Abstract
Despite continuous efforts to prevent cardiovascular diseases (CVDs), heart failure prevails as the number one cause of death in developed countries. To properly treat CVDs, scientists had to take a closer look at the factors that contribute to their pathogenesis and either modernize current pharmaceuticals or develop brand new treatments. Enhancement of current drugs, such as tolvaptan and omecamtiv mecarbil, sheds new light on already-known therapies. Tolvaptan, a vasopressin antagonist, could be adopted in heart failure therapy as it reduces pre- and afterload by decreasing systolic blood pressure and blood volume. Omecamtiv mecarbil, which is a myosin binding peptide, could aid cardiac contractility. The next generation vasodilators, serelaxin and ularitide, are based on naturally occurring peptides and they reduce peripheral vascular resistance and increase the cardiac index. In combination with their anti-inflammatory properties, they could turn out to be extremely potent drugs for heart failure treatment. Cardiotrophin has exceeded many researchers’ expectations, as evidence suggests that it could cause sarcomere hypertrophy without excessive proliferation of connective tissue. Rapid progress in gene therapy has caused it to finally be considered as one of the viable options for the treatment of CVDs. This novel therapeutic approach could restore stable heart function either by restoring depleted membrane proteins or by balancing the intracellular calcium concentration. Although it has been set back by problems concerning its long-term effects, it is still highly likely to succeed.
Introduction
Cardiovascular disease (CVD) is currently one of the most common causes of mortality and morbidity in developed countries.Citation1 In the last 20 years, strong emphasis was placed on improving survival and quality of life in patients with heart failure (HF); however, despite these great efforts, the HF 1-year mortality rate has only slightly declined and the 5-year mortality rate has not declined at all over the last 10 years.Citation2
The current goals of HF research include the short-term improvement of clinical status and quality of life as well as the long-term targets of reducing all-cause readmission and, most importantly, reducing mortality. Thus, the emphasis is on identifying drugs with detrimental long-term effects.Citation3
The standard therapy for HF involves the use of inotropic agents, vasodilators, and loop diuretics. These drugs, in combination with angiotensin-converting-enzyme inhibitors and beta-blockers, are effective forms of evidence-based therapies. The existing medical therapies for HF have brought about moderate success (52.6% of patients die within 5 years);Citation2 however, there is still an unmet need for new pharmaceuticals that could be beneficial in HF treatment.Citation4
This article aims to summarize the clinical progress of new therapeutic agents that could become standard HF therapies in the future ().
Table 1 An overview of the presented novel therapeutic methods with respect to their mechanism of action and latest stage of clinical development
Omecamtiv mecarbil
Treatment guidelines state that positive inotropic agents may be a therapeutic option in some specific types of HF, such as in patients with a low ejection volume, cardiogenic shock or low tissue perfusion. However, administering inotropic agents has no positive effects on mortality or hospitalization time, and these substances also have proarrhythmic effects.Citation5 The use of standard inotropic agents increases cAMP levels in cardiomyocytes through various mechanisms. They produce a higher calcium concentration in the cytoplasm, which leads to better contractility; however, this is also accompanied by a higher oxygen use, which could have negative effects on patients’ health.Citation6–Citation8 These disadvantages of inotropic agents mean that there is a strong incentive to develop new and safer substances with similar pharmacological effects. One such substance could be the specific cardiac myosin activator, omecamtiv mecarbil.Citation6–Citation10
Omecamtiv mecarbil binds to the catalytic domain of myosin with high affinity and shifts the equilibrium of ATP hydrolysis toward ADP-P during the stroke. The change of equilibrium increases the number of myosin heads ready to bind to actin filaments. This results in a more powerful stroke without increasing the calcium level or oxygen use in cardiomyocytes ().Citation6,Citation7,Citation9
Figure 1 Mechanism of the impact of OM on muscle contraction.(/p)(/p)Notes: OM binds to myosin filaments and shifts the equilibrium of ATP hydrolysis toward the ADP-P state. OM increases the number of myosin filaments ready to bind actin filaments, in turn increasing the stroke power.(/p)(/p)Abbreviations: OM, omecamtiv mecarbil; ATP, adenosine triphosphate; ADP, adenosine diphosphate; P, inorganic phosphate.
