Abstract
Purpose
We aimed to evaluate the association of serum uric acid on admission with long-term outcome of critically ill patients.
Materials and methods
We conducted a retrospective cohort study using data extracted from the Medical Information Mart for Intensive Care III database. The primary endpoint was 90-day mortality. Propensity score matching (PSM) was performed, and multivariate Cox regression analysis was used to adjust for potential confounders. Receiver operating characteristic (ROC) curves were also used to assess the mortality predictions.
Results
A total of 2,123 patients were included finally with a PSM cohort consisting of 556 90-day non-survivors matched 1:1 with 556 90-day survivors. No statistically significant difference of median admission uric acid was observed between the two groups (survivors 5.50 mg/dL vs non-survivors 5.60 mg/dL, p=0.536). ROC area under the curve was 0.511 (95% confidence interval [CI] 0.477–0.545), suggesting that uric acid had poor discriminative powers for predicting 90-day mortality. No significant association between uric acid and 90-day mortality was found (hazard ratio 1.00, 95% CI 0.98–1.03, p=0.6835).
Conclusion
Serum uric acid on intensive care unit admission failed to predict 90-day mortality of critically ill patients.
Introduction
Uric acid, the end product of an exogenous pool of purines, which functions as either an antioxidant or a pro-oxidant, has been reported as a predictor of outcomes in multiple diseases.Citation1–Citation4 Related research studies focused mainly on cardiovascular disease and found that uric acid might serve as a biomarker of severity of coronary artery disease in patients with acute coronary syndrome, cardiovascular mortality, 1-year mortality of patients with acute coronary syndromes treated with percutaneous coronary intervention, and might improve the prognostic accuracy of some clinical models.Citation5–Citation8 The prognostic and predictive value of uric acid was also explored in type 2 diabetic patients and patients who had open heart surgery.Citation9,Citation10 However, the value of initial serum uric acid on admission in critically ill patients seems limited. Akbar et al reported that elevated uric acid levels in patients with sepsis are associated with an increased risk of acute kidney injury and acute respiratory distress syndrome, but Zhu et al found that there was no correlation between the initial levels of serum uric acid and prognosis of infection in critically ill patients.Citation11,Citation12 Meanwhile, it has been reported that no relationship was found between serum uric acid and short-term mortality of critically ill patients.Citation13,Citation14 To the best of our knowledge, there is no research to evaluate the association of serum uric acid on intensive care unit (ICU) admission with long-term outcome of critically ill patients. Thus, we performed a retrospective cohort study using a modifiable data mining technique applied to the publicly available Medical Information Mart for Intensive Care III (MIMIC-III) database, aiming to clarify whether there is an association between admission serum uric acid levels and long-term outcome.Citation15
Materials and methods
Study design and data sources
We conducted a retrospective cohort study using data extracted from the MIMIC-III database, which is a large publicly available database consisting of de-identified health-related data of patients who had stayed in the ICU of Beth Israel Deaconess Medical Center between 2001 and 2012. Access to database has been approved by the institutional review boards of both Beth Israel Deaconess Medical Center and Massachusetts Institute of Technology Affiliates. No informed consent was required on the de-identified patients.
Participants
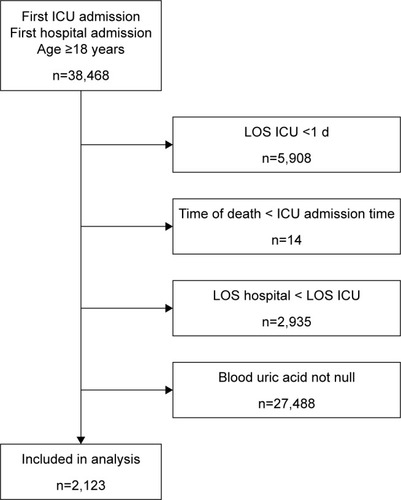
Adult patients (aged ≥18 years) of first hospital admission and first ICU admission were considered and included, but patients staying at ICU for <1 day and patients without admission serum uric acid records were excluded. In addition, patients whose death was earlier than ICU admission time and patients whose length of hospital stay was less than length of ICU stays were excluded in order to exclude potential typographical errors and records of organ donor account ().
