patient received vancomycin hydrochloride intravenously (19 days) for MRSA and fluconazole intravenously (18 days) combined with oral fluconazole and switched to voriconazole later for C. albicans. Intra-articular injection was used in our patient only. Surgeries were applied including OSR (patient 1), RA (patient 2), TSR (patient 3) and DAIR (patient 4). The final outcome was favorable in 2 (50%) patients with no relapse (patients 1 and 4). But one was positive with RA (patient 2) and another died after TSR (patient 3).
Table 1 Demographic characteristics, treatment, outcome of patients with MRSA and Candida PJI
Discussion
This is the first case report of MRSA and Candidal PJIs treated with DAIR. PJI is a serious complication of arthroplasty associated with significant mortality.Citation16–Citation18 The rarity of MRSA and Candida infections limits our understanding of the management and outcome in these patients.Citation19
The present case had several risk factors including high frequency of door openings in the operating theater,Citation20 fluid resuscitation over 2,000 mL,Citation21 3 units of allogeneic red blood cells and 200 mL fresh frozen plasma transfusion during the operation. The Pulido et al study showed patients receiving allogenic transfusions were regarded as an independent risk factor for PJI which was 2.1 times more likely to develop PJI compared with no transfusion.Citation22
In addition, the complexity of arthroplasty, such as the use of hinged implants in our case, may also contribute to high complication rate.Citation23 Furthermore, the infection rate using a rotating hinge design was enormous with 11% in primary and 9% in revision cases.Citation24,Citation25
Also in our review, these four patients had several identifiable risk factors for PJI, either systemic diseases or local comorbidities, such as poorly controlled diabetes mellitus, male gender, chronic renal disease, diagnosis of post-traumatic arthritis, prior surgical procedure in the affected joint, which was consistent with potential risk factors for development of PJI described in The International Consensus on Periprosthetic Joint Infection.Citation48
With incidence of MRSA PJI and Candidal PJI being on an upward trend, the management of these patients is a major challenge to most orthopedic surgeons.Citation26 No consensus currently exists on the optimal antibiotic therapy and surgical intervention for PJI due to MRSA and Candida species. Although reimplantation protocol resulted in a viable option for patients with infections by common organisms, it might be accompanied by a high recurrence rate, especially in patients with antibiotic resistant organisms.
Our case is unique since the patient had successful eradication of infection by using open debridement, continuous lavage for MRSA infection and intra-articular washout for C. albicans. During the debridement operation, washout with hydrogen peroxide, soaking with 10% PVP-I for 15 min and pulsed lavage was used.
In vitro, PVP-I has been proved to be effective against a wide range of pathogens, including Gram-positive and Gram-negative bacteria, bacillus, protozoa and viruses.Citation27,Citation28 Giacometti et alCitation29 demonstrated that PVP-I at the concentration of 1,000 mg/L for 30 min or 10,000 mg/L for 15 min, respectively, was able to inhibit the growth of methicillin sensitive S. aureus (MSSA), MRSA and Pseudomonas aeruginosa.
In addition, continuous lavage was used after complete debridement in our patient. Mont et al study shows that in patients with acute PJI, multiple debridement and retention of the components can result in low morbidity with high success rates.Citation30 However, Tsumura et al demonstrated that multiple operations would damage the skin and create anxieties in patients but continuous irrigation is an effective method to cure early-stage infection of TKA.Citation31 This method might be especially useful in controlling infection and decreasing the number of surgical interventions. It also remains uncertain about when to remove the drainage tube, how to confirm the absence of organism and how to prevent superinfection.
More importantly, intra-articular washout combined with oral antifungal agents was performed in our patient to control C. albicans. Salvati et al also reported that the proper antibiotic concentration in joint fluid could not be maintained by antibiotic-impregnated cement.Citation32–Citation34 However, it had been proved that intra-articular delivery of antibiotics produced peak concentrations that many orders of magnitude higher than intravenous administration and trough concentrations which have remained at the therapeutic dose for 24 h. Similar methods of intra-articular antibiotic delivery have been reported effectively in previous literature to treat acute and chronic-infected TKA.Citation35–Citation38 Revision combined with intra-articular vancomycin hydrochloride administration to control infection in MRSA-infected TKA is also shown to be safe and effective.Citation39,Citation40 OSR combined with intra-articular delivery of fluconzole was reported to successfully treat fungal PJI.Citation16 However, to our knowledge, this case is the first to adopt this intra-articular delivery method of voriconazole without the removal of the implant. The agents must be adjusted according to the results of susceptibility testing because fungal biofilms are more complex than other pathogens.Citation41 In our treatment experience, voriconazole is more effective than fluconazole when applied in the treatment of C. albicans infections. The reason may be that voriconazole is a more active regimen to suppress the recovery of viable colony-forming units (CFUs) from the model at 24 h. In addition, voriconazole-exposed catheters exhibited a significantly lower burden of organisms, revealed by sonicated catheter cultures at 48 h, versus the fluconazole for isolated C. albicans.Citation42
Lastly, the authors consider whether a prolonged time of local susceptible antibiotics delivery in acute PJI might be the major issue in treatment and induce a better outcome than radical revision surgery, which has also been shown effective in some of the earlier in vitro and clinical reports, but it needs further study to prove the hypothesis.Citation43,Citation44 Therefore, risk factors like intra-articular corticosteroid injection, high frequency of door openings in the operating room,Citation45 excessive blood loss and allogeneic red blood cell transfusion should be avoided, and our method (DAIR) could be an alternative to tackle the challenge of MRSA and Candidal PJI. Particularly, repeated intra-articular washout with susceptible drugs and a prolonged duration of oral antibiotics was essential for microbial control.