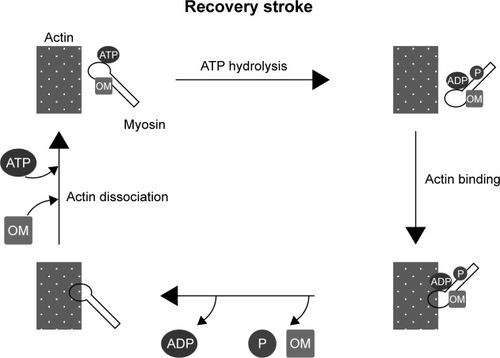
The Acute Treatment with Omecamtiv Mecarbil to Increase Contractility in Acute Heart Failure (ATOMIC-HF) study was a phase 2 prospective trial that compared a placebo and omecamtiv mecarbil in patients with acute HF. The results showed that use of the cardiac myosin activator as a treatment did not match the predicted dyspnea relief, except in the higher administration group; however, omecamtiv mecarbil increased systolic ejection time (SET) and was well tolerated.Citation7,Citation8,Citation11
The Chronic Oral Study of Myosin Activation to Increase Contractility in Heart Failure (COSMIC-HF) study showed better results for the treatment of chronic HF with omecamtiv mecarbil. This was a phase 2 trial that was designed to show the pharmacokinetics and optimal oral dose of omecamtiv mecarbil in the treatment of chronic HF. Changes in SET, stroke volume, end-diastolic diameter and N-terminal prohormone of brain natriuretic peptide (NT-proBNP) levels were also examined.Citation7,Citation11 Patients received a placebo or different dose of omecamtiv mecarbil. The results showed that the therapy was safe and well tolerated. It also led to an increased SET and stroke volume but decreased the heart rate, NT-proBNP level, and left ventricular volume.Citation7 These results were very promising, therefore there are huge expectations for phase 3 trials in a larger group of patients.Citation7,Citation10,Citation11
The Multicenter Study to Assess the Efficacy and Safety of Omecamtiv Mecarbil on Mortality and Morbidity in Subjects With Chronic Heart Failure With Reduced Ejection Fraction (GALACTIC-HF) study is a phase 3 clinical trial that is estimated to conclude in 2021. The study will include 8,000 patients with HF and its main outcome is to measure the time of the first HF event or CV death during omecamtiv mecarbil therapy.Citation9
Ularitide
The natriuretic peptide hormone family includes ANP, BNP, CNP, DNP and urodilatin. There are three main natriuretic peptide receptors (NPRs): NPR-A, NPR-B, and NPR-C.Citation12 Elevated levels of natriuretic peptides (BNP .35 pg/mL or NT-proBNP .125 pg/mL) are indicative of HF.Citation3 Downregu-lation or desensitization of NPRs leads to beneficial effects in HF treatment, which makes them a target for pharmacological therapy.Citation13
Ularitide is a synthetic analog of urodilatin, which is a kidney peptide hormone secreted by the distal tubule, collecting duct cells in response to increased pressure. It binds to NPR-A and results in increased diuresis and natriuresis ().Citation14 Other pharmacological effects of ularitide include vasodilation and inhibition of the renin-angiotensin-aldosterone system.Citation15,Citation16 Clinically, it demonstrates prolonged activity in comparison with atrial peptides.Citation17
Figure 2 Mechanism of action of urodilatin.(/p)(/p)Note: The consequences of NPR-A receptor activation include increased cGMP production, resulting in lowered intracellular calcium level which reduces the smooth muscle tonus.(/p)(/p)Abbreviations: AVP, arginine vasopressin; GTP, guanosine triphosphate; cGMP, cyclic guanosine monophosphate; NPR-A, natriuretic peptide receptor A.
