Abstract
Purpose
To estimate the high-risk human papillomavirus (HR-HPV) prevalence in a hospital-based population using the Cervista® and to determine the clinical value and significance of Cervista for cervical cancer screening in Fujian Province, China.
Patients and methods
In a hospital-based population, a total of 10,771 women from the Fujian Province were screened for cervical cancer and precancerous lesions using the thinprep cytologic test (TCT) and/or the Cervista. Women with HR-HPV infection and/or abnormal TCT were referred for colposcopy and biopsy. Pathological diagnosis was used as the gold standard.
Results
The overall HR-HPV prevalence was 16.57%. Among 10,229 cases, 976 had abnormal cytology results, of which, the HR-HPV positivity rate was 60.35% in this opportunistic screening population. The most common HR-HPV infection style was a simple infection. The most common species was A9 which was also the most prevalent species in all age. The women with CIN2+ (high-grade squamous intraepithelial lesion [HSIL]), especially cancer, were mostly concentrated in the age from 51 to 60 years old. The peak of CIN1 (low-grade squamous intraepithelial lesion, LSIL) prevalence was in the women aged 31–40. When using CIN1+, CIN2+ and CIN3+ as observed endpoints, the sensitivities were 86.07%, 92.73%, and 93.30% and negative likelihood ratio (NPV) were 99.15%, 99.75% and 99.83%, respectively. Cervista and TCT co-testing achieved the highest sensitivity and the lowest NLR.
Conclusion
The Cervista could be easily introduced in clinical practice in combination with TCT for cervical cancer screening in China. Patients with species A9 infection require a more actively clinical intervention.
Introduction
Cervical cancer is the third most frequent cancer among women globally, especially in developing countries. It is estimated that there were 528,000 newly diagnosed cases and 265,653 deaths in 2012 worldwide.Citation1 In China, the age-standardized incidence rate (ASIR) and mortality rate (ASMR) of cervical cancer were estimated at 10.3 and 2.6 per 100,000 women, respectively, in 2013; these rates are higher than those found in other developed countries.Citation2 Cervical cancer has become one of the major health hazards for women. Fujian Province is on the southern coast of China, and the burden of cervical cancer is heavy there. Despite the successful implementation of cytopathologic screening programs, Chen et al estimate that 98,900 women will be diagnosed with cervical cancer, and 30,500 women died from the disease in China during 2015.Citation3
In the beginning, HPV was used to triage abnormal cytology.Citation4 Subsequently, in 2011, the American Cancer Society (ACS), the American Society for Colposcopy and Cervical Pathology (ASCCP) and the American Society for Clinical Pathology (ASCP) stated that in most clinical settings, women aged 30–65 years should be screened with co-testing.Citation5 Recently, the Society of Gynecologic Oncology (SGO)/ASCCP guidelines propose to use HR-HPV testing alone as a primary screening way, which also recommend to use genotyping for HPV-16 and -18 to triage HR-HPV positive women.Citation6 The benefits of HPV testing in primary cervical cancer screening have been demonstrated in several randomized controlled trials.Citation7,Citation8 A plethora of evidence indicates that high-risk human papillomaviruses (HR-HPVs) are important factors in cervical cancer.Citation9,Citation10 At present, the detection methods for HPV include Hybrid Capture 2 (HC2, Qiagen, Gaithersburg, MD, USA), Cervista® (Hologic, Bedford, MA, USA), Aptima (Hologic), Cobas (Roche, Pleasanton, CA, USA) and others.Citation11 Early in 2009, HPV types were classified categorically as carcinogenic (Group 1), probably carcinogenic (Group 2A), possibly carcinogenic (Group 2B), not classifiable (Group 3), or probably not carcinogenic (Group 4) by the International Agency for Research on Cancer (IARC) Monograph Working Group. Group 1 contained 13 HPV types (type 51, 56, 66, 18, 39, 45, 59, 16, 31, 33, 35, 52 and 58) and Group 2A included 68 HPV types.Citation12 In March 2009, the Cervista HR-HPV assay, which includes three species of HR-HPV (14 HR-HPV types) that were all involved in Group 1 and Group 2A carcinogens, became the second HPV assay approved by the FDA as a triage test for women with atypical squamous cells of undetermined significance (ASC-US) and as an adjunctive test with cervical cytology for routine screening in women aged 30 years or older. This test was also approved in China by the China Food and Drug Administration (CFDA) at the end of 2011 and was clinically available in 2012. There is limited evidence in the literature on the clinical significance of Cervista, especially on its use in China. The Cervista HR-HPV test was a qualitative test for the detection of DNA from 14 HR-HPV types that were used in China for 5 years. We then presented one result regarding Cervista from Fujian Province Cervical Lesion Screening Cohorts (FCLSCs), involving several cervical screening cohorts that included more than 140,000 hospital-based and community-based patients who were tested by multiple HPV detection assays in Fujian Provincial Maternity and Children’s Health Hospital.
