Abstract
Background
Epicardial adipose tissue (EAT) is considered an important source of bioactive molecules that can influence coronary arteries directly and is related to the concurrent presence of both obstructive coronary stenosis and myocardial ischemia independently. Non-alcoholic fatty liver disease (NAFLD) has become an emergent health problem worldwide.
Aim
This cross-sectional study aimed to address the relationship between the volume of EAT and NAFLD and other cardiovascular risk factors in the general population.
Materials and methods
In this study, we selected a total of 2,238 participants aged at least 40 years from the Jidong community in Tangshan, China. The 64-slice CT was used to survey the volume of EAT and liver ultrasonography was used for the diagnosis of NAFLD. The study cohorts were compared according to EAT volume.
Results
Cardiovascular risk factors, such as coronary artery calcium score, carotid intima-media thickness, NAFLD, and ideal cardiovascular health metrics were also found to be related to EAT. In multivariate logistic regression analysis, NAFLD groups showed significant association with higher EAT volume, after correcting for main cardiovascular disease risk factors (OR [95% CI], 1.407 [1.117, 1.773]).
Conclusion
Our findings in a general community population provide evidence that EAT is strongly associated with NAFLD and other cardiovascular risk factors.
Introduction
Epicardial adipose tissue (EAT) and intra-abdominal fat are both derived from brown adipose tissue during embryonic stage.Citation1 As a special form of visceral adipose tissue deposited around the heart, EAT can be found in considerable quantities around sub-epicardial coronary arteries.Citation2 EAT forms a layer between the pericardium and the myocardium, surrounding the heart with no fascial layer separating this adipose tissue from the coronary artery wall and the surface of myocardium.Citation3 Epicardial fat and myocardium share a same unobstructed microcirculation, suggesting potential interaction among these tissues. Under physiological conditions, epicardial fat has metabolic, thermogenic (as brown fat), and mechanical (cardio-protective) characteristics.Citation4 Studies in cadavers showed that coronary atherosclerotic plaque tends to be more noticeable on the arterial side in contact with EAT deposits; the dissected epicardial fat weight is positively correlated to that of the myocardium.Citation5,Citation6 Moreover, EAT is considered a rich source of bioactive molecules that can influence coronary arteries directly,Citation7 and is related to the concurrent presence of both obstructive coronary stenosis and myocardial ischemia.Citation8
Non-alcoholic fatty liver disease (NAFLD) has become a worldwide health concern. Approximately 20%–30% of the general population are affected by NAFLD.Citation9 NAFLD patients are at a higher risk of developing cardiovascular changes earlier in life.Citation10 A previous study suggest that NAFLD is an independent cardiovascular disease (CVD) risk factor.Citation11 Several studies have also indicated that CVD is the main cause of death in NAFLD patients.Citation12,Citation13 However, the association of NAFLD with CVD may be indirect and due to generalized obesity or ectopic fat, especially EAT. Whether NAFLD could affect visceral adipose tissue outside of the liver has not been studied extensively.
In this study, we aimed to test the relationship between the volume of EAT and NAFLD and cardiovascular risk factors using the diagnosis results of 2,238 people from a northern Chinese community. We confirmed that EAT contributes to the pathogenesis of CVD and is associated with cardiovascular risk factors.
Materials and methods
Ethics statement
This study was performed in accordance with the ethical guidelines of China’s regulations and guidelines on good clinical practice and the declaration of Helsinki; the Ethics Committee of the Jidong Oilfield Hospital approved it. All participants provided written informed consent before the start of the study. “Jidong study” is hosted by Capital Medical University. Beijing Recdata Technology Co., Ltd. is responsible for the field management and operation of the project, which is implemented by Jidong Oilfield Hospital. The aim of this project is to establish the first long-term follow-up sub-health cohort in China to observe and explore the occurrence and development of systemic disease in a community population. Beijing Recdata Technology Co., Ltd is responsible for the collection and analysis of clinical data, and the preservation of biological samples. Our research has obtained permission from Capital Medical University, Beijing Recdata Technology Co., Ltd., and Jidong Oilfield Hospital.