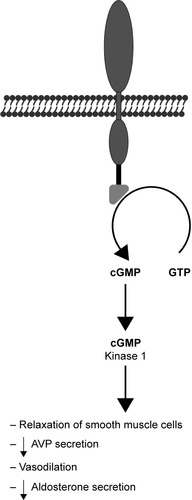
Initial phase 1 studies found that ularitide decreases pulmonary capillary wedge pressure (PCWP) in healthy menCitation18 and decreases systemic vascular resistance and PCWP and increases cardiac index and stroke volume in patients with acute HF (AHF).Citation19 These preliminary studies were followed by the phase 2 SIRIUS I study, which explored the potential clinical value of ularitide. Patients subjected to ularitide treatment showed improved PCWP, reduced NT-proBNP, and improved dyspnea without renal function effects.Citation20
SIRIUS II was a randomized controlled trial in 221 patients which further defined the hemodynamic effects of ularitide infusion. Its secondary endpoints included PCWP changes and patient-assessed dyspnea improvements. All ularitide groups showed significant reductions in PCWP and dyspnea, and the higher-dose groups also showed reductions in systemic vascular resistance and increased cardiac index at 6 hours. As in the SIRIUS I study, a dose-dependent reduction in systolic blood pressure (SBP) was noted in some patients.Citation21 TRUE-AHF was a randomized double-blind parallel-group placebo-controlled trial in 2,157 patients. The goal of the study was to evaluate the effect of ularitide on CV mortality and 48-hour clinical status. Secondary endpoints included, among others, the length of stay, events of worsening HF, and proBNP changes.Citation22 The drug produced satisfactory hemodynamic effects such as reduced SBP and decreased NT-proBNP levels; however, it did not significantly impact any of the primary or secondary endpoints. Death from CV causes occurred in 236 patients in the ularitide group and 225 patients in the placebo group.Citation23 Therefore the findings of this study indicate that ularitide therapy can produce beneficial hemodynamic effects and reduce cardiac wall stress; however, it does not reduce myocardial injury or affect disease progression as indicated by the absence of change in the risk of CV death.Citation24
Serelaxin
Relaxin is a member of the relaxin family, which includes peptides that are structurally similar to insulin. It was first extracted from the corpus luteum of pigs and has since been shown to play a role in modulating CV changes in pregnancy. Four relaxin receptors have been identified to date (RXFP1–4), all of which are G protein–coupled receptors. Their presence in the heart, lung, kidney, and blood cells is particularly important in CV therapy. Through stimulation of nitric oxide and VEGF production and inhibition of vasoconstrictors, relaxin can increase plasma volume and cardiac output and decrease blood pressure and vascular resistance (). These results from animal models make relaxin a potential candidate for HF treatment.Citation4,Citation25
Figure 3 Effect of relaxin binding with RXFP family receptor.(/p)(/p)Note: Mediated by G-proteins, the response involves activation of adenylate cyclase, increase in cAMP accumulation, as well as NO production.(/p)(/p)Abbreviations: RXFP, relaxin family peptide; ATP, adenosine triphosphate; cAMP, cyclic adenosine monophosphate; PKA, protein kinase A; Gαi3, Gαi subunit 3; GαoB, Gαo subunit B; GαS, Gα subunit S; β, Gβ subunit; γ, Gγ subunit; PI3K, Phosphatidylinositol-3 kinases; AKT, protein kinase B; PKC, protein kinase C; eNOS, endothelial nitric oxide synthase; NO, nitric oxide.
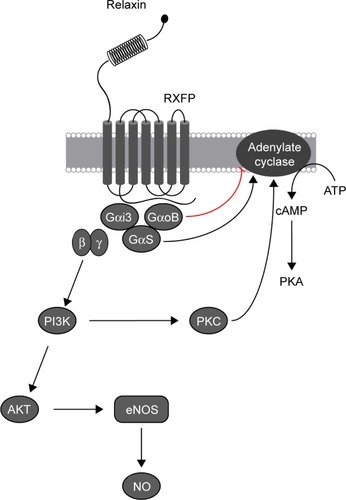
Serelaxin is a synthetic recombinant of relaxin that displays identical hemodynamic properties. Initial investigation of relaxin in humans began with phase 1 trials, which examined the drug’s safety and tolerability, whereas the subsequent phase 2 trials focused on its hemodynamic effect and clinical efficacy. The primary phase 1 trials assessed if serelaxin was capable of increasing renal plasma flow and natriuresis,Citation26 reducing PCWP, improving the cardiac index, and reducing vascular resistance.Citation27 These preliminary studies were followed by a dose-finding pilot trial called pre-RELAX-AHF, which was a placebo-controlled, parallel-group phase 2 study that enrolled 234 patients with AHF. The results from this study revealed that a 30 µg/kg/day serelaxin infusion produced the best dyspnea improvement with a reduction in CV death or readmission due to heart or renal failure.Citation28
These promising results initiated a phase 3 trial (RELAX-AHF) that was completed in 2012. The study enrolled 1,161 patients hospitalized for AHF, all of whom suffered from dyspnea, congestion, and elevated BNP levels. Each dose produced a clinical benefit; however, a 48-hour infusion of serelaxin at a dose of 30 µg/kg/day presented the greatest improvement in HF symptoms with sparse adverse effects. Investigators found that there was a great reduction in patient-assessed dyspnea over 5 days. The 60-day survival, death, or readmission rate did not significantly differ in serelaxin and placebo groups; however, it reduced HF worsening by 47% by day 5 and both all-cause and CV 180-day mortality by 37%. However, hypotensive episodes were noted in the sere-laxin group.Citation29 In light of the results of the RELAX-AHF trial, two global phase 3 trials were designed: RELAX-AHF-2 and RELAX-AHF-ASIA.