Variables
We applied Structured Query Language to extract data from the database mainly by using codes from the MIMIC Code Repository.Citation16,Citation24 Age, sex, ICU mortality and hospital mortality, length of ICU stay and length of hospital stay, 28-day mortality and 90-day mortality, admission serum uric acid (admission was defined as within 24 hours after ICU admission), Simplified Acute Physiology Score II (SAPS II), the Elixhauser comorbidities, and the Elixhauser Comorbidity Index (State Inpatient Database [SID]30) were extracted or calculated.Citation17–Citation19 Missing components for the calculation of SAPS II were treated as normal (usually 0). Because the database has had date of birth of patients who are older than 89 years shifted to exactly 300 years before to obscure their age, we corrected them (age −300+89) before analysis.
Outcome measures
Ninety-day mortality after ICU admission was chosen as the primary end point, and 28-day mortality, hospital mortality, and ICU mortality were secondary outcomes. ICU mortality was determined only by the first ICU stay.
Propensity score matching (PSM)
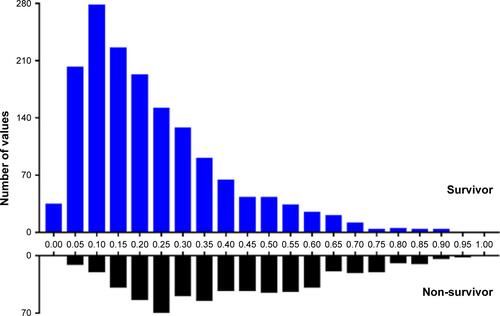
We grouped the study subjects as survivors and non-survivors according to their 90-day survival status after ICU admission. The propensity score for each patient was calculated to estimate their probability of death during the first 90 days after ICU admission by using multivariable logistic regression models given the following covariates: gender, age, SAPS II, Elixhauser Comorbidity Index (SID30), sepsis (based on International Classification of Diseases, Ninth Revision [ICD-9] codes), mechanical ventilation on the first day, renal replacement therapy on the first day, congestive heart failure, cardiac arrhythmias, valvular disease, pulmonary circulation disorder, peripheral vascular disorder, hypertension, paralysis, other neurological disease, chronic pulmonary disease, uncomplicated diabetes, complicated diabetes, hypothyroidism, renal failure, liver disease, peptic ulcer, acquired immune deficiency syndrome, lymphoma, metastatic cancer, solid tumor, rheumatoid arthritis, coagulopathy, obesity, weight loss, fluid and electrolyte disorders, blood loss anemia, deficiency anemia, alcohol abuse, drug abuse, psychoses, and depression. Matching was performed with the use of a 1:1 matching protocol without replacement (greedy-matching algorithm), with a caliper width equal to 0.05 of the standard deviation of the logit of the propensity score. The overlap of the distribution of the propensity scores across survivors and non-survivors groups is shown in Figure S1.
Statistical analysis
For continuous variables, data were expressed as median and interquartile range (IQR) unless otherwise stated. For categorical variables, data were shown as numbers and percentages. Comparison of continuous and categorical variables was performed using Kruskal-Wallis and chi-square (or Fisher’s exact) tests, respectively. We used receiver operating characteristic (ROC) curves to evaluate the prognostic predictive value of serum uric acid for 90-day mortality and other outcomes and used the Kaplan–Meier (K-M) method and log-rank tests to compare survival differences among patients of different admission serum uric acid levels. Variables associated with 90-day mortality were evaluated by univariate Cox regression analysis, and those with a p-value <0.1 were considered in multivariable Cox regression model. Considering the expected collinearity between comorbidities and the Elixhauser Comorbidity Index (SID30), we would choose only either one of them to be enrolled into one adjusted model when variables are potentially significant (p<0.1) in univariate analysis. Age was not included in the multivariable regression analysis since it was factored into SAPS II. Multivariable Cox regression model was performed to evaluate the association of serum uric acid on 90-day mortality and 28-day mortality, and multivariable logistic regression model was used to examine the association between hospital mortality and ICU mortality. p-values of <0.05 were considered to indicate statistical significance. Empower(R) (www.empowerstats.com; X&Y solutions, Inc., Boston, MA, USA) and R software, version 3.4.3 (http://www.r-project.org; R Foundation for Statistical Computing, Vienna, Austria) were used for statistical analyses.
