Abstract
Background
Recent studies have shown that Toll-like receptors (TLRs) may be associated with cancers. The aim of this meta-analysis is to summarize the predicting role of TLRs for survival in patients with a variety of carcinomas.
Materials and methods
Eligible studies were identified and assessed for quality through multiple search strategies. We collected data from studies investigating the relationship between the expression level of TLRs and survival in cancer patients. Studies were pooled and combined hazard ratios (HRs) of TLRs for survival were analyzed.
Results
A total of 24 studies, including 2,812 patients with various cancers, were identified for the meta-analysis. Importantly, this meta-analysis showed that higher expression levels of TLR4 or TLR7 in tumor tissues could predict poorer survival, with the pooled HR being 1.29 (95% CI: 1.17, 1.42) and 1.71 (95% CI: 1.38, 2.12), respectively. However, higher expression of TLR9 had no significant association with outcome as HR was 0.84 (95% CI: 0.62, 1.115). Heterogeneity existed in TLR4 and TLR9 studies (P-value <0.001) but not in TLR7 studies (P-value >0.05).
Conclusion
The expression level of TLR4 or TLR7 in cancerous tissue may have a prognosis value in patients with various cancers.
Keywords:
Introduction
Cancers constitute an enormous burden on modern society. About 14.1 million new cancer patients have been identified and 8.2 million deaths occurred in 2012 worldwide based on GLOBOCAN estimates. The burden is expected to increase because of the growing population and aging.Citation1 In general, early diagnosis and specific therapy are crucial for better survival in cancer patients. Thereby, cancer biomarkers are important for improving outcome. Currently, some biomarkers have been applied in cancer diagnosis and monitored to evaluate the therapeutic effects, including genes or proteins related to cell proliferation, apoptosis, signal recognition, and transduction. However, cancer therapy is still a great challenge to date and precision medicine may be a potential trend to resolve it. Therefore, a cancer patient should be diagnosed more in detail and more biomarkers should be tested.
Toll-like receptors (TLRs) are a family of evolutionarily conserved pattern recognition receptors (PRRs), which participate in immunologic first-line host defense against pathogens by recognizing pathogen-associated molecular patterns (PAMPs). Different PRRs react with specific PAMPs, leading to distinct expression patterns, specific signaling pathways, and distinct antipathogen responses. Till now there are 13 TLRs described in mammals (ten receptors in humans and 12 in mice). Some TLRs reside at the plasma membrane, where they recognize molecular components located on the surface of pathogens. By contrast, others exist intracellularly, where they mediate recognition of nucleic acids.Citation2 Recently, many studies revealed that TLRs play a cardinal role in the homeostasis of the human immune system. TLRs could recognize PAMPs and produce inflammatory cytokines to establish an effective defense system for the protection of the host.Citation3,Citation4 However, the abnormal activation of TLRs could jeopardize normal physiologic processes and may contribute to some diseases.Citation5,Citation6 Nowadays, more and more significant evidence suggested the important role for TLRs in human cancer, and inflammatory and immune diseases.Citation7–Citation9
Recent studies indicated that tumor cells had dysregulated expression of TLRs and TLRs signaling promoted tumor growth and immune evasion.Citation10 Cammarota et al found that colorectal cancer (CRC) patients with higher TLR4 expression had a significantly increased risk of disease progression and those with very high levels of TLR4 in the tumor stroma relapsed significantly earlier than those with lower expression levels.Citation11 However, Eiro et al reported that TLR4 expression by tumor cells was significantly associated with a lower rate of tumor recurrence in CRCCitation12; whereas high TLR4 expression was significantly associated with a shortened relapse-free survival (P=0.001) in cutaneous malignant melanoma (CMM).Citation13 In addition, Grimm et al found that the survival in the highly expressing TLR7 and TLR8 subgroups was significantly poorer than that of the lowly expressing TLR7 and TLR8 subgroups in CRC.Citation14 Therefore, to date, several TLRs were investigated in clinical prognosis studies and the results for a special TLR were inconsistent. It is timely and necessary for us to evaluate the overall risk of the expression of TLRs in patients with cancer.