RELAX-AHF-ASIA was a randomized, double-blind, placebo-controlled phase 3 trial that aimed to evaluate the effects of serelaxin in Asian patients, unlike previous trials, which targeted primarily Caucasian patients. The study aimed to recruit 1,520 patients with AHF and assess the symptom relief and clinical outcomes with the endpoint being treatment success, treatment failure, or no change in status. The secondary endpoints included HF worsening by day 5 and deaths by day 180.Citation30
RELAX-AHF-2 aimed to confirm the beneficial effects of serelaxin on 180-day CV death and worsening HF by day 5, with secondary endpoints including 180-day all-cause mortality, CV death or rehospitalization, and length of hospitalization. Patients were randomized to receive 48-hour serelaxin infusions (30 µg/kg/day) or a placebo in addition to their standard therapy.Citation31 However, RELAX-AHF-2 did not meet any of its endpoints. There was no difference in CV mortality at 180 days and the reduction of worsening HF was not statistically significant. Serelaxin also failed to meet its secondary endpoints (ie, all-cause 180-day mortality, length of hospitalization, and rehospitalization due to heart or renal failure). The results of the study indicate that serelaxin is a safe but inefficacious drug (ie, it does not improve the outcomes compared with the placebo). These results were contrary to the findings of the RELAX-AHF trial, where a hemodynamic improvement was accompanied by a reduction in mortality.Citation32
Tolvaptan
One consequence of HF is fluid retention, which leads to edemas and increased pulmonary congestion. The main causes of fluid retention are sodium retention and neurohu-moral abnormalities.Citation33,Citation34 Arginine vasopressin (AVP) levels increase during HF, and higher levels of AVP are characteristic of the advanced stage of this disease. The main role is played by the nonosmotic regulatory pathway. Reduced cardiac output and decreased blood pressure lead to activation of baroreceptors and the renin-angiotensin-aldosterone system, which leads to increased AVP release from the pituitary gland.Citation35
Increased vasopressin levels and the use of loop diuretics can cause hyponatremia.Citation34 Loop diuretics are commonly used in HF treatment;Citation33,Citation36,Citation37 however, up to 30% of patients are resistant to this type of therapy. Other side effects of loop diuretic therapy include electrolyte imbalance or hypotension.Citation34,Citation37,Citation38
Tolvaptan is an oral vasopressin type 2 receptor antagonist that affects collecting ducts and increases aquaresis. Urine volume is increased but there is no change in the excretion of electrolytes.Citation33–Citation35,Citation39 Studies show that tolvaptan increases serum sodium levels in patients with hyponatremiaCitation34,Citation35,Citation37,Citation39 and has a lower tendency for worsening renal function than standard diuretics.Citation36,Citation38
Many studies assessed the beneficial effects of tolvaptan in the treatment of HF. For example, the Efficacy of Vasopressin Antagonism in Heart Failure: Outcome Study with Tolvaptan (EVEREST) trial checked the long- and short-term safety and efficacy of tolvaptan administration in addition to standard HF treatment. This was a double-blind, placebo-controlled test in 1,567 patients with HF. Patients were asked to complete the Kansas City Cardiomyopathy Questionnaire to assess their health-related quality of life (HRQOL) at 1 week after discharge. The results showed dyspnea relief in most patients; however, there was no improvement in the HRQOL score. There was also no association with mortality or dyspnea as assessed by a physician.Citation40
The Qualification of Efficacy and Safety in the Study of Tolvaptan in Cardiac Oedema (QUEST) study was a prospective observational study. Patients were treated with tolvaptan (administered daily) for a standard observation time (eg, 14 days). The trial assessed HF symptoms, such as lower limb edema, dyspnea, pulmonary congestion, jugular venous distention, and hepatomegaly, during the treatment. Body weight and urine volume were also evaluated. All data were recorded from baseline to the end of treatment at day 14. The results showed a shrinking prevalence of these symptoms, a reduction in body weight, and an increase in diuresis; however, some patients developed hypernatremia with higher doses of tolvaptan.Citation37
CT-1
CT-1 is a novel drug that is based on gp130, which forms part of the receptors for cytokines including IL-6, IL-11, and CT-1. These cytokines and their receptors are commonly associated with inflammation.