The purpose of this study was to estimate the HR-HPV prevalence within a hospital-based population using the Cervista high-risk human papillomavirus (HR-HPV) assay and to evaluate the clinical performance characteristics, including pathological diagnosis in different age stratification, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the Cervista HR-HPV test for the detection of CIN2 or worse (CIN2+), and CIN3 or worse (CIN3+) cervical lesions in women in Fujian Province, China.
Patients and methods
Study population
All specimens were collected using plastic cervical swabs from women visiting Fujian Provincial Maternity and Children’s Health Hospital. The study was approved by the Ethics Committee of Fujian Provincial Maternity and Children’s Hospital, Affiliated hospital of Fujian Medical University. All the participants were written informed consent, and that this study was conducted in accordance with the Declaration of Helsinki. The purpose was to estimate the overall HPV prevalence, species, the extent of multiple infections, and clinical significance in the detection of CIN and cervical invasive cancer. The population eligible for this study included 10,771 women, a total of 1,023 were excluded due to pregnancy (n=35), age under 21 (n=32), miss or invalid cytology test (n=395), history of histological diagnosis and treatment of CIN (n=51), lack of consistent follow-up with Cervista or other cytology test (n=29) and miss pathological results or reject biopsy (n=481). The women received HPV screening by gynecological practitioners between March 2012 and December 2016. The population consisted of hospital staff, policewomen, teachers, workers, civil servants, and retirees. The participants were required to fulfill the following criteria: 1) sexually active women aged 21 years or older, 2) no previous histological diagnosis and treatment of gynecological diseases, 3) willingness to undergo HPV testing. All patients provided informed consent.
Cervical specimen collection
Cervical cells were obtained from the cervix of all the women. Ecto- and endocervical specimens were collected by Cervex broom in two separate vials of PreservCyt® Liquid (Hologic). Subsequently, each sample was processed for cytology and HR-HPV assays, respectively.
ThinPrep liquid-based cytology
A slide for the cytologic study was made. The cervical cytologic examination was performed using the automated imaging system (Hologic, Inc., San Diego, CA, USA) and was reviewed by two experienced cytotechnologists and cytopathologists. The results were reported following the Bethesda 2001 system.Citation13 Samples were classified as: negative for intraepithelial lesion or malignancy (NILM), atypical squamous cells of undetermined significance (ASC-US), low-grade squamous intraepithelial lesion (LSIL), atypical squamous cells, not possible to exclude high-grade squamous intraepithelial lesion (ASC-H), high-grade squamous intra-epithelial lesion (HSIL), squamous cervical cancer (SCC), atypical glandular cells (AGC), and adenocarcinoma in situ (AIS). If the diagnosis differed between two cytopathologists, the cervical samples were reviewed again and a consensus diagnosis was obtained.