Materials and methods
We performed this meta-analysis following the guidelines of the Meta-analysis of Observational Studies in Epidemiology group (MOOSE).Citation15
Search strategy
To identify the relevant studies, we carefully searched online PubMed from 1966 to July 31, 2017. Two sets of keywords were used, namely “Toll-like receptors and cancer and prognosis” and “TLR, cancer, prognosis”. The studies were regarded as eligible, as follows: 1) they studied the associations between TLRs and cancers; 2) they were designed as case-controlled ones, which means that they compare prognosis in patients with different TLR expression levels; 3) they reported data or figures about survival analysis and the follow-up duration cannot be less than 6 months. Articles were discarded when they met the following criteria: 1) no relationship with TLRs and survival; 2) review articles or letters; 3) key information missing (such as hazard ratio [HR] data or sample size). When duplicate studies were retrieved, the studies having reported HRs, or involving more patients (usually the latest one) were included in our analysis. Thus, the overlap between cohorts and overestimation of the overall HR could be avoided.
Information of all identified studies such as titles, abstracts, and full texts were carefully distinguished by two reviewers (Wang and Zhang) and double checked by Zhao. In case of key information missing, we sent emails to the authors for additional information for the meta-analytic calculations.
Quality assessment
According to a critical review checklist of the Dutch Cochrane Center proposed by MOOSE, we systematically assessed the quality of all the studies included.Citation15 Six key items were used to assess the study quality, including 1) clear definition of study population; 2) clear definition of cancer type; 3) clear definition of study design; 4) clear definition of measurement of TLRs; 5) clear definition of outcome assessment, such as overall survival (OS), recurrence-free survival, or disease-free survival; 6) sufficient period of follow-up, not <6 months. If a study did not mention all these six points, it was excluded so as not to compromise the quality of the meta-analysis.
Data extraction and statistical analysis
Data from each of the included study were extracted independently by two authors (Wang and Zhang). Any inconsistencies in the data extraction were discussed with Zhao to reach consensus. The following information was collected from each eligible study: 1) publication details: first author’s last name, publication year, country of origin; 2) characteristics of the studied population: sample size, age, sex, and type of disease; 3) study design: method to detect TLRs; follow-up duration; HR of elevated TLRs’ expression for survival, as well as their 95% CI and P-value. The simplest method consisted of the direct collection of HRs and their 95% CI from the original literature, with a HR of more than 1 being associated with a poorer outcome. If the HR and P-value were not available in the literature, they were calculated from the numbers of patient deaths in each group. When information was only available as Kaplan–Meier curves, data were extracted from the graphical survival plots and estimation of the HR was then performed using the described method.Citation16 Lower expressions of TLRs were chosen as baseline; if not, we will recalculate the HR by reciprocal.
Statistical analysis
Statistical heterogeneity across the studies was evaluated by the I-squared statistic and the significance of the heterogeneity was determined using the Cochran’s Q test. A P-value of <0.05 was considered significant. A random-effect model (Der Simonian and Laird method) was used when heterogeneity was observed (P<0.05), while the fixed-effect model was applied in the absence of between-study heterogeneity (P≥0.05). Publication bias was evaluated using the funnel plot with the Egger’s bias indicator test.Citation17 All statistical analyses were carried out by using Review Manager 5.3 (Cochrane, London, UK) and “Stata: Data Analysis and Statistical software” Version 12 (StataCorp LP, College Station, TX, USA).
Results
In PubMed, totally 200 publications were identified by the initial search strategy. After manually screening the titles, abstracts, and critical data, 149 records were excluded because they were review articles, letters, laboratory studies, or studies irrelevant to the current meta-analysis. Among 51 studies selected for detailed evaluation, nine of them about TLR2, TLR3, or TLR5 were excluded because there were insufficient studies for meta-analysis. Other 12 studies were excluded based on HR values missing. Ultimately, 24 studiesCitation11–Citation14,Citation18–Citation38 were included in our analysis, with four of them investigated two or three TLRs synchronously. Among them, 12,Citation11–Citation13,Citation18–Citation26 five,Citation13,Citation14,Citation27–Citation29 and elevenCitation13,Citation18,Citation30–Citation38 studies focused on TLR4, TLR7, and TLR9, respectively. The flow diagram of the study selection process is shown in .
Figure 1 Flow diagram of the study selection process.