CT-1 is often connected with pathological hypertrophy of the heart,Citation41 and elevated CT-1 plasma levels are associated with myocardial fibrosis.Citation42 This substance is believed to play a key role in the stimulation of fibroblasts. Induction of cardiomyocyte hypertrophy and collagen synthesis is also connected with higher CT-1 levels. Furthermore, there is also a positive correlation between CT-1 plasma levels and heart valve disease.Citation42 According to recent research, CT-1 is important for physiological hypertrophy of the heart, eg, research conducted on cardiomyocytes showed an instant modification in cell function and structure and a significant change in the length-to-width ratio. This change was different from those caused by pathological hypertrophy agents such as α-adrenergic agonists.Citation43 Importantly, CT-1 also slows down the pathological hypertrophy induced by the aforementioned agents. The curbing of this unfavorable process is also observed in people who practice regular aerobic exercise. Therefore it is possible that hypertrophy is also curbed naturally (after exercise) by CT-1.Citation43
CT-1 not only influences single cardiomyocytes but it also affects the whole cardiac muscle. These changes are reminiscent of those observed in sportsmen (eg, heart hypertrophy). Cardiac wall thickening occurred without changes in the internal diameter of the ventricle.Citation43 The changes brought about by isoproterenol or phenylephrine are not as beneficial as those caused by CT-1, and isoproterenol and phenylephrine produce a negative change in the left-to-right ventricle internal diameter ratio.
Another difference between physiological and pathological hypertrophy is reversibility. Only changes caused by CT-1 therapy are fully reversible, which resembles physiological hypertrophy.
The molecular process responsible for the difference between CT-1 and phenylephrine activity is probably associated with the STAT1:STAT3 balance.Citation44 STAT3 is anti-inflammatory (probably activated by CT-1), while STAT1 is proinflammatory (probably activated by α-adrenergic agonists). Caspases are also important for CT-1 activity, and knockout of CASP-3 or CASP-9 stops the positive effect of CT-1 stimulation.Citation43 This suggests that beneficial hypertrophy depends on gp130, CASP-3, CASP-9, and STAT3 domination over STAT1. Another effect of CT-1 stimulation (probably via STAT3)Citation45 is angiogenesis, which is vital for providing energy for sarcomeres (oxygen consumption after CT-1 therapy is increased).Citation43 CK2, which is a caspase inhibitor, is activated by CT-1 and is the reason why CT-1-induced caspase activation is restricted and does not cause pathological changes.Citation43
Another research group has shown a positive influence of CT-1 on human health.Citation46 CT-1 depletion in a murine model can produce insulin resistance and lead to many subsequent negative consequences; however, CT-1 delivery can prevent insulin resistance. CT-1 also affects the regulation of food intake and leads to a noticeable reduction in consumption.
A separate study also showed the importance of CT-1 as a CV insufficiency therapy. A drug that could simultaneously promote beneficial heart remodeling, impede pathological changes, and fight against obesity (induced by increased food intake and insulin resistance) would solve the two main problems in those suffering from CV insufficiency.
Gene therapy
Gene therapy is believed to be a great hope for rare congenital diseases; however, many scientists think this is an unreal expectation due to its lack of spectacular success. Cardiology could also benefit from gene therapy. Supplementation of genes for molecules such as SERCA2a, SUMO1 (S100A1, a calcium sensor), and PP1 inhibitor-1 (an upstream regulator of SERCA2a via inhibition of PP 1) could be the future of modern cardiology. Other ways to treat CV insufficiency include upregulating the β-adrenergic receptor (by stopping GRK2 from using the genes of its inhibitors)Citation47 and overexpressing microRNAs or other types of RNA to regulate gene expression and prevent hypertrophy and fibrosis.Citation48,Citation49 The regulation of calcium levels inside cardiomyocytes is often imbalanced in the heart of a person with CV insufficiency, therefore proteins that regulate calcium levels are the main targets of gene therapy. The use of SERCA2a, which transfers Ca2+ from the cytosol to the lumen of the sarcoplasmic reticulum, in gene therapy bodes very well. Supplying a cell with genes for SERCA2a restores the intracellular calcium concentration in different phases of myocardial activity (). A 12-month observational study concluded that a high-dose treatment significantly decreases the risk of CV events (HR =0.12; P=0.003) and reduces the average time of hospitalization 11-fold vs a placebo (P=0.05).Citation50 An additional 3-year experiment proved that this gene therapy is safe and reduces the risk of CV events.Citation51
Figure 4 Mechanism of action of SERCA2a.(/p)(/p)Note: This ATPase pump transfers calcium ions from the sarcolemma to the sarcoplasmic reticulum.