Cervista HR-HPV test
This is a qualitative test detecting 14 HR-HPV types. The assay uses three separate oligonucleotide mixtures: mix 1 (species A5/A6) contains probes for HPV 51, 56 and 66; mix 2 (species A7) contains probes for HPV 18, 39, 45, 59 and 68, and mix 3 (species A9) contains probes for HPV 16, 31, 33, 35, 52 and 58. In these three mixes, oligonucleotides for the human histone 2 gene (HIST2H2BE) are also present as an internal control for the presence of sufficient genomic DNA. Then, Invader Chemistry (Hologic, Inc.) was applied, consisting of two simultaneous isothermal and signal-amplification reactions to detect specific nucleic acid sequences.Citation14
Histology
The women who were HPV-positive and/or had abnormal thinPrep liquid-based cytology (with a grade higher than ASC-US) were referred for colposcopy and biopsy. Specimens were collected from these women by punch biopsy or loop electrosurgical excision procedure cone biopsy (LEEP). Specimens were fixed in 10% formalin and were routinely processed for paraffin embedding. Then, 4 µm thick histological sections were cut and stained with hematoxylin and eosin using standard methods. Then, cervical biopsy specimens were histologically examined and classified according to the cervical intraepithelial neoplasia system. All samples with a primary histology result of CIN2+ were reviewed by an independent expert. In case of a discrepant review reading, a second histology review was performed. If two out of three diagnoses were identical, the result was considered final.
Statistical methods
The performance characteristics of the screening tests were evaluated by calculating sensitivity, specificity, PPV, NPV, PLR, and NLR according to the standard definitions for CIN1, CIN2, CIN3, and invasive cervical cancer. All confidence intervals (CIs) were exact binomial confidence intervals. In addition, an analysis exploring test characteristics at different cut points for Cervista was undertaken by Fisher’s exact test. These results were then plotted. All data analyses were performed using SPSS 20.0 (IBM, Chicago, IL, USA).
Results
Prevalence of HR-HPV infection
In this study, 10,771 women underwent the Cervista HR-HPV test. A flowchart describing the selection of the study cohort with inclusion/exclusion criteria can be found in . The overall HR-HPV infection rate in this population was 16.57% (1,695/10,229, CI: 15.85%–17.31%) when excluding 1,023 cases who did not conform to the standard criteria or displayed invalid or missing information. The positivity of simple species was 14.30% (1,463/10,229), including 2.90% (297/10,229) of species A5/A6, 3.07% (314/10,229) of species A7 and 8.33% (852/10,229) of species A9. The positivity of multiple species was 2.27% (232/10,624), including double species positivity of 1.78% (182/10,229) and triple species positivity of 0.49% (50/10,229) (). The HR-HPV positive rate was 11.95% (1,106/9,253) in women with NILM and 60.35% (589/976) in women with abnormal cytological results. The HR-HPV prevalence was 45.69% (223/488) in patients diagnosed with ASC-US, 75% (15/20) in patients with ASC-H, 87.50% (182/208) in patients with LSIL, 97.12% (135/139) in patients with ≥ HSIL, and 27.73% (33/119) in patients with AGC. In the HR-HPV infected population, species A9 was detected in 1,064 cases, accounting for 62.78% (1,064/1,695) of all positive specimens (including mixed infection). ().
Figure 1 Flowchart of participants in the study.
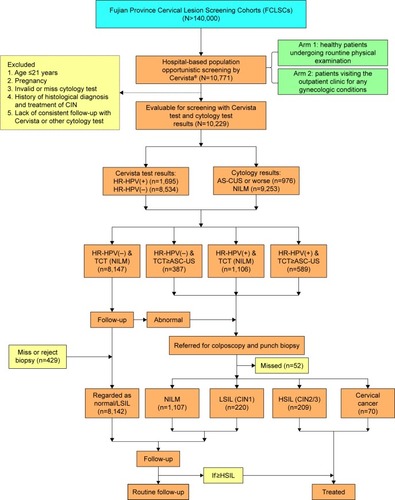
Figure 2 Prevalence of different HR-HPV infection styles and patients with different pathological results.
Abbreviations: CIN, cervical intraepithelial neoplasia; HR-HPV, high-risk human papillomavirus; NILM, negative for intraepithelial lesion or malignancy; SI, simple infection; DI, double infection; TI, triple infection.