Results
Patient characteristics
A total of 2,123 patients were included (). As shown in , the median age of the study patients was 64.09 years (IQR 51.39–75.74 years) and 1,219 of the 2,123 cases (57.42%) were male. The median admission serum uric acid was 5.40 mg/dL (IQR 3.80–7.90 mg/dL) with a median SAPS II score of 39 (IQR 30–49). Among them, 239 (11.26%) patients were diagnosed with sepsis based on ICD-9 codes and 1,032 (48.61%) patients required mechanical ventilation on admission. The five most common comorbidities were fluid and electrolyte disorders (38.48%), congestive heart failure (20.35%), deficiency anemia (20.16%), cardiac arrhythmias (20.07%), and coagulopathy (19.31%). The 90-day mortality was 27.23% with 578 non-survivors and 1,545 survivors. The length of ICU stay and hospital stay was 3.79 (IQR 2.01–9.19) and 14.69 (IQR 8.05–26.37) days, respectively. Non-survivors had significantly higher SAPS II (p<0.001). No statistically significant difference was observed in serum uric acid between survivors and non-survivors.
Table 1 Characteristics and comparison between survivors and non-survivors of all patients
Characteristics of the PSM cohort
A total of 556 non-survivors were successfully matched with one control. Characteristics of PSM cohort are shown in . There was no statistically significant difference between survivors and non-survivors in age, gender, SAPS II on admission, Elixhauser Comorbidity Index (SID30), and comorbidities (p>0.05), and no statistically significant difference was found on serum uric acid between survivors and non-survivors.
Table 2 Characteristics and comparison between survivors and non-survivors of PSM cohort
Survival status of patients with different serum uric acid levels on admission
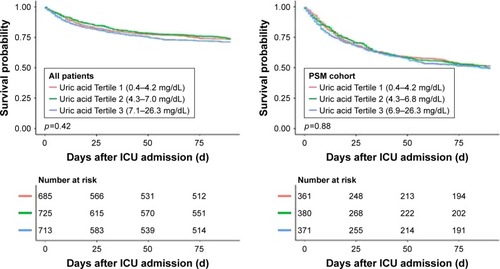
Patients were grouped according to their serum uric acid levels on admission. The K-M survival curves presented in showed that there was no difference in the survival rate among different serum uric acid levels on admission (log-rank test: p=0.88) after PSM. The K-M survival curves of 28-day mortality are shown in Figure S2.
ROC curve analysis
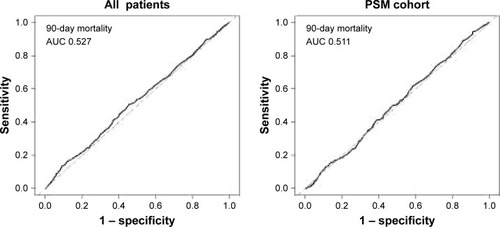
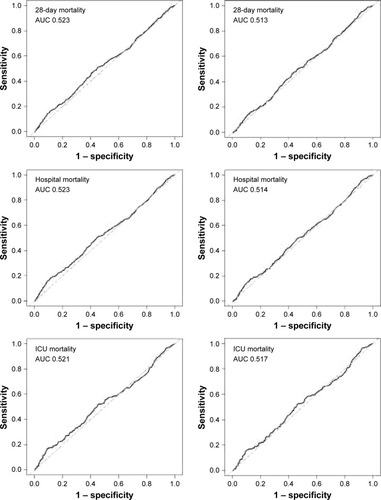
As shown in , the area under the ROC curve (AUC) of admission serum uric acid for discrimination of 90-day survivors and non-survivors was 0.522 (95% confidence interval [CI] 0.494–0.550) and 0.511 (95% CI 0.477–0.545) for all patients and PSM cohort, respectively. ROC curve analysis of other outcomes also indicated a poor predictive value of serum uric acid.
Association between serum uric acid levels on admission and ICU outcomes
Results of univariate Cox regression analysis of all patients and PSM cohort are presented in Tables S1 and S2, respectively. As shown in , multivariable regression analysis of PSM cohort indicated that serum uric acid was not an independent risk factor of 90-day mortality (hazard ratio [HR] 1.00, 95% CI 0.98–1.03, p=0.6835), 28-day mortality (HR 1.01, 95% CI 0.98–1.04, p=0.4894), hospital mortality (odds ratio [OR] 1.01, 95% CI 0.97–1.04, p=0.6099), and ICU mortality (OR 1.01, 95% CI 0.97–1.05, p=0.6934). Results of regression analysis of all patients are also shown in .