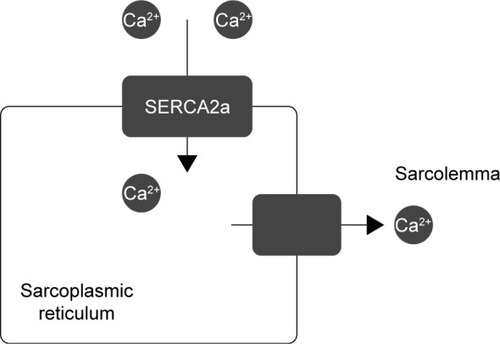
A percutaneous technique was used to insert genes into patients’ hearts; however, the main disadvantage of this method is that it is invasive. AAV1, AAV6, and AAV9 can be used as vectors in gene therapy;Citation52–Citation54 however, further trials must be conducted to determine the best way to transfer DNA to cardiomyocytes.
The two main problems of gene therapy for CVDs include: 1) the reaction of the patient’s immune system, which could prevent AAV penetration, and 2) worsening of the therapy’s effect over a prolonged period of time. These problems probably led to the results being far below the expectations of more optimistic researchers. Despite good results in the first stages of the study, CUPID 2 did not reach an endpoint with a substantial improvement in patient conditions.Citation55
This last result should not discourage researchers, as vector modifications and technical improvements may lead to more refined therapies in the near future. Overcoming the present-day problems of gene therapy would be a great milestone, not only for cardiology but for medicine as a whole. Increasing numbers of gene therapy tests, not only in cardiology, is a good sign that high hopes are fully justified.
Conclusion
CVD is undoubtedly one of the biggest challenges for modern medicine. Increasing numbers of people suffering from CVD requires scientists to develop new therapeutic methods. Increasing knowledge of the pathological mechanisms of heart disease means that it is possible to employ therapies that take advantage of new pharmaceutical targets. The continuous progress of physiology and cardiology increases the chances of finding a highly successful therapy for the treatment of HF.
The outcomes of the aforementioned therapeutic methods are promising (the latest clinical trials summarized in ); however, all require further research before they become efficacious therapeutic options. It should be mentioned that patients enrolled in clinical trials were treated not only with clinical trial medication, but also received standard HF treatment.
Table 2 A summary of the latest clinical trials with respect to their outcomes and observed adverse effects
Acknowledgments
The authors would like to thank Mikołaj Kozłowski for the figures, which helped greatly to improve the quality of the paper.
Disclosure
The authors report no conflicts of interest in this work.
References
- BraunwaldEResearch advances in heart failure: a compendiumCirc Res2013113663364523888056
- BenjaminEJBlahaMJChiuveSEAmerican Heart Association Statistics Committee and Stroke Statistics Subcommittee, 2017. Heart Disease and Stroke Statistics-2017 Update: a report from the American Heart AssociationCirculation2017135e146e60328122885
- PonikowskiPVoorsAAAnkerSDESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESCEur Heart J2016201618891975
- GhoshRKBanerjeeKTummalaRBallSRavakhahKGuptaASerelaxin in acute heart failure: most recent update on clinical and preclinical evidenceCardiovasc Ther2017351556327727514
- AljundiAHSMohammedSFKPatelAInotropic agents use in patients hospitalized with acute decompensated heart failure: a retrospective analysis from a 22-year registry in a Middle-Eastern Country (1991–2013)BMC Cardiovasc Disord20161614726892533
- TariqSAronowWUse of inotropic agents in treatment of systolic heart failureInt J Mol Sci20151612290602906826690127
- GreenbergBNovel therapies for heart failure-where do they stand?Circ J18912016801882
- TeerlinkJRFelkerGMMcmurrayJJVAcute treatment with omecamtiv mecarbil to increase contractility in acute heart failure: The ATOMIC-AHF StudyJ Am Coll Cardiol201667121444145527012405
- Planelles-HerreroVJHartmanJJRobert-PaganinJMalikFIHoudusseAMechanistic and structural basis for activation of cardiac myosin force production by omecamtiv mecarbilNat Commun20178119028775348
- HashemSTibertiMForniliAAllosteric modulation of cardiac myosin dynamics by omecamtiv mecarbilPLoS Comput Biol20171311e100582629108014