Study population
Participants were recruited from the Jidong community in Tangshan, Hebei, China which mainly consisted of employees of Jidong Co. Ltd. Participants who were willing to provide informed consent were included in the study. From July 2013 to August 2014, 9,078 residents aged 20 years or older were invited to participate in this study at the time of their annual physical examination. Among 9,078 participants, 2,928 subjects who were aged 40 years and above, with complete diagnostic history of coronary CT, carotid artery ultrasound, medical history, and abdominal ultrasound were selected. Two hundred and fifty-eight individuals with a history of CVD (including heart failure, myocardial infarction, atrial fibrillation or any other heart disease), cancer, and stroke; 333 subjects with excessive alcohol consumption (10 g/day for women and ≥20 g/day for men for more than a year); and 99 with positive HbsAg were excluded. A total of 2,238 participants were finally included in the analysis ().
A reliable questionnaire expressly designed for this study was used to collect clinical data from all participants. Demographic information (eg, age, gender, household income, level of education, marital status, smoking status, physical activity, and history of diseases) of all participants was collected. A patient was considered diagnosed with hyperlipidemia based on a history of cholesterol, cholesterol lowering medication, a total cholesterol (TC) level >220 mg/dL, triglycerides >150 mg/dL, or low-density lipoprotein (LDL) >160 mg/dL. Those with a self-reported history, current treatment with insulin, oral hypoglycemic agents or fasting blood glucose (FBG) level >126 mg/dL were categorized as having diabetes mellitus (DM).Citation24
Hypertension diagnosis was given in the presence of at least one of the following: 1) SBP >140 mmHg or DBP >90 mmHg, 2) history of antihypertensive medication, or 3) history of arterial hypertension. Individuals were classified as being normal weight (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2) or obese (≥30 kg/m2).Citation14
Automated analyzers (AU400; Olympus Corporation, Tokyo, Japan) were used to analyze samples in the central laboratory of Jidong Oilfield Hospital. Many biological indicators were included such as TC, FBG, serum triglycerides (TG), serum high-density lipoprotein (HDL) cholesterol, LDL cholesterol, AST, Cr, and ALT.
CT imaging and analysis of EAT volume, coronary artery calcium (CAC) score
CAC score was defined as at least four contiguous pixels with a CT density of >130 HU (the Agatston method was used). Total Agatston CAC score was calculated as the sum of all coronary arteries, which was separately calculated according to the Agatston method.Citation25
EAT volume was assessed using the same method. EAT was defined as all adipose tissue enclosed by the visceral pericardium, including all fat directly surrounding the coronary arteries. Contiguous dimensional voxels between the HU limits of −195 to −45 were defined as fat voxels; these limits can be modified by the user if deemed appropriate.Citation26
Ultrasonography examinations
Liver ultrasonography was used to diagnose fatty liver, by a high-resolution B-mode topographic ultrasound system with a 3.5 MHz probe (ACUSON X300; Siemens, Munich, Germany). According to Chinese Association for the Study of Liver Disease and the Asia-Pacific Working Party on NAFLD, diagnosis was based on at least two of three abnormal findings: 1) diffusely increased liver near field ultrasound echo; 2) vascular blurring and the gradual attenuation of far field ultrasound echo; 3) liver echo greater than kidney.Citation27,Citation28
A sonographer performed carotid intima-media thickness (CIMT) measurements using the same B-mode topographic ultrasound system with a 3.5 MHz probe (ACUSON X300). The operator measured CIMT on the far wall of the right and left common carotid arteries, 1.5 cm proximal to the bifurcation. The greater value of the right and left common CIMT was used for analysis, CIMT ≥0.8 mm was defined as abnormal.Citation29,Citation30
Cardiovascular health metrics assessment
According to the American Heart Association guidelines, we defined the seven cardiovascular health metrics in two levels: “poor” =0 score and “ideal” =1 score.Citation31 The dietary intake metric was classified as ideal (meet the recommended), or poor (did not meet the recommended). The smoking metric was classified as poor (current smoking), or ideal (never- or quit-smoking for >12 months). Physical activity was classified as poor (0–149 min/week of moderate intensity or 0–74 min/week of vigorous intensity), or ideal (≥150 min/week of moderate intensity or ≥75 min/week of vigorous intensity). BMI was classified as poor (≥25 kg/m2), or ideal (<25 kg/m2). Blood pressure was classified as poor (SBP of ≥120 mmHg or DBP of ≥80 mmHg), or ideal (SBP of <120 mmHg, DBP of <80 mmHg, and untreated). FBG level was classified as poor (100–125 mg/dL and treated to goal), or ideal (<100 mg/dL and untreated). TC status was classified as poor (≥200 mg/dL or treated to goal), or ideal (<200 mg/dL and untreated).