In , we summarized the main features of 24 eligible studies. The recruited 2,812 participants were globally from Italy, Spain, Finland, Croatia, Germany, France, Poland, USA, Korea, Japan, and China. The patients were suffering from a variety of carcinomas, including pancreatic cancer/pancreatic ductal adenocarcinoma, CRC, esophageal adenocarcinoma, hepatocellular carcinoma, CMM, ovarian epithelial cancers, breast cancer (BC), adenoid cystic carcinoma, oral squamous cell carcinoma/oral tongue squamous cell carcinoma, mucoepidermoid carcinoma, non-small-cell lung cancer, renal cell carcinoma, prostate cancer (ProC), and chronic lymphocytic leukemia (CLL). Immunohistochemistry was used in the majority of studies (23/24) to measure the expression of TLRs. Although all of the studies applied dichotomy to compare the difference of survival, the TLR cutoff values were not consistent. Seven studies used median as the cutoff value, seven studies compared positive to negative, and the other ten compared higher expression to lower expression. In addition, all of the studies were retrospective.
Table 1 Summary table of the studies included in this meta-analysis
Subsequently, the heterogeneity of the included studies was assessed. There appeared to be heterogeneity between studies for TLR4 (P<0.0001) and TLR9 (P<0.00001) (). Therefore, a random model was applied to calculate a pooled HR and its 95% CI. On the other hand, a fixed model was applied to calculate TLR7’s pooled HRs and 95% CI according to the homogeneity (P-value >0.05). We found that higher expression levels of TLR4 and TLR7 predicted poorer survival, with the pooled HR being 1.29 (95% CI: 1.17, 1.42) and 1.71 (95% CI: 1.38, 2.12) (). However, higher expression of TLR9 could not predict poorer survival (HR: 0.84; 95% CI: 0.62, 1.115) (). In addition, considering the difference between solid tumors and leukemia, we reperformed a meta-analysis about TLR9 after deleting the study about CLL. The result remained not significant (HR: 0.81; 95% CI: 0.58, 1.28). Furthermore, we also analyzed the association between the expression level of TLRs and OS alone. The data showed that higher expression levels of TLR4 and TLR7 predicted worse OS, with the pooled HR being 1.23 (95% CI: 1.11, 1.37) and 1.78 (95% CI: 1.36, 2.33), whereas higher expression of TLR9 had no prognostic value. Therefore, in a summary, the data suggested that TLR4 and TLR7 may have predictive value for cancer outcome.
Figure 2 Forest plots of studies evaluating hazard ratios of higher expression of TLR4 (A), TLR7 (B), and TLR9 (C) as compared to lower expression in various cancers.
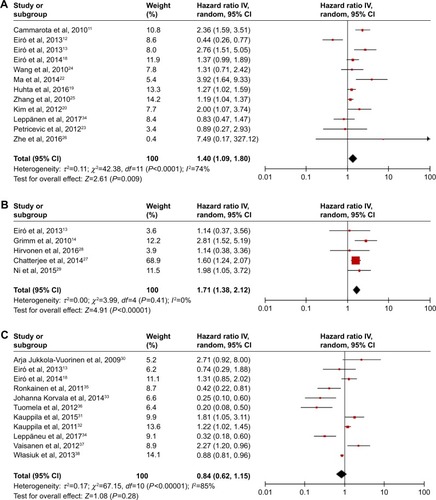
Table 2 Comparison of the predicting value of TLRs’ expression in patients
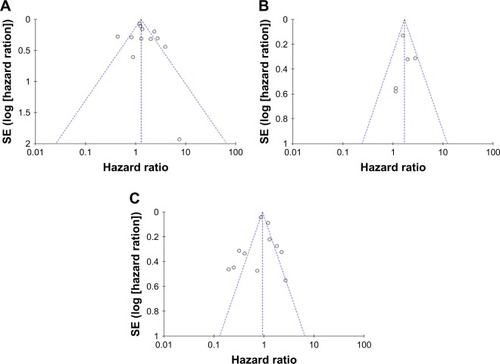
Finally, as shown in and , publication bias of the included studies was evaluated by funnel plots and Egger’s tests. In TLR4, TLR7, and TLR9 meta-analysis, the P-values of Egger’s regression intercepts were 0.410, 0.957, and 0.757, respectively. There was no publication bias existing in the studies because the funnel plots were almost symmetric and the P-values of Egger’s regression were more than 0.05.