- StarlingRCCardiac myosin activators for the treatment of heart failure stop now or push aheadJ Am Coll Cardiol201667121456145827012406
- GassanovNBiesenbachECaglayanENatriuretic peptides in therapy for decompensated heart failureEur J Clin Pharmacol201268322323021901345
- PotterLRAbbey-HoschSDickeyDMNatriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functionsEndocr Rev2006271477216291870
- AnkerSDPonikowskiPMitrovicVPeacockWFFilippatosGUlaritide for the treatment of acute decompensated heart failure: from preclinical to clinical studiesEur Heart J2015361271572325670819
- BestleMHOlsenNVChristensenPJensenBVBiePCardiovascular, endocrine, and renal effects of urodilatin in normal humansAm J Physiol19992763 Pt 2R684R69510070128
- SchmittMGunaruwanPPayneNEffects of exogenous and endogenous natriuretic peptides on forearm vascular function in chronic heart failureArterioscler Thromb Vasc Biol200424591191715001459
- SaxenhoferHRaselliAWeidmannPUrodilatin, a natriuretic factor from kidneys, can modify renal and cardiovascular function in menAm J Physiol19902595 Pt 2F832F8382146885
- KentschMLudwigDDrummerCGerzerRMüller-EschGHaemodynamic and renal effects of urodilatin in healthy volunteersEur J Clin Invest19922253193251317296
- KentschMLudwigDDrummerCGerzerRMüller-EschGHaemodynamic and renal effects of urodilatin bolus injections in patients with congestive heart failureEur J Clin Invest199222106626691333960
- MitrovicVLüssHNitscheKEffects of the renal natriuretic peptide urodilatin (ularitide) in patients with decompensated chronic heart failure: a double-blind, placebo-controlled, ascending-dose trialAm Heart J20051506123916338265
- MitrovicVSeferovicPMSimeunovicDHaemodynamic and clinical effects of ularitide in decompensated heart failureEur Heart J200627232823283217074775
- PackerMHolcombRAbrahamWTRationale for and design of the TRUE-AHF trial: the effects of ularitide on the short-term clinical course and long-term mortality of patients with acute heart failureEur J Heart Fail201719567368127862700
- PackerMO’ConnorCMcmurrayJJVEffect of ularitide on cardiovascular mortality in acute heart failureN Engl J Med2017376201956196428402745
- PackerMShort- and long-term effect of immediate vasodilator therapy in acute decompensated heart failurePresented at American Heart Association Scientific Session11132016New Orleans, LA
- BathgateRAHallsMLvan der WesthuizenETCallanderGEKocanMSummersRJRelaxin family peptides and their receptorsPhysiol Rev201393140548023303914
- SmithMCDanielsonLAConradKPDavisonJMInfluence of recombinant human relaxin on renal hemodynamics in healthy volunteersJ Am Soc Nephrol200617113192319717035617
- DschietzigTTeichmanSUnemoriEIntravenous recombinant human relaxin in compensated heart failure: a safety, tol-erability, and pharmacodynamic trialJ Card Fail200915318219019327619
- TeerlinkJRMetraMFelkerGMRelaxin for the treatment of patients with acute heart failure (Pre-RELAX-AHF): a multicentre, randomised, placebo-controlled, parallel-group, dose-finding phase IIb studyThe Lancet2009373967314291439
- TeerlinkJRCotterGDavisonBARELAXin in Acute Heart Failure (RELAX-AHF) InvestigatorsSerelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trialLancet2013381293923141816
- SatoNLamCSTeerlinkJREvaluating the efficacy, safety, and tolerability of serelaxin when added to standard therapy in asian patients with acute heart failure: design and rationale of RELAX-AHF-ASIA trialJ Card Fail2017231637127825893
- TeerlinkJRVoorsAAPonikowskiPSerelaxin in addition to standard therapy in acute heart failure: rationale and design of the RELAX-AHF-2 studyEur J Heart Fail201719680080928452195
- TeerlinkJSerelaxin in acute heart failure presented at Heart Failure 2017 and the 4th World Congress on Acute Heart FailureParis, France29 April, 2017
- InomataTIkedaYKidaKEffects of additive tolvaptan vs. Increased furosemide on heart failure with diuretic resistance and renal impairment – results from the K-STAR studyCirc J2018821159167