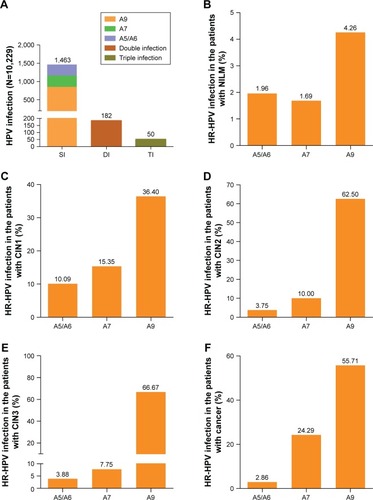
Table 1 The distribution of HR-HPV infection in different cytology results (N=10,229) (n (%))
Prevalence of cytological abnormality
The patients with abnormal cytology and/or HR-HPV infection were referred for colposcopy and biopsy. The prevalence of cytological results with diagnosis of ASC-US or worse was 9.54% (976/10,229, 95% CI: 8.98%–10.13%), including 45.90% ASC-US (448/976), 2.05% ASC-H (20/976), 21.31% LSIL (208/976), 14.24% ≥ HSIL (139/976), 12.19% AGC (119/976). In this study, there was 23.57% (70/297) abnormal cytology in the simple species A5/A6 positive, 30.57% (96/314) in the simple species A7 positive, 38.38% (327/852) in the simple species A9 positive. Simple A9 species was the most prevalent in the population with different cytology. The simple species A9 infection was the most prevalent with a rate of 47.47% (525/1,106) in patients with NILM, 54.26% (121/223) in patients with ASC-US, 80.00% (12/15) in patients with ASC-H, 45.05% (82/182) in patients with LSIL, 71.11% (96/135) in patients with ≥ HSIL and 45.45% (15/33) in patients with AGC, respectively. ().
Triage of HR-HPV in the patients with a different pathological diagnosis
In general, 8,147 patients were negative for both cytology and HR-HPV infection while 1,601 cases with HR-HPV positive and/or abnormal cytology were referred for colposcopy and biopsy whose pathological results was used as a final diagnosis standard. HR-HPV infection rate was 9.06% (837/9,242) in patients with cervicitis or NILM, 77.63% (177/228) in patients with CIN1, 91.25% (73/80) in patients with CIN2, 91.41% (117/128) in patients with CIN3 and 97.12% (68/70) in patients with cancer, respectively. According to the pathological results, simple species A9 infection rates in patients with CIN1, CIN2, CIN3, and cancer were 36.40% (83/228), 62.50% (50/80), 66.41% (85/128) and 55.71% (39/70), respectively. (). Overall, the detection rate of CIN2+ and CIN3+ lesions in the HPV-positive species was significantly higher than that of the HR-HPV-negative species (CIN2+=7,290.07, P<0.001; CIN3+=2,528.13, P<0.001). The detection rate of CIN2+ or CIN3+ lesions in the HR-HPV-positive species was significantly higher than that of the HR-HPV-negative species in patients with cervicitis or NILM, ASC-US and AGC. However, the detection rate of CIN2+ lesions in the HR-HPV-positive species was significantly higher than that of the HR-HPV-negative species in the patients with LSIL, however, the detection rate of CIN3+ lesions in HR-HPV-positive species and HR-HPV-negative species showed no significant difference. Moreover, the detection rates of CIN2+ or CIN3+ lesions in the HR-HPV-positive species and HR-HPV-negative species in the patients with ASC-H and ≥ HISL were not significantly different ().
Table 2 Compare HR-HPV infection and cytology with pathological diagnosis in 9,748 patients
Age-specific proportions for HR-HPV infection and high-grade cervical lesion
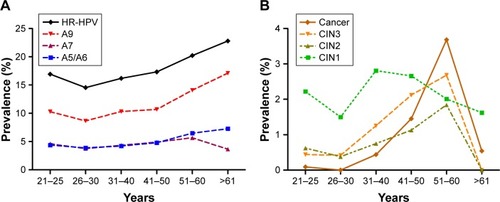
The HR-HPV infection rates in different age groups were 16.93% (21–25 years), 14.55% (26–30 years), 16.18% (31–40 years), 17.33% (41–50 years), 20.23% (51–60 years) and 22.80% (>61 years), respectively. The trend of A9 species and A5/A6 species infection rates was similar to the HR-HPV infection rate. But A7 species infection rate significantly dropped in the >61 years old group. Obviously, the prevalence of A9 species was the highest in the HR-HPV infection (). According to the pathological diagnosis, the women with CIN2+ (HSIL) were mostly concentrated in the age from 51 to 60 years old. Notably, the prevalence of cervical cancer was the highest in the patients aged 51–60. The peak of CIN1 (LSIL) prevalence was in the women aged 31–40 ().