Table 3 Association of uric acid with 90-day mortality, 28-day mortality, ICU mortality, and hospital mortality
Discussion
For the first time, the present study evaluated the association between serum uric acid on ICU admission and long-term outcome of critically ill patients. Results of the study indicated that serum uric acid on admission cannot predict long-term outcome of critically ill patients.
It is interesting to find no correlation between serum uric acid with clinical outcomes of critically ill patients, since many studies had reported the prognostic predictive value of serum uric acid in many clinical conditions. For example, uric acid was found to be an independent predictor of cardiovascular outcomes and increase prognostic accuracy of Cox models in hypertensives with normal renal function which allowed a risk reclassification according to a recent report of Perticone et al.Citation8 Given serum uric acid is increased in respiratory disease, especially in the presence of hypoxia and systemic inflammation, many researchers wondered whether it could serve as a biomarker of prognostic predictive value.Citation20 Nagaya et al reported that serum uric acid levels correlate with the severity and the mortality of primary pulmonary hypertension.Citation21 Bartziokas et al found that serum uric acid was associated with increased 30-day mortality and risk for future acute exacerbation of chronic obstructive pulmonary disease.Citation22 Ergun et al reported that high serum uric acid levels are predictive for not only long-term mortality but also for short-term mortality.Citation23 However, in terms of critically ill patients, only a few studies were conducted to explore the value of uric acid and most of the conclusions were negative.Citation12–Citation14 Considering that most of the previous studies evaluated only the short-term outcomes with limited sample sizes, we conducted this present study aiming to evaluate the predictive value of serum uric acid for long-term outcome of critically ill patients. In our study, we included over 2,000 patients which made enough adjustment for confounders available and improved statistical power. Meanwhile, we performed PSM to further minimize the potential selection bias. Results of all patients and PSM cohort were consistent and provided a solid conclusion of the association between serum uric acid and 90-day mortality for critically ill patients, although negative. We also examined some short-term outcomes in the study, and the results were consistent with previous studies.
Although the findings in our study were informative, there were several limitations in the present study. First, given the observational nature of our study, it is not possible to adjust all potential confounders. Although we considered many variables known to affect the outcomes, unmeasured confounders may have affected our results. As we know, the reference value of serum uric acid is different between male and female; hence, gender must be considered in the K-M survival curves. In fact, the results were consistent even after grouped by sex (data not shown), but there were still other potential confounders such as renal replacement therapy on the first day, fluid and electrolyte disorders, which made it difficult to take all these confounders into consideration in the K-M curves. And since there were too many specific primary diagnoses for all the patients, we categorized the primary diseases as several comorbidities ( and ) to make it easier to adjust and analyze. However, it was indisputable that some unmeasured confounders such as gout, uremia, and other uric acid metabolic disorder might still have affected the results. In addition, as a retrospective database study, it was difficult to account for the potential effect of therapy before ICU admission on serum uric acid levels, because such information was usually not documented. Thus, further well-designed prospective study is needed to confirm our results. Second, the present study included data from only one ICU center, which might limit the external applicability of the study results. Third, we found no association between serum uric acid on admission and long-term outcomes of critically ill patients, but whether the changes of serum uric acid would be associated with the clinical outcomes of the patients remained unknown.
Conclusion
This large retrospective cohort study found that there was no statistically significant association of admission serum uric acid with 90-day mortality of ICU patients, providing a stronger confirmation of the controversial issue. However, further prospective basic and clinical research studies are still needed especially to reveal the underlined mechanisms and to evaluate the potential predictive value of changes of uric acid.
Author contributions
Qingui Chen designed the study and was the primary author of the manuscript. Qinchang Chen and Kai Huang mainly performed data extraction and statistical analysis. All authors contributed toward data analysis, drafting, and critically revising the paper, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Acknowledgments
The study was supported by National Natural Science Foundation of China (No 81670066), Major Science and Technology Planning Project of Guangdong Province (No 2016A020216009), Natural Science Foundation of Guangdong Province China (No 2015A030310346), and Special Funds for the Cultivation of Guangdong College Students’ Scientific and Technological Innovation (“Climbing Program” Special Funds).
Supplementary materials
Figure S2 Kaplan–Meier survival curve of 28-day mortality.
Abbreviations: ICU, intensive care unit; PSM, propensity score matching.