Discussion
This systemic review and meta-analysis, which recruited 24 studies and 2,812 patients, showed that higher expression of TLR4 or TLR7 did indeed predict poor survival in patients with a variety of carcinomas. The analysis of TLR9, however, could not get the significant results.
In general, the meta-analysis as performed in this study had a number of inherent limitations, so the conclusion should be tempered for several reasons. First, although the pooled risks of TLR4 and TLR7 were statistically significant, they were not strong, with HRs of 1.4 and 1.71, respectively. Empirically, HR >2 is considered strongly predictive.Citation39 Second, as only five studies were included for analysis with a relatively sample size of 645, the meta-analysis result of TLR7 was less powerful. More studies should be conducted in future to evaluate the prognostic value of TLRs in cancers. Third, one study detected the total expression of TLR9 in CLL, which was different from other malignant solid tumors.
Marked heterogeneity of subjects existed in TLR4 and TLR9 groups. The heterogeneity of the populations was probably due to the difference in baseline characteristics of patients (age, stage, sex, race, or country), the duration of follow-up, cancer type, the cutoff value of TLRs, and so on. For example, several studies utilized median expression as the cutoff, while others used positive vs negative or low vs high. Even for those studies using median value in their laboratory or hospital as the cutoff value, the accurate values were different, and thereby, this analysis could not provide a clear clue about how high is high. As such differences might have a residual confounding effect within these studies, we attempted to minimize the effect by using a random-effect model. In addition, the prognostic value may also be weakened because all cancers were grouped together without identifying some particular cancers having increased exposure to commensal bacteria. For routine clinical application in future, more studies should be conducted and the above-mentioned problems should be solved by further experimental studies.
Our data demonstrated that TLR4 and TLR7 were promising biomarkers of cancers, while TLR9 may not be appropriate for monitoring clinical outcome. Other than TLR4, TLR7, and TLR9, researchers also reported other TLRs related to cancers. Elevated expression of some TLRs has been reported in many tumor cells and tissues.Citation5 Ironically, overexpression of TLRs has been paradoxically found in many tumor cases. Grimm et al found that high expression of TLR8 was an independent prognostic factor for worse outcome in multivariate analysis.Citation14 Makinen et al found that high or strong TLR2 expression was correlated with deeper tumor invasion, whereas negative or mild TLR5 expression predicted poor disease-specific survival.Citation40 Gonzalez-Reyes et al reported that tumors with high TLR3 expression were significantly associated with higher probability of metastasis in BC and with higher probability of biochemical recurrence in ProC.Citation41,Citation42 The above studies suggested that the other TLRs may also play predicting roles in various cancers. For the current meta-analysis, however, we did not conduct further analysis because of the limited study number.
Although dysregulation of TLRs was found in many malignant tumors, the biologic function of TLRs in tumor formation and development remains obscure because of the limited duration of research after they were identified in the latest decade. Interestingly, despite divergent ligands and receptors, two major pathways are used by TLR family, one is mediated by myeloid differentiation primary-response protein 88 (MyD88) and the other is independent of MyD88. All TLRs except TLR3 use a common signaling pathway through the adaptor molecule MyD88.Citation43,Citation44 TLRs that are activated by their individual ligands could recruit MyD88 and subsequently activate the downstream targets, including nuclear factor of kappa B, mitogen-associated protein kinase, and interferon regulatory factors.Citation2 Furthermore, recently, numerous studies have demonstrated that TLRs could be involved in antitumor or protumor responses.Citation45,Citation46 For example, TLR4-deficient mice have showed enhanced tumorigenesis in inducible model of lung cancer, skin cancer, and BC. Injection of TLR4 agonists, such as OM-174 and OK-132, could repress tumor formation in mice. Such results suggested that TLR4 may have antitumor effects. However, other studies about cancer cells showed that TLR4 stimulation could induce tumor cell proliferation and suppress apoptosis. Hence, to date, the accurate effects of TLRs in cancer still remain unclear and more experiments should be conducted in the future.
Conclusion
Our meta-analysis, representing a quantified synthesis of all published studies, has shown that the elevated TLR4 and TLR7 expression is significantly associated with poor survival in patients with various types of carcinoma. More clinical investigations should be conducted before TLRs can be implemented in the routine clinical management of cancer.