Figure 3 Prevalence of HR-HPV infection and a cervical lesion in different age stratification.
Abbreviations: CIN, cervical intraepithelial neoplasia; HR-HPV, high-risk human papillomavirus.
Diagnostic efficiency of Cervista HR-HPV, thinprep cytologic test (TCT), and co-testing (TCT combined with Cervista HR-HPV) in cervical cancer
The sensitivity, specificity, PPV, NPV, PLR, and NLR were analyzed for different screening methods by HR-HPV, TCT, or co-testing (TCT+ HR-HPV) when used to predict CIN1+, CIN2+, and CIN3+ as critical endpoints (). The results show that the sensitivity of co-testing and HR-HPV was higher than that of TCT, especially in CIN2+ and CIN3+. With the increase in cervical lesions, the sensitivity of the HPV, TCT, and co-testing rose, but the specificity was reduced. The sensitivity and NPV were the highest and NLR was the lowest in the method of HR-HPV+ TCT sequential screening. Moreover, as listed in , the co-test as primary screening could detect 239 cases with CIN2+ at the baseline, 35 cases in the 1-year recall, and five cases in the follow-up. As for CIN3+, 174 cases were detected at the baseline, 23 cases in the 1-year recall, and two cases in the follow-up.
Figure 4 Combining Cervista® with TCT to detect CIN2+/CIN3+.
Abbreviations: CIN2+, cervical intraepithelial neoplasia 2 or worse; CIN3+, cervical intraepithelial neoplasia 3 or worse
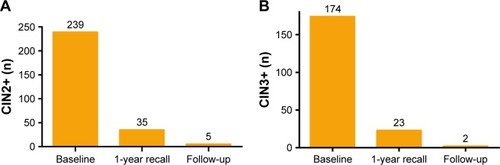
Table 3 Comparing cytology and HR-HPV with co-testing in different degree of the cervical lesion (N=9,748)
Discussion
A retrospective evaluation of cervical screening data from 56,501 specimens subjected to both cytologic examination and HR-HPV DNA testing suggested the rate of abnormal cytology with ASC-US or worse was 11.7% and the HR-HPV infection rate was 15.9% by Cervista HR-HPV assay in USA.Citation14 In China, there are not sufficient data on the national infection rates of HR-HPV. Based on opportunistic screenings in a population of 19,018 women in Beijing, the rate of abnormal cytology was 6.48% and the rate of HPV infection was 19.1% by high-risk HPV genotyping real-time PCR kit (Shanghai ZJ Bio-Tech Co., Ltd., Shanghai, China).Citation15 A multicenter study including 30,207 women from 17 population-based studies throughout China reported an HR-HPV infection rate of 17.7% and age-standardized HR-HPV prevalence was 16.8% by HC2 (Qiagen) in 2012.Citation16 In our study, based on a local center hospital population, the HR-HPV overall infection rate was 16.57% when detected by Cervista, and the rate of abnormal cervical cytology was 9.54%, consistent with the data from single-center or multicenter studies in China.
According to the IARC Monograph Working Group, the HR-HPV gene types of species A7 and A9 were included in Group 1 and Group 2A.Citation12 Most studies indicated women who were HR-HPV negative were at lower risk of CIN3, cancer, and cancer death over a 10-year period.Citation17,Citation18 There is profound importance in detecting the species A7 and A9 in China, in which cervical cancer screening has not yet been universally adopted. In this study, the most common style of HR-HPV infection was simple HR-HPV species, among which the most common species was A9 followed by A7. Moreover, women with abnormal cytology were mostly infected with species A9 or A7, especially in ≥ HSIL. Previously, our team reported the most common HR-HPV types were HPV-16, -52, -58, -18, -53, -33, and -51, ranked from highest to lowest, and HPV-16, -18, -58, -59, and -33 were the top five prevalent genotypes in cervical cancer; however, HPV-16, -18, -59, -45, and -33 were the top five highest risk factors for cancer in Fujian Province.Citation19 The risk of species A9 has been confirmed in many studies and Cervista A9 even replaced the full Cervista assay as the primary screening modality.Citation20 Our recent research also showed Cervista A9 was a good primary screening strategy.Citation21 This study also suggested that simple species A9 infection was overwhelming in women with CIN2, CIN3 and cancer, and all the infection rates were higher than 50%. Therefore, species A9 could be used to triage patients who were HPV-positive but who without substantial abnormal cytology. The benefits are not only that patients could obtain better precise screening and avoid overtreatment but also that the psychological burden regarding cervical cancer could be reduced sharply.