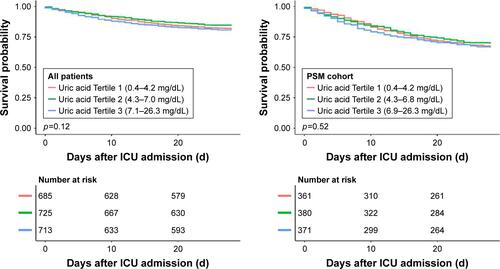
Table S1 Univariate Cox regression analysis of all patients on 90-day mortality
Table S2 Univariate Cox regression analysis of PSM cohort on 90-day mortality
Disclosure
The authors report no conflicts of interest in this work.
References
- El RidiRTallimaHPhysiological functions and pathogenic potential of uric acid: a reviewJ Adv Res20178548749328748115
- MartinonFUpdate on biology: uric acid and the activation of immune and inflammatory cellsCurr Rheumatol Rep201012213514120425023
- AmesBNCathcartRSchwiersEHochsteinPUric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesisProc Natl Acad Sci U S A19817811685868626947260
- SautinYYJohnsonRJUric acid: the oxidant-antioxidant paradoxNucleosides Nucleotides Nucleic Acids200827660861918600514
- DuranMKalayNAkpekMHigh levels of serum uric acid predict severity of coronary artery disease in patients with acute coronary syndromeAngiology201263644845222096206
- DuttaAHenleyWPillingLCWallaceRBMelzerDUric acid measurement improves prediction of cardiovascular mortality in later lifeJ Am Geriatr Soc201361331932623496291
- NdrepepaGBraunSHaaseHUPrognostic value of uric acid in patients with acute coronary syndromesAm J Cardiol201210991260126522325088
- PerticoneMTripepiGMaioRRisk reclassification ability of uric acid for cardiovascular outcomes in essential hypertensionInt J Cardiol201724347347828528984
- ZoppiniGTargherGNegriCElevated serum uric acid concentrations independently predict cardiovascular mortality in type 2 diabetic patientsDiabetes Care20093291716172019542211
- GaipovASolakYTurkmenKSerum uric acid may predict development of progressive acute kidney injury after open heart surgeryRen Fail20153719610225347234
- AkbarSRLongDMHussainKHyperuricemia: an early marker for severity of illness in sepsisInt J Nephrol2015201530102126294973
- ZhuHCCaoRLThe relationship between serum levels of uric acid and prognosis of infection in critically ill patientsWorld J Emerg Med20123318619025215061
- HoomanNMehrazmaMNakhaiiSThe value of serum uric acid as a mortality prediction in critically ill childrenIran J Pediatr201020332332923056724
- AminiahidashtiHBozorgiFMousaviSJSedighiOGorjiAMRashidianHSerum uric acid level in relation to severity of the disease and mortality of critically ill patientsJ Lab Physicians201791424628042216
- JohnsonAEPollardTJShenLMIMIC-III, a freely accessible critical care databaseSci Data2016316003527219127
- JohnsonAStoneDJCeliLAPollardTJThe MIMIC code repository: enabling reproducibility in critical care researchJ Am Med Inform Assoc20172513239
- Le GallJRLemeshowSSaulnierFA new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter studyJAMA199327024295729638254858
- SteinerCElixhauserASchnaierJThe healthcare cost and utilization project: an overviewEff Clin Pract20025314315112088294
- ThompsonNRFanYDaltonJEA new Elixhauser-based comorbidity summary measure to predict in-hospital mortalityMed Care201553437437925769057
- RuggieroCCherubiniABleAUric acid and inflammatory markersEur Heart J200627101174118116611671
- NagayaNUematsuMSatohTSerum uric acid levels correlate with the severity and the mortality of primary pulmonary hypertensionAm J Respir Crit Care Med1999160248749210430718
- BartziokasKPapaioannouAILoukidesSSerum uric acid as a predictor of mortality and future exacerbations of COPDEur Respir J2014431435323645404
- ErgunRErganBDoes serum uric acid levels predict in-hospital mortality in severe COPD exacerbations?Eur Respir J201546Suppl 59PA395
- MIMIC Code Repository [homepage on the Internet]CambridgeLaboratory for Computational Physiology, Massachusetts Institute of Technology2018 [updated March 15, 2018]. Available from: http://github.com/MIT-LCP/mimic-codeAccessed April 10, 2018