Abstract
Despite major advances in therapeutic interventions and the availability of detailed treatment guidelines, a high proportion of patients with symptomatic asthma remain uncontrolled. Asthma management is largely guided by the Global Initiative for Asthma (GINA) strategy and is based on a backbone of inhaled corticosteroid (ICS) therapy with the use of additional therapies to achieve disease control. Inhaled long-acting bronchodilators alone and in combination are the preferred add-on treatment options. Although long-acting muscarinic antagonists (LAMAs) are a relatively recent addition to disease management recommendations for asthma, tiotropium has been extensively studied in a large clinical trial program. In Europe and the United States, tiotropium is approved for patients aged ≥6 years and uncontrolled on medium- to high-dose ICS/long-acting β2-agonists at GINA Steps 4 and 5 with a history of exacerbations. Evidence supports the efficacy of tiotropium Respimat® in adults in terms of lung function and asthma control, with a safety profile comparable with that of placebo across a range of asthma severities. Similarly, clinical trials in patients aged 1–17 years have shown improvements in lung function and trends toward improved asthma control. Furthermore, its efficacy makes tiotropium relatively easy to incorporate into routine clinical practice, irrespective of allergic status and without the need for patient phenotyping. Tiotropium is a cost-effective treatment that may offer an important alternative to other, more expensive add-on therapies. This review discusses the potential future position of LAMAs in clinical practice by considering the continuously evolving evidence. Prominence is given to tiotropium, the only LAMA supported by a structured clinical trial program in asthma to date, while also considering other recommended treatment options for patients with uncontrolled asthma. The importance of effective patient/caregiver–clinician communication and shared decision-making in enhancing treatment adherence is also highlighted.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Despite major advances in therapeutic interventions, asthma remains a challenge globally.Citation1 The 2016 Global Burden of Disease update reported a global prevalence of asthma of 339 million people and estimated years lived with disability (YLD) as 13 million in 2016, with an increase of 3.6% in age-adjusted YLD rates in the last decade.Citation2 In a European survey, the prevalence of diagnosed asthma in adults across five countries was estimated at 5.8%; of these patients, ~50% were not well controlled.Citation3 In the United Kingdom, it is estimated that over 60% of adults with asthma being treated with at least an inhaled corticosteroid (ICS) combined with a long-acting β2-agonist (LABA) (Global Initiative for Asthma [GINA] Step 3) remain uncontrolled.Citation4 Similarly, poor levels of asthma control of over 50% are reported for children and adolescents.Citation5 Uncontrolled asthma confers a considerable impact on health care systems. Data from the REcognise Asthma and LInk to Symptoms and Experience survey of 8,000 European patients aged 18–50 years show that, of the 45% of patients with uncontrolled asthma, 24% had visited the emergency department and 12% had been hospitalized over the previous 12 months.Citation6 The frequency of exacerbations is a major risk factor for the development of chronic and progressive disease in children and adolescents.Citation7
The long-term goals of asthma management are symptom control, minimization of future risk of exacerbations, and reduction of airflow limitation while simultaneously minimizing treatment side effects.Citation8 There is a need for more treatment options to allow these goals to be achieved in patients who remain symptomatic with the highest therapy regimens.Citation8 This review discusses current recommendations and recent developments in asthma management; it also considers the role of anticholinergic therapy and the future position of long-acting muscarinic antagonists (LAMAs) in the management of asthma across all age groups, while taking into account other recommended treatment options for patients with uncontrolled asthma. The discussion will also include an overview of the evidence to date for the benefits of tiotropium as the most extensively studied LAMA in the management of asthma in adult and pediatric/adolescent patients and the potential impact of these findings for future clinical practice.
Current treatment recommendations for asthma
The 2018 GINA reportCitation8 recommends a stepwise approach in the treatment of all patients with asthma, details of which are discussed elsewhere in this review series.Citation9,Citation10 Briefly, treatment algorithms are the same for adolescents and adults, recommending initial low-dose ICS with increasing ICS dose and/or other add-on treatments until control is achieved. Until recently, add-on maintenance therapies for these age groups included LABAs and leukotriene receptor antagonists (LTRA).Citation8 Therapeutic recommendations differ for children aged 6–11 years, with preference for the use of moderate-dose ICS (double the “low” daily dose over combination ICS/LABA at Step 3). If this strategy is ineffective, it is recommended that the child be referred for expert assessment and advice at further treatment steps.Citation8 In children aged <6 years, the preferred asthma control medication is low-dose ICS, such as 200 µg budesonide or equivalent, plus as-needed short-acting β2-agonists (SABAs) as reliever medication. However, ICS monotherapy often only confers partial control in children aged <6 years and, due to lack of data, treatment options for these patients are limited to doubling of low-dose ICS and addition of an LTRA, with specialist referral required if this strategy fails.Citation8
The recent label approval of tiotropium for use in patients aged ≥6 years with severe asthma in both EuropeCitation11 and the United StatesCitation12 reflects an awareness of the need for effective therapies for this younger population. However, there is still an important unmet clinical need for additional evidence-based second-line therapies in young children with uncontrolled symptomatic asthma.