When squamous intraepithelial lesions of cervix occurred, the detection of CIN2+ by TCT was 87.46% (244/279); in particular, the detection rate of CIN2+ was 10.71% (44/411) in patients with ASC-US, 55.56% (10/18) in patients with ASC-H, 24.73% (46/186) in patients with LSIL and 95.52% (128/134) in patients with ≥ HSIL. A similar trend was observed in the detection of CIN3+ by TCT. While the detection of CIN2+ was only 12.17% (14/115) in patients with AGC. Due to the only two patients with AIS detected by TCT, we did not calculate the percentage (). Thus, the diagnostic specificity of cytology aimed at high-grade squamous intraepithelial lesions of the cervix showed significant advantage but not in AGC lesions. Several studies suggested that abnormal cytological findings in the normal population can lead to an erroneous diagnosis of ASC-US, AGC, or even LSIL.Citation11,Citation22–Citation24 Therefore, co-testing showed particularly important in cervical cancer screen. Then, how effective was the clinical application of the Cervista test in China, especially in Fujian province?
As seen in , the detection rate of CIN2+ by HR-HPV was 92.83 (259/279); in particular, the detection rate of CIN2+ was 88.64% (39/44) in patients with ASC-US, 90.00% (9/10) in patients with ASC-H, 95.65% (44/46) in patients with LSIL, and 96.88% (124/128) in patients with ≥ HSIL in the squamous intraepithelial lesions of the cervix. Moreover, a similar trend was revealed for the detection of CIN3+ by HR-HPV. Nevertheless, the HR-HPV-positive women had a higher incidence of CIN2+ and CIN3+ cervical lesions than the HR-HPV-negative in the patients with NILM, ASC-US when the squamous intraepithelial lesions of the cervix happened. It was worth noting that the HR-HPV-positive women had a higher incidence of CIN2+ but not CIN3+ cervical lesions than the HR-HPV-negative in the patients with LSIL. However, the incidence of CIN2+ and CIN3+ cervical lesions between HR-HPV-positive and HR-HPV-negative women showed no significant difference when their cytology diagnoses were ≥ HSIL. This evidence reconfirmed that HR-HPV showed no large advantage when triaging high-grade cervical lesions and cytology could triage patients directly. This observation has also been confirmed in many other studies.Citation25–Citation28
Similarly, the detection rate of CIN2+ and CIN3+ was 85.71% (12/14) and 78.57% (11/14), respectively, in patients with AGC. The HR-HPV-positive women had a higher incidence of CIN2+ and CIN3+ cervical lesions than the HR-HPV-negative in the patients with AGC. Therefore, HR-HPV showed advantages compared with TCT when screening AGC of the cervix. On one hand, HR-HPV triaged patients with NILM or ASC effectively. On the other hand, patients with CIN2+ and CIN3+ cervical lesions when their cytology diagnosis was AGC could be distinguished by HR-HPV. Liu et alCitation29 collected a total of 169 paraffin-embedded specimens of cervical adenocarcinoma from nine hospitals in seven regions across China. They detected 14 types of HR-HPV in whole tissue sections (WTSs). HPV16 was the most common type, and the second most common were HPV18 and HPV52. The HPV positive rate was 50.8% and 66.7% for the simple infection and multiple infections, respectively, after laser capture microdissection (LCM). That was a better proof that HR-HPV could reliably identify AGC and cervical adenocarcinoma.