Data analysis
Statistical analysis was performed with SAS software (version 9.4; SAS Institute Inc., Cary, NC, USA). Continuous variables were presented as mean ± SD and compared using ANOVA or Student’s t-test. Categorical variables were described as percentages and compared using chi-squared test; ordinal variables were compared using nonparametric test. Multiple logistic regression was used to test the association between presence of NAFLD and EAT volume. All statistical tests were 2-sided with significance level α=0.05.
Results
showed the flow chart of the enrolled participants who met the requirements. From July 2013 to August 2014, 9,078 Jidong residents aged 20 years or older were invited to participate in this study at the time of their annual physical examination at the Jidong Oilfield Hospital. Finally, a total of 2,238 participants were included in the analysis.
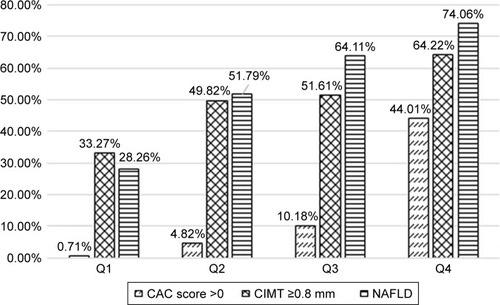
showed that percentage of NAFLD, CIMT ≥0.8 mm and CAC score >0 all increased with increasing EAT volume. The prevalence of NAFLD in four categories was 28.26%, 51.79%, 64.11%, and 74.06%. The prevalence of CIMT ≥0.8 mm was 33.27%, 49.82%, 51.61%, and 64.22% in four categories, respectively. The prevalence of CAC score >0 in four categories was 0.71%, 4.82%, 10.18%, and 44.01%.
Figure 2 Prevalence of non-alcoholic fatty liver disease, CIMT ≥0.8 mm, and CAC score >0, by epicardial adipose tissue quartiles.
showed the baseline characteristics of the study participants, grouped according to quartiles of EAT volume. The mean age of the study participants was 55.6±7.8 years (mean ± SD); 43.88% (982 patients) was within this range. The median EAT volume (interquartile) was 127.46 cm3 (93.87 cm3, 172.72 cm3). The prevalence of NAFLD was 28.26%, 51.79%, 64.11%, and 74.06% in the four quartiles, respectively (P<0.001). Compared to those with lower volume of EAT, subjects with higher volume of EAT tended to be older, have a larger waist and hip circumference, higher weight, blood pressure, LDL level, FBG level, BMI, triglycerides, and lower HDL level. Furthermore, the percentage of DM, hypertension, and hyperlipidemia increased with increasing EAT volume (P<0.001).
Table 1 Baseline characteristics of the entire cohort stratified by quartiles of epicardial adipose tissue
showed cardiovascular risk factors of the entire cohort stratified by quartiles of epicardial adipose tissue. Compared to those with lower volume of EAT, subjects with higher volume of EAT tended to have lower ideal cardiovascular health metrics, higher CIMT, and higher CAC scores compared to those with lower prevalence of NAFLD (P<0.001).