Acknowledgments
The analysis was sponsored by Natural Science Foundation of Shanghai (17ZR1408000), National Natural Science Foundation of China (81502059), Shanghai Rising-Star Program (16QB1402900), and Science and Technology Development Foundation of Pudong New District, Shanghai, China (PKJ2015-S29).
Disclosure
The authors report no conflicts of interest in this work.
References
- TorreLABrayFSiegelRLFerlayJLortet-TieulentJJemalAGlobal cancer statistics, 2012CA Cancer J Clin20156528710825651787
- AkiraSUematsuSTakeuchiOPathogen recognition and innate immunityCell2006124478380116497588
- ShankaranVIkedaHBruceATIFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicityNature200141068321107111111323675
- KawaiTAkiraSThe role of pattern-recognition receptors in innate immunity: update on Toll-like receptorsNat Immunol201011537338420404851
- EySOuchiTThe application of Toll like receptors for cancer therapyJ Int J Biol Sci201067675681
- KeoghBParkerAEToll-like receptors as targets for immune disordersTrends Pharmacol Sci201132743544221529972
- LiewFYXuDBrintEKO’NeillLANegative regulation of toll-like receptor-mediated immune responsesNat Rev Immunol20055644645815928677
- TaniguchiNKawaharaKYoneKHigh mobility group box chromosomal protein 1 plays a role in the pathogenesis of rheumatoid arthritis as a novel cytokineArthritis Rheum200348497198112687539
- SoEYOuchiTThe application of Toll like receptors for cancer therapyInt J Biol Sci20106767568121060729
- IwasakiAMedzhitovRToll-like receptor control of the adaptive immune responsesNat Immunol200451098799515454922
- CammarotaRBertoliniVPennesiGThe tumor microenvironment of colorectal cancer: stromal TLR-4 expression as a potential prognostic markerJ Transl Med2010811221059221
- EiróNGonzálezLGonzálezLOToll-like receptor-4 expression by stromal fibroblasts is associated with poor prognosis in colorectal cancerJ Immunother201336634234923799413
- EiróNOviesCFernandez-GarciaBExpression of TLR3, 4, 7 and 9 in cutaneous malignant melanoma: relationship with clinicopathological characteristics and prognosisArch Dermatol Res20133051596723179584
- GrimmMKimMRosenwaldAToll-like receptor (TLR) 7 and TLR8 expression on CD133+ cells in colorectal cancer points to a specific role for inflammation-induced TLRs in tumourigenesis and tumour progressionEur J Cancer201046152849285720728343
- StroupDFBerlinJAMortonSCMeta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) groupJAMA2000283152008201210789670
- ParmarMKTorriVStewartLExtracting summary statistics to perform meta-analyses of the published literature for survival endpointsStat Med19981724281528349921604
- EggerMDavey SmithGSchneiderMMinderCBias in meta-analysis detected by a simple, graphical testBMJ199731571096296349310563
- EiróNAltadillAJuárezLMToll-like receptors 3, 4 and 9 in hepatocellular carcinoma: relationship with clinicopathological characteristics and prognosisHepatol Res201444776977823742263
- HuhtaHHelminenOLehenkariPPSaarnioJKarttunenTJKauppilaJHToll-like receptors 1, 2, 4 and 6 in esophageal epithelium, Barrett’s esophagus, dysplasia and adenocarcinomaOncotarget2016717236582366727008696
- KimKHJoMSSuhDSExpression and significance of the TLR4/MyD88 signaling pathway in ovarian epithelial cancersWorld J Surg Oncol20121019322985132
- LeppänenJHelminenOHuhtaHHigh toll-like receptor (TLR) 9 expression is associated with better prognosis in surgically treated pancreatic cancer patientsVirchows Archiv2017470440141028191612
- MaFJLiuZBHuXPrognostic value of myeloid differentiation primary response 88 and Toll-like receptor 4 in breast cancer patientsPLoS One2014910e11163925360699
- PetricevicBVrbanecDJakic-RazumovicJExpression of Toll-like receptor 4 and beta 1 integrin in breast cancerMed Oncol201229248649421400218