Consent
The patient provided written informed consent specifically for the publication of the case details and any accompanying images.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81472136).
Disclosure
The authors report no conflicts of interest in this work.
References
- SousaRJBarreiraPMLeitePTSantosACRamosMHOliveiraAFPreoperative Staphylococcus aureus screening/decolonization protocol before total joint arthroplasty – results of a small prospective randomized trialJ Arthroplasty201631123423926362785
- SalgadoCDDashSCanteyJRMarculescuCEHigher risk of failure of methicillin-resistant Staphylococcus aureus prosthetic joint infectionsClin Orthop Relat Res2007461485317534195
- SennevilleEJoulieDLegoutLOutcome and predictors of treatment failure in total hip/knee prosthetic joint infections due to Staphylococcus aureusClin Infect Dis201153433434021810745
- KuiperJWWillinkRTMoojenDJvan den BekeromMPColenSTreatment of acute periprosthetic infections with prosthesis retention: Review of current conceptsWorld J Orthop20145566767625405096
- JakobsOSchoofBKlatteTOFungal periprosthetic joint infection in total knee arthroplasty: a systematic reviewOrthop Rev (Pavia)201571562325874061
- ShaikhAAHaC-WParkY-GParkY-BTwo-stage approach to primary TKA in infected arthritic knees using intraoperatively molded articulating cement spacersClin Orthop Relat Res201447272201220724599649
- BradburyTFehringTKTauntonMThe fate of acute methicillin-resistant Staphylococcus aureus periprosthetic knee infections treated by open debridement and retention of componentsJ Arthroplasty2009246 Suppl101104
- CushingRDFulgenziWRSynovial fluid levels of fluconazole in a patient with Candida parapsilosis prosthetic joint infection who had an excellent clinical responseJ Arthroplasty19971289509458262
- SimonianPTBrauseBDWickiewiczTLCandida infection after total knee arthroplasty. Management without resection or amphotericin BJ Arthroplasty19971278258299355014
- BrooksDHPupparoFSuccessful salvage of a primary total knee arthroplasty infected with Candida parapsilosisJ Arthroplasty19981367077129741450
- WadaMBabaHImuraSProsthetic knee Candida parapsilosis infectionJ Arthroplasty19981344794829645532
- SiddiquiMMLoNNAb RahmanSChinPLChiaSLYeoSJTwo-year outcome of early deep MRSA infections after primary total knee arthroplasty: a joint registry reviewJ Arthroplasty2013281444822682043
- TriantafyllopoulosGKSoranoglouVMemtsoudisSGPoultsidesLAImplant retention after acute and hematogenous periprosthetic hip and knee infections: Whom, when and how?World J Orthop20167954655227672567
- AboltinsCAPageMABuisingKLTreatment of staphylococcal prosthetic joint infections with debridement, prosthesis retention and oral rifampicin and fusidic acidClin Microbiol Infect200713658659117331125
- ChiuFYChenCMSurgical debridement and parenteral antibiotics in infected revision total knee arthroplastyClin Orthop Relat Res200746113013517438469
- JiBZhangXXuBGuoWMuWCaoLSingle-stage revision for chronic fungal periprosthetic joint infection: An average of 5 years of follow-upJ Arthroplasty20173282523253028478188
- ZawadzkiNWangYShaoHReadmission due to infection following total hip and total knee procedures: A retrospective studyMedicine (Baltimore)20179638e796128930833
- KuiperJWvan den BekeromMPvan der StappenJNoltePAColenS2-stage revision recommended for treatment of fungal hip and knee prosthetic joint infectionsActa Orthop201384651752324171675
- MatsumotoTIshidaKTsumuraNTreatment of 50 deep infections after total knee arthroplastyOrthopedics2015386e529e53526091228
- PanahiPStrohMCasperDSParviziJAustinMSOperating room traffic is a major concern during total joint arthroplastyClin Orthop Relat Res2012470102690269422302655
- EverhartJSAltneuECalhounJHMedical comorbidities are independent preoperative risk factors for surgical infection after total joint arthroplastyClin Orthop Relat Res2013471103112311923519927
- PulidoLGhanemEJoshiAPurtillJJParviziJPeriprosthetic joint infection: the incidence, timing, and predisposing factorsClin Orthop Relat Res200846671710171518421542
- GuenounBLatargezLFreslonMDefossezGSalasNGayetLEComplications following rotating hinge Endo-Modell (Link) knee arthroplastyOrthop Traumatol Surg Res200995752953619837642