Table 2 Cardiovascular risk factors of the entire cohort stratified by quartiles of epicardial adipose tissue
showed that the OR (95% CI) of EAT volume affected the CIMT and CAC score. For all participants, statistically significant correlations/associations were observed, in the crude model as well as three other models adjusted for confounding factors such as traditional risk factors and sociological features (model 1: for CIMT >0.8 mm, 1.033, [1.023, 1.044], P<0.0001; for CAC score >0, 1.230, [1.204, 1.258], P<0.0001; model 2 adjusted for gender and age: for CIMT >0.8 mm, 1.006, [0.998, 1.015], P=0.1371; for CAC score >0, 1.213, [1.186, 1.242], P<0.0001; model 3: adjusted for level of education and income, smoking status, diabetes, and hypertension on the basis of model 2: for CIMT >0.8 mm, 1.004, [0.996, 1.013], P=0.3083; for CAC score >0, 1.214, [1.186, 1.243], P<0.0001; model 4: adjusted for BMI, on the basis of model 3: for CIMT >0.8 mm, 1.001, [0.993, 1.009], P=0.8114; for CAC score >0, 1.26, [1.225, 1.295], P<0.001).
Table 3 The OR (95% CI) of EAT affected CIMT and CAC score
showed the multivariate logistic regression analysis of the OR [95% CI] of groups with NAFLD for higher EAT volume groups (volume ≥127.46 cm3). For all participants, statistically significant correlations/associations were observed in the crude model as well as three other models adjusted for confounding factors (model 1: 3.346, [2.811, 3.983], P<0.0001; model 2 adjusted for gender and age: 3.073, [2.547, 3.708], P<0.0001; model 3: adjusted for level of education, smoking status and income, diabetes and hypertension on the basis of model 2: 2.787, [2.787, 2.296], P<0.0001; model 4: adjusted for hip circumference, abdominal circumference, TG, LDL, HDL, ALT, BMI, physical activity on the basis of model 3: 1.407, [1.117, 1.773], P<0.0001).
Table 4 Multivariate logistic regression analysis of risk for higher EAT volume elevation between groups with or without nonalcoholic fatty liver disease
Discussion
This population-based, cross-sectional study’s main finding was that EAT is independently correlated with NAFLD and CAC score in a community population. Increased EAT volume was strongly correlated with increased NAFLD percentage and many other cardiovascular risk factors, such as CAC score and CIMT and cardiovascular health, in this study. Subjects with higher volume of EAT tended to be older, have a larger waist and hip circumference, higher weight, blood pressure, LDL level, BMI, FBG level, triglycerides, and lower HDL level as well as higher CIMT, lower ideal cardiovascular health metrics, and higher CAC score compared to those with lower prevalence of NAFLD. BMI is associated with both EAT and NAFLD, subjects with higher volume of EAT and NAFLD tended to have a higher BMI. The higher volume of EAT groups had a higher rate of being overweight or obese, and higher rate of NAFLD. showed that the OR (95% CI) of EAT affected CIMT and CAC score, in model 4 (adjusted for BMI on the basis of model 3: for CIMT >0.8 mm, 1.001, [0.993, 1.009], P=0.8114; for CAC score >0, 1.26, [1.225, 1.295], P<0.001), thus distribution of fat (such as EAT) may be more appropriate than BMI.
Furthermore, percentage of DM, hypertension, and hyperlipidemia increased with increasing EAT volume. It is also confirmed that participants with NAFLD have a higher risk of the aggravation of EAT volume. To the best of our knowledge, this study is the first to show the relation between NAFLD and the volume of EAT in a community population. We thus hypothesized that NAFLD contributes to the pathogenesis of CVD via the aggravation of EAT. We addressed the potential connection between the volume of EAT and NAFLD in a community population.