After age stratification, there were two peaks of HR-HPV prevalence in the women who were 21–25 years and >60 years, respectively. The result was consistent with the data of cervical cancer screening in Zhejiang Province.Citation30 While the prevalence of A7 and A5/A6 infection was similar. A9 species infection was the most popular. The prevalence of HSIL (CIN2+) was increasing with age. There was a same peak of HSIL (CIN2+) in the women aged 51–60 years, which was consistent with the time-dependent carcinogenesis of HPV. However, the peak of LSIL (CIN1) was in the population aged 31–40 years. But the prevalence of LSIL (CIN1) showed a downward trend with age after 40 years. Thereout, we need to pay more attention to the elderly women with A9 species infection in clinical.
Our study showed that the Cervista HR-HPV assay and cervical tissue pathology were positively correlated, indicating that the Cervista technology can accurately detect HR-HPV infection in cervical lesions. In particular, the species A9 and A7 HR-HPV infection would be more detected. The success of HR-HPV testing in clinical practice is largely dependent on its high sensitivity, NPV, and lowest NLR. Therefore, false-negative results should be particularly worrying. A combination of HPV detection and cytology can improve the sensitivity and the NPV of the screening for cervical cancer and CIN. In this study, the use of TCT cytology combined with the Cervista HR-HPV test was evaluated. When the detection endpoint was CIN1+, CIN2+ and CIN3+ lesions, the PPV and specificity of both tests combined were higher than either test alone. Boers et alCitation31 claimed that both the clinical sensitivity and specificity of the Cervista HPV HR test for high-risk human papillomavirus (HPV) detection were not inferior to those of the Hybrid Capture 2 (HC2) test. Moreover, we missed 14.34% (40/279) women with CIN2+ and 12.56% (25/199) women with CIN3+ in the first round co-testing. When 1-year recall, we missed 1.79% (5/279) women with CIN2+ and 1.01% (2/199) women with CIN3+. Thus, Cervista could be used in co-testing to screen cervical lesion.
There are limited data in the literature regarding the clinical use of Cervista in China. Our results showed that the Cervista HR-HPV test was sensitive, specificity, and reliable screening method. It not only could screen out the cervical lesion of squamous epithelium cell but also of gland cell. Gynecologists should take active clinical intervention when the patient infected with species A9.
Conclusion
The Cervista could be easily introduced in clinical practice in combination with TCT for cervical cancer screening in China. Patients with species A9 infection require a more actively clinical intervention.
Author contributions
Conceptualization: P Sun, X Mao. Methodology: P Sun, X Mao, G Ruan. Software: X Mao, B Dong, L Che.n Validation: P Sun, X Mao, G Ruan, S X.u Formal analysis: P Sun, X Mao, L Chen. Data curation: X Mao, G Ruan, B Dong, S Xu, F Lin. Writing – original draft: X Mao. Writing – review and editing: P Sun. Funding acquisition: P Sun, B Dong. All authors contributed toward data analysis, drafting and revising the paper, gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Acknowledgments
This work was supported by grants from the Fujian Provincial Health and Family Planning Commission Innovation Project (grant no. 2009-CXB-33) and the Natural Science Foundation of Fujian Province (grant no. 2017J01232).
Disclosure
The authors report no conflicts of interest in this work.