- PoseAAlmenarLGaviraJJBenefit of tolvaptan in the management of hyponatraemia in patients with diuretic-refractory congestive heart failure: the SEMI-SEC projectESC Heart Failure20174213013728451449
- VinodPKrishnappaVChauvinAMKhareARainaRCardiorenal syndrome: role of arginine vasopressin and vaptans in heart failureCardiol Res201783879528725324
- NomotoHSatohYKamiyamaMMechanisms of diuresis for acute decompensated heart failure by tolvaptanInt Heart J201758459360028701677
- KinugawaKSatoNInomataTEfficacy and safety of tolvaptan in heart failure patients with volume overload – an Interim result of post-marketing surveillance in JapanCirc J20147884485224670835
- SağSAydın KaderliAYıldızAUse of tolvaptan in patients hospitalized for worsening chronic heart failure with severe hyponatre-mia: The initial experience at a single-center in TurkeyTurk Kardiyol Dern Ars201745541542528694395
- UemuraYShibataRTakemotoKSafety and efficacy of long-term use of tolvaptan in patients with heart failure and chronic kidney diseaseCirc J201781111736173828883217
- AmbrosyAPKhanHUdelsonJEChanges in dyspnea status during hospitalization and postdischarge health-related quality of life in patients hospitalized for heart failure: findings from the EVEREST trialCirc Heart Fail201695e00245827140204
- LópezBGonzálezAQuerejetaRLarmanMRábagoGDíezJAssociation of cardiotrophin-1 with myocardial fibrosis in hypertensive patients with heart failureHypertension201463348348924366078
- CalabròPLimongelliGRieglerLNovel insights into the role of cardiotrophin-1 in cardiovascular diseasesJ Mol Cell Cardiol200946214214819059413
- Abdul-GhaniMSuenCJiangBCardiotrophin 1 stimulates beneficial myogenic and vascular remodeling of the heartCell Research201727101195121528785017
- RegisGPensaSBoselliDNovelliFPoliVUps and downs: the STAT1:STAT3 seesaw of Interferon and gp130 receptor signallingSemin Cell Dev Biol200819435135918620071
- ObanaMMaedaMTakedaKTherapeutic activation of signal transducer and activator of transcription 3 by interleukin-11 ameliorates cardiac fibrosis after myocardial infarctionCirculation2010121568469120100971
- Moreno-AliagaMJPérez-EcharriNMarcos-GómezBCardiotrophin-1 is a key regulator of glucose and lipid metabolismCell Metab201114224225321803294
- WilliamsMLHataJASchroderJTargeted beta-adrenergic receptor kinase (betaARK1) inhibition by gene transfer in failing human heartsCirculation2004109131590159315051637
- KarakikesIChaanineAHKangSTherapeutic cardiac-targeted delivery of miR-1 reverses pressure overload-induced cardiac hypertrophy and attenuates pathological remodelingJ Am Heart Assoc201322e00007823612897
- GanesanJRamanujamDSassiYMiR-378 controls cardiac hypertrophy by combined repression of mitogen-activated protein kinase pathway factorsCirculation2013127212097210623625957
- JessupMGreenbergBManciniDCalcium upregulation by per-cutaneous administration of gene therapy in cardiac disease (CUPID)Circulation2011124330431321709064
- ZseboKYaroshinskyARudyJJLong-Term Effects of AAV1/SERCA2a Gene Transfer in Patients With Severe Heart FailureCirculation Research2014114110110824065463
- BeeriRChaputMGuerreroJLGene delivery of sarcoplasmic reticulum calcium ATPase inhibits ventricular remodeling in ischemic mitral regurgitationCirc Heart Fail20103562763420634484
- FishKMLadageDKawaseYAAV9.I-1c Delivered via direct coronary infusion in a porcine model of heart failure improves contractility and mitigates adverse remodelingCirculation: Heart Failure20136231031723271792
- HammoudiNIshikawaKHajjarRJAdeno-associated virus-mediated gene therapy in cardiovascular diseaseCurrent Opinion in Cardiology201530322823425783685
- GreenbergBButlerJFelkerGMCalcium upregulation by percutaneous administration of gene therapy in patients with cardiac disease (CUPID 2): a randomised, multinational, double-blind, placebo-controlled, phase 2b trialThe Lancet20163871002411781186