Our findings are consistent with previous studies. In the Heinz Nixdorf Recall Study, with 4,093 participants, Mahabadi et al found a significant association of cardiovascular risk factors with EAT volume in univariable and multivariable analyses.Citation7 In a study involving 100 gender-controlled patients who were 50 years old with biopsy-proven NAFLD, patients with NAFLD tended to have increased arterial stiffness and EAT thickness.Citation14 The interaction between heart function and liver fat has been proven by studies reporting an association between epicardial fat thickness and NAFLD.Citation15,Citation16 Perseghin et al reported that men with newly diagnosed fatty liver despite normal left ventricular (LV) morphological features and systolic and diastolic functions had increased epicardial fat and abnormal LV energy metabolism.Citation17 In East Asia, a study with 1,473 participants discovered a significant association between increased EAT thickness and coronary artery calcification in Korean adults.Citation18
The inflammatory state of NAFLD might affect the homeostasis of various organs as demonstrated for systemic atherosclerosis. Some clinical studies showed that ectopic fat depots have an important role in the pathogenesis of NAFLD.Citation19,Citation20 EAT accumulation is part of the metabolic disorders re-elevated with the occurrence of fatty liver. High EAT may epitomize the severity of metabolic disorders, as emphasized by its association with liver fibrosis and nonalcoholic steatohepatitis, and may also be involved in the development of target organ damage, especially blood vessels, the heart, and the liver.Citation21
Some experimental studies have shown the possible inflammatory mediators. An animal study pointed out that the rate of fatty acid liberated by EAT is about twice that of the perirenal depots and pericardial.Citation32 In humans, a protein secreted by adipose tissue is associated with increased cardiovascular risk.Citation22 Abnormal right ventricle geometry and increased LV mass are associated with increased volume of epicardial fat.Citation23 In epicardial fat tissue of patients with CVD, a dense inflammatory infiltrate (mainly comprising macrophages) is commonly present. The EAT transcriptome includes B-cell-associated factors, T-cell and macrophage markers, TGF-β2, and multiple chemokine ligands and chemokine receptors.Citation1 The epicardial inflammatory molecules’ secretion increases the arterial wall thickness, and is likely to promote atherogenesis. The atherogenic effects of epicardial fat tissue are caused by its intense proinflammatory activity, and also by its anatomical vicinity to the plaque.Citation4
Conclusion
The strengths of this study are its sample size and its population-based design. However, its cross-sectional design limits the strength of the confidence of the correlation. CT is better than competing imaging modalities, which offers great spatial resolution of 0.4–0.6 mm. The present study was unable to isolate NAFLD’s direct effect on the development of CVD or cardiovascular events, thus further research is required. The relationship between the volume of EAT and inflammatory station of EAT, and the plasma biomarkers should be researched and we will focus on this in future.
In conclusion, our findings in a general community population provided evidence that the layer of EAT is strongly linked to NAFLD and cardiovascular risk factors. The findings may also imply the possibility of NAFLD contributing to the pathogenesis of CVD via the aggravation of EAT.
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
This work was supported by grants from the Medical and Health Science and Technology Innovation Project of Chinese Academy of Medical Sciences (no 201612M1009).
Disclosure
The authors report no conflicts of interest in this work.
References
- MazurekTZhangLZalewskiAHuman epicardial adipose tissue is a source of inflammatory mediatorsCirculation2003108202460246614581396
- ChatterjeeTKStollLLDenningGMProinflammatory phenotype of perivascular adipocytes: influence of high-fat feedingCirc Res2009104454154919122178
- HirataYTabataMKurobeHCoronary atherosclerosis is associated with macrophage polarization in epicardial adipose tissueJ Am Coll Cardiol201158324825521737014
- IacobellisGLocal and systemic effects of the multifaceted epicardial adipose tissue depotNat Rev Endocrinol201511636337125850659
- PratiFArbustiniELabellarteAEccentric atherosclerotic plaques with positive remodelling have a pericardial distribution: a permissive role of epicardial fat? A three-dimensional intravascular ultrasound study of left anterior descending artery lesionsEur Heart J200324432933612581680