- WangELQianZRNakasonoMHigh expression of Toll-like receptor 4/myeloid differentiation factor 88 signals correlates with poor prognosis in colorectal cancerBr J Cancer2010102590891520145615
- ZhangJJWuHSWangLTianYZhangJHWuHLExpression and significance of TLR4 and HIF-1alpha in pancreatic ductal adeno-carcinomaWorld J Gastroenterol201016232881288820556833
- ZheYLiYLiuDSuDMLiuJGLiHYExtracellular HSP70-peptide complexes promote the proliferation of hepatocellular carcinoma cells via TLR2/4/JNK1/2MAPK pathwayTumour Biol20163710139511395927492456
- ChatterjeeSCrozetLDamotteDTLR7 promotes tumor progression, chemotherapy resistance, and poor clinical outcomes in non-small cell lung cancerCancer Res201474185008501825074614
- HirvonenKBäckLHaglundCToll-like receptor 5 and 7 expression in adenoid cystic carcinoma of major salivary glandsTumour Biol2016378109591096426888781
- NiYHDingLZhangDYHouYYHuangXHuQDistinct expression patterns of Toll-like receptor 7 in tumour cells and fibroblast-like cells in oral squamous cell carcinomaHistopathology201567573073925828894
- Jukkola-VuorinenARahkoEVuopalaKSToll-like receptor-9 expression is inversely correlated with estrogen receptor status in breast cancerJ Innate Immun200911596820375566
- KauppilaJHKorvalaJSiiriläKToll-like receptor 9 mediates invasion and predicts prognosis in squamous cell carcinoma of the mobile tongueJ Oral Pathol Med201544857157725338738
- KauppilaJHTakalaHSelanderKSLehenkariPPSaarnioJKarttunenTJIncreased Toll-like receptor 9 expression indicates adverse prognosis in oesophageal adenocarcinomaHistopathology201159464364922014045
- KorvalaJHarjulaTSiiriläKToll-like receptor 9 expression in mucoepidermoid salivary gland carcinoma may associate with good prognosisJ Oral Pathol Med201443753053724484266
- LeppänenJHelminenOHuhtaHHigh toll-like receptor (TLR) 9 expression is associated with better prognosis in surgically treated pancreatic cancer patientsVirchows Arch2017470440141028191612
- RonkainenHHirvikoskiPKauppilaSAbsent Toll-like receptor-9 expression predicts poor prognosis in renal cell carcinomaJ Exp Clin Cancer Res2011308421929816
- TuomelaJSandholmJKarihtalaPLow TLR9 expression defines an aggressive subtype of triple-negative breast cancerBreast Cancer Res Treat2012135248149322847512
- VäisänenMRJukkola-VuorinenAVuopalaKSSelanderKSVaaralaMHExpression of Toll-like receptor-9 is associated with poor progression-free survival in prostate cancerOncol Lett2013551659166323761830
- WłasiukPTomczakWZającMDmoszyńskaAGiannopoulosKTotal expression of HLA-G and TLR-9 in chronic lymphocytic leukemia patientsHum Immunol201374121592159723994589
- HayesDFIsaacsCStearnsVPrognostic factors in breast cancer: current and new predictors of metastasisJ Mammary Gland Biol Neoplasia20016437539212013528
- MäkinenLKAtulaTHäyryVPredictive role of Toll-like receptors 2, 4, and 9 in oral tongue squamous cell carcinomaOral Oncol20155119610225264223
- González-ReyesSMarínLGonzálezLStudy of TLR3, TLR4 and TLR9 in breast carcinomas and their association with metastasisBMC Cancer20101066521129170
- González-ReyesSFernándezJMGonzálezLOStudy of TLR3, TLR4, and TLR9 in prostate carcinomas and their association with biochemical recurrenceCancer Immunol Immunother201160221722620978888
- BryantCESymmonsMGayNJToll-like receptor signalling through macromolecular protein complexesMol Immunol201563216216525081091
- KoppEMedzhitovRRecognition of microbial infection by Toll-like receptorsCurr Opin Immunol200315439640112900270
- DajonMIribarrenKCremerIToll-like receptor stimulation in cancer: a pro- and anti-tumor double-edged swordImmunobiology201722218910027349597
- Moradi-MarjanehRHassanianSMFiujiHToll like receptor signaling pathway as a potential therapeutic target in colorectal cancerJ Cell Physiol201823385613562229150944