References
- FerlayJSoerjomataramIDikshitRCancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012Int J Cancer20151365e359e38625220842
- JiangXTangHChenTEpidemiology of gynecologic cancers in ChinaJ Gynecol Oncol2018291e729185265
- ChenWZhengRBaadePDCancer statistics in China, 2015CA Cancer J Clin201666211513226808342
- US Preventive Services Task ForceCurrySJKristAHScreening for cervical cancer: US Preventive Services Task Force Recommendation StatementJAMA2018320767468630140884
- ChelmowDCervical cancer screening and preventionAm Coll Obstet Gynecol2016128923925
- SaslowDSolomonDLawsonHWAmerican Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancerCA Cancer J Clin201262314717222422631
- RoncoGDillnerJElfströmKMEfficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trialsLancet2014383991652453224192252
- MeijerCJBerkhofJCastlePEGuidelines for human papillomavirus DNA test requirements for primary cervical cancer screening in women 30 years and olderInt J Cancer2009124351652018973271
- WalboomersJMJacobsMVManosMMHuman papillomavirus is a necessary cause of invasive cervical cancer worldwideJ Pathol19991891121910451482
- MeijerCJSnijdersPJCastlePEClinical utility of HPV genotypingGynecol Oncol20061031121716934860
- GuanPHowell-JonesRLiNHuman papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancerInt J Cancer2012131102349235922323075
- BouvardVBaanRStraifKA review of human carcinogens–Part B: biological agentsLancet Oncol200910432132219350698
- Diane SolomonDDKurmanRMoriartyAThe 2001 Bethesda SystemJAMA20022872114211911966386
- YouensKEHoslerGAWashingtonPJJeneveinEPMurphyKMClinical experience with the Cervista HPV HR assay: correlation of cytology and HPV status from 56,501 specimensJ Mol Diagn201113216016621354050
- LiYHuangKJiPLSongLLiuHTPlJCervical infection of oncogenic human papillomavirus (HPV) types in Beijing, ChinaBiomed Environ Sci2016291073474127927273
- ZhaoFHLewkowitzAKHuSYPrevalence of human papilloma-virus and cervical intraepithelial neoplasia in China: a pooled analysis of 17 population-based studiesInt J Cancer2012131122929293822488743
- Mulindi MwanahamuntuHHParhamGPHPV screening for cervical cancer in rural IndiaN Engl J Med20093613305306
- SchiffmanMWacholderSMark SchiffmanSWFrom India to the world−a better way to prevent cervical cancerN Engl J Med2009360141453145519339726
- SunPSongYRuanGClinical validation of the PCR-reverse dot blot human papillomavirus genotyping test in cervical lesions from Chinese women in the Fujian province: a hospital-based population studyJ Gynecol Oncol2017285e5028657218
- ZhaoJduHBelinsonJLEvaluation of The Cervista HPV A9 group in screening patients for cervical cancerJ Med Screen2016231384326466824
- RuanGSongYDongBCervical cancer screening using the Cervista high-risk human papillomavirus test: opportunistic screening of a hospital-based population in Fujian province, ChinaCancer Manag Res2018103227323530233239
- KennedyAWSalmieriSSWirthSLBiscottiCVTuasonLJTravarcaMJResults of the clinical evaluation of atypical glandular cells of undetermined significance (AGCUS) detected on cervical cytology screeningGynecol Oncol199663114188898161
- StolerMHSchiffmanMAtypical Squamous Cells of Undetermined Significance-Low-grade Squamous Intraepithelial Lesion Triage Study (ALTS) GroupInterobserver reproducibility of cervical cytologic and histologic interpretations: realistic estimates from the ASCUS-LSIL Triage StudyJAMA2001285111500150511255427
- ScheungraberCKleekampNSchneiderAManagement of low-grade squamous intraepithelial lesions of the uterine cervixBr J Cancer200490597597814997192
- TrachtJMDavisADFascianoDNEltoumIADiscrepant HPV/cytology co-testing results: Are there differences between cytology-negative versus HPV-negative cervical intraepithelial neoplasia?Cancer Cytopathol20171251079580528817235
- SideriMIgidbashianSBoveriSAge distribution of HPV genotypes in cervical intraepithelial neoplasiaGynecol Oncol2011121351051321396686
- LiZMZengLQPengXHAnalysis of clinical and pathological characteristics of high-risk HPV-negative carcinoma of the uterine cervixZhonghua Fu Chan Ke Za Zhi201651968368727671050
- LiuHFLiuMXuYLYlXAnalysis of high-risk HPV infection and cervical cytologic screening in HIV positive womenZhonghua Fu Chan Ke Za Zhi2016511073473827788739
- LiuBWuZNLiuXYDistribution of human papillomavirus (HPV) among HPV positive cervical adenocarcinoma cases detected by laser capture microdissection (LCM)Zhonghua Zhong Liu Za Zhi201638427728227087374
- WuQZhaoXFuYA cross-sectional study on HPV testing with type 16/18 genotyping for cervical cancer screening in 11,064 Chinese womenCancer Med2017651091110128378404
- BoersASlagter-MenkemaLvan HemelBMComparing the Cervista HPV HR test and Hybrid Capture 2 assay in a Dutch screening population: improved specificity of the Cervista HPV HR test by changing the cut-offPLoS One201497e10193025051098