- YerramasuADeyDVenurajuSIncreased volume of epicardial fat is an independent risk factor for accelerated progression of sub-clinical coronary atherosclerosisAtherosclerosis2012220122323022015177
- MahabadiAABergMHLehmannNAssociation of epicardial fat with cardiovascular risk factors and incident myocardial infarction in the general population: the Heinz Nixdorf Recall StudyJ Am Coll Cardiol201361131388139523433560
- NakazatoRDeyDChengVYEpicardial fat volume and concurrent presence of both myocardial ischemia and obstructive coronary artery diseaseAtherosclerosis2012221242242622305262
- PettaSMuratoreCCraxìANon-alcoholic fatty liver disease pathogenesis: the present and the futureDig Liver Dis200941961562519223251
- WuRHouFWangXNonalcoholic Fatty Liver Disease and Coronary Artery Calcification in a Northern Chinese Population: a Cross Sectional StudySci Rep201771993328855585
- BhatiaLSCurzenNPByrneCDNonalcoholic fatty liver disease and vascular riskCurr Opin Cardiol201227442042822596186
- LazoMHernaezRBonekampSNon-alcoholic fatty liver disease and mortality among US adults: prospective cohort studyBMJ2011343d689122102439
- SöderbergCStålPAsklingJDecreased survival of subjects with elevated liver function tests during a 28-year follow-upHepatology201051259560220014114
- PettaSArganoCColombaDEpicardial fat, cardiac geometry and cardiac function in patients with non-alcoholic fatty liver disease: association with the severity of liver diseaseJ Hepatol201562492893325445395
- IacobellisGBarbariniGLetiziaCBarbaroGEpicardial fat thickness and nonalcoholic fatty liver disease in obese subjectsObesity201422233233624115757
- ColakYKarabayCYTuncerIRelation of epicardial adipose tissue and carotid intima-media thickness in patients with nonalcoholic fatty liver diseaseEur J Gastroenterol Hepatol201224661361822495402
- PerseghinGLattuadaGde CobelliFIncreased mediastinal fat and impaired left ventricular energy metabolism in young men with newly found fatty liverHepatology2008471515817955548
- KimBJCheongESKangJGKimBSKangJHRelationship of epicardial fat thickness and nonalcoholic fatty liver disease to coronary artery calcification: From the CAESAR studyJ Clin Lipidol2016103e611e626
- van der PoortenDMilnerKLHuiJVisceral fat: a key mediator of steatohepatitis in metabolic liver diseaseHepatology200848244945718627003
- CheungOKapoorAPuriPThe impact of fat distribution on the severity of nonalcoholic fatty liver disease and metabolic syndromeHepatology20074641091110017610277
- FracanzaniALPisanoGConsonniDEpicardial Adipose Tissue (EAT) Thickness Is Associated with Cardiovascular and Liver Damage in Nonalcoholic Fatty Liver DiseasePLoS One2016119e016247327627804
- PischonTGirmanCJHotamisligilGSRifaiNHuFBRimmEBPlasma adiponectin levels and risk of myocardial infarction in menJAMA2004291141730173715082700
- IacobellisGRelation of epicardial fat thickness to right ventricular cavity size in obese subjectsAm J Cardiol2009104111601160219932799
- SongQLiuXWangAAssociations between non-traditional lipid measures and risk for type 2 diabetes mellitus in a Chinese community population: a cross-sectional studyLipids Health Dis2016157027044245
- AgatstonASJanowitzWRHildnerFJZusmerNRViamonteMDetranoRQuantification of coronary artery calcium using ultrafast computed tomographyJ Am Coll Cardiol19901548278322407762
- GorterPMvan LindertASde VosAMQuantification of epicardial and peri-coronary fat using cardiac computed tomography; reproducibility and relation with obesity and metabolic syndrome in patients suspected of coronary artery diseaseAtherosclerosis2008197289690317884060
- FanJGJiaJDLiYMGuidelines for the diagnosis and management of nonalcoholic fatty liver disease: update 2010: (published in Chinese on Chinese Journal of Hepatology 2010;18:163–166)J Dig Dis2011121384421276207
- FarrellGCChitturiSLauGKSollanoJDAsia-Pacific Working Party on NAFLD. Guidelines for the assessment and management of non-alcoholic fatty liver disease in the Asia-Pacific region: executive summaryJ Gastroenterol Hepatol200722677577717565629
- HuangYBiYXuMNonalcoholic fatty liver disease is associated with atherosclerosis in middle-aged and elderly ChineseArterioscler Thromb Vasc Biol20123292321232622814750
- LuijendijkPLuHHeynnemanFBIncreased carotid intima-media thickness predicts cardiovascular events in aortic coarctationInt J Cardiol2014176377678125205481
- Lloyd-JonesDMHongYLabartheDDefining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyondCirculation2010121458661320089546
- MarchingtonJMMattacksCAPondCMAdipose tissue in the mammalian heart and pericardium: structure, foetal development and biochemical propertiesComp Biochem Physiol B19899422252322591189