- SteckelHKlingerHMBaumsMHSchultzWLong-term results of the Blauth knee prosthesis – current status of hinged knee prosthesesZ Orthop Ihre Grenzgeb20051431303515754229
- PetrouGPetrouHTilkeridisCMedium-term results with a primary cemented rotating-hinge total knee replacement. A 7- to 15-year follow-upJ Bone Joint Surg Br200486681381715330020
- GouldIMReillyJBunyanDWalkerACosts of healthcare-associated methicillin-resistant Staphylococcus aureus and its controlClin Microbiol Infect201016121721172820825434
- MessagerSGoddardPADettmarPWMaillardJ-YComparison of two in vivo and two ex vivo tests to assess the antibacterial activity of several antisepticsJ Hosp Infect200458211512115474182
- WutzlerPSauerbreiAKlöckingRBrögmannBReimerKVirucidal activity and cytotoxicity of the liposomal formulation of povidone-iodineAntiviral Res2002542899712062394
- GiacomettiACirioniOGregantiGAntiseptic compounds still active against bacterial strains isolated from surgical wound infections despite increasing antibiotic resistanceEur J Clin Microbiol Infect Dis200221755355612172750
- MontMAWaldmanBBanerjeeCPachecoIHHungerfordDSMultiple irrigation, debridement, and retention of components in infected total knee arthroplastyJ Arthroplasty19971244264339195319
- TsumuraHIkedaSOnoTItonagaITairaHTorisuTSynovectomy, debridement, and continuous irrigation for infected total knee arthroplastyInt Orthop200529211311615685455
- SalvatiEACallaghanJJBrauseBDKleinRFSmallRDReimplantation in infection. Elution of gentamicin from cement and beadsClin Orthop Relat Res198620783933720107
- Anguita-AlonsoPRouseMSPiperKEJacofskyDJOsmonDRPatelRComparative study of antimicrobial release kinetics from polymethylmethacrylateClin Orthop Relat Res200644523924416474225
- HaleemAABerryDJHanssenADMid-term to long-term followup of two-stage reimplantation for infected total knee arthroplastyClin Orthop Relat Res20044283539
- WhitesideLANayfehTALaZearRRoyMEReinfected revised TKA resolves with an aggressive protocol and antibiotic infusionClin Orthop Relat Res2012470123624321948323
- DavenportKTrainaSPerryCTreatment of acutely infected arthroplasty with local antibioticsJ Arthroplasty1991621791831875210
- PerryCRHulseyREMannFAMillerGAPearsonRLTreatment of acutely infected arthroplasties with incision, drainage, and local antibiotics delivered via an implantable pumpClin Orthop Relat Res19922812162231499215
- FukagawaSMatsudaSMiuraHOkazakiKTashiroYIwamotoYHigh-dose antibiotic infusion for infected knee prosthesis without implant removalJ Orthop Sci201015447047620721714
- WhitesideLAPeppersMNayfehTARoyMEMethicillin-resistant Staphylococcus aureus in TKA treated with revision and direct intra-articular antibiotic infusionClin Orthop Relat Res20114691263320390472
- WhitesideLARoyMENayfehTAIntra-articular infusion: a direct approach to treatment of infected total knee arthroplastyBone Joint J201698-B1 Suppl A313626733638
- ChandraJKuhnDMMukherjeePKHoyerLLMcCormickTGhannoumMABiofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistanceJ Bacteriol2001183185385539411514524
- LewisREKontoyiannisDPDarouicheRORaadIIPrinceRAAntifungal activity of amphotericin B, fluconazole, and voriconazole in an in vitro model of Candida catheter-related bloodstream infectionAntimicrob Agents Chemother200246113499350512384356
- DusaneDHDiamondSMKnechtCSEffects of loading concentration, blood and synovial fluid on antibiotic release and anti-biofilm activity of bone cement beadsJ Control Release2017248243228087408
- HowlinRPBrayfordMJWebbJSCooperJJAikenSSStoodleyPAntibiotic-loaded synthetic calcium sulfate beads for prevention of bacterial colonization and biofilm formation in periprosthetic infectionsAntimicrob Agents Chemother201559111112025313221
- SadrizadehSPantelicJShermanMClarkJAboualiOAirborne particle dispersion to an operating room environment during sliding and hinged door openingJ Infect Public Health2018
- KlatteTOKendoffDKamathAFSingle-stage revision for fungal peri-prosthetic joint infection: A single-centre experienceBone Joint J201496-B449249624692616
- UengSWLeeCYHuCCHsiehPHChangYWhat is the success of treatment of hip and knee candidal periprosthetic joint infection?Clin Orthop Relat Res201347193002300923633184
- ZmistowskiBDella ValleCBauerTWDiagnosis of periprosthetic joint infectionJ Orthop Res201432S98S10724464903