Recent developments in asthma management
Asthma management continues to evolve as new treatment options are developed, approved, and incorporated into treatment recommendations. The GINA strategy currently includes the addition of the LAMA tiotropium as a Step 4–5 add-on treatment for patients aged ≥12 years with a history of exacerbations, before escalation to treatment with biologics or low-dose oral corticosteroids (OCS) (where applicable).Citation8 An extensive trial program, designed to establish the efficacy and safety of tiotropium Respimat® Soft Mist™ inhaler (Boehringer Ingelheim Pharma GmbH & Co. KG, Ingelheim am Rhein, Germany),Citation13–Citation19 has helped in the quest to address the unmet need of patients who remain symptomatic despite available therapies, resulting in the label approval in adult patients in Singapore (aged ≥18 years), Japan (aged ≥15 years), and, more recently, in Europe and the United States for patients aged ≥6 years.Citation11,Citation12 Given that tiotropium is a well-tolerated and efficacious add-on to at least medium-dose ICS plus one or more controller medications in clinical trials in children aged <12 years,Citation17,Citation20,Citation21 assessing its use for the treatment of patients aged <6 years, in whom alternative controller treatments are limited, could be extremely valuable due to the high unmet medical need in this age group. An increasing number of biologic therapies targeting exacerbations are emerging. Antibody-based therapies are available to treat patients with moderate-to-severe allergic asthma aged >6 years,Citation22 or for adolescentsCitation22 and adults,Citation23,Citation24 as add-on options at GINA Step 5. Omalizumab (Novartis Pharma Services AG, Basel, Switzerland) is a humanized monoclonal anti-immunoglobulin E (IgE) antibody initially approved in 2003 for the treatment of moderate-to-severe allergic asthma. By binding to the fragment region of free IgE, it prevents its binding and allergen-induced cross-linking of high-affinity receptors on effector cells.Citation22 More recently, the humanized anti- IL–5 monoclonal antibodies reslizumab (Teva UK Ltd, Harlow, UK), mepolizumab (GlaxoSmithKline UK, Uxbridge, Middlesex, UK), and benralizumab (AstraZeneca UK Ltd, Luton, Bedfordshire, UK) have been approved for the treatment of severe eosinophilic asthma in adults.Citation25–Citation27 Dupilumab (Regeneron Pharmaceuticals, Inc., Guildford, Surrey, UK),Citation28,Citation29 which targets the alpha chain of the IL-4 receptor, thereby inhibiting binding of both IL-4 and IL-13, has also demonstrated improved outcomes in adult patients with severe or moderate-to-severe asthma, including lung function, exacerbations, and symptoms.Citation30 Treatment with biologics requires either subcutaneous or, in some cases, intravenous injections by health care professionals at regular intervals, as IgE levels and eosinophil numbers can be rapidly restored.Citation31 Although these therapies have proven safety profiles, important limiting factors include the inconvenience caused to the patient due to the repeated visits for therapy and the personal financial cost of these visits. Their use also requires patient phenotyping and specific biomarker analysis to ensure the appropriate selection of patients who are most likely to benefit from the different biologic options.Citation32 This requires additional testing, thereby increasing the complexity of prescribing an already costly therapy. Inhaled therapies offer a more autonomous option for asthma management as well being more cost effective and should therefore be thoroughly explored before considering biologics or OCS as therapeutic options.
Further options for Step 4–5 add-on treatment
In adults with symptomatic severe asthma with poor control and/or frequent exacerbations despite good inhaler technique and treatment adherence, low-dose OCS may be considered.Citation8 However, OCS are frequently associated with substantial side effects after both frequent short-term and long-term use;Citation33,Citation34 this may account for the reluctance to prescribe OCS generally, not just in younger, symptomatic severe asthma patients.Citation35 Another recommended alternative for symptomatic severe adults is bronchial thermoplasty – a nonpharmacological intervention that claims to reduce the amount of smooth muscle in the airway walls, making it less likely that the airways will become narrow in the future.Citation8,Citation36 Although bronchial thermoplasty has shown some improvements mainly in reducing exacerbations in adults with severe asthma, more long-term evidence from well-controlled clinical trials is required to establish the procedure, particularly in severe asthma.Citation36
A subpopulation of adult patients with persistent symptomatic asthma who may benefit from treatment with macrolides has yet to be precisely defined.Citation37 Clinical trials with azithromycin found a decrease in exacerbations in patients treated compared with those prescribed placebo, as well as an improvement in asthma-related quality of life.Citation35 Macrolides could also be considered another potential treatment to achieve disease control in patients who remain poorly controlled despite optimal inhaled treatment and who do not qualify for biologics. In particular, the effects of long-term therapy with macrolides on community microbial resistance are, as yet, undetermined and remain a major concern.
Cholinergic activity in asthma pathophysiology
Understanding the role of cholinergic signaling in the airways is important to gain a clear overview of the significance of LAMAs in asthma management. The neurotransmitter acetylcholine (ACh) is released by the parasympathetic nervous system and regulates airway tone, smooth muscle contraction, and mucus secretion.Citation38 ACh exerts its effects primarily via interaction with muscarinic (M) receptors on the airway smooth muscle (ASM).Citation38 It is postulated that functional antagonism due to crosstalk between M receptors and β2-adrenoceptors may exert an influence on β2-agonist-induced muscle relaxation.Citation39 Thus, muscarinic antagonists may provide bronchodilation in a way that is both complementary and different to β2-agonists.
Anticholinergics, both short-acting and long-acting, have long been used in the management of respiratory disease, particularly chronic obstructive pulmonary disease (COPD). Tiotropium has been approved for use in COPD for over 10 years and has the most extensive research available in asthma for a LAMA. As such, its mechanism of action as a bronchodilator is well established in these respiratory diseases. In the airways, tiotropium binds competitively and reversibly to the M1 and M3 receptors on ASM,Citation40 inhibiting the action of ACh, thereby indirectly leading to smooth muscle relaxation.Citation40,Citation41 Compared with the short-acting muscarinic antagonist (SAMA) ipratropium, tiotropium dissociates much more slowly from the M1 and M3 receptors, making it a more potent bronchodilator.Citation42 Its >24-hour duration of action has been confirmed by several clinical studies in COPD.Citation42,Citation43
Clinical evidence with anticholinergics
Short-acting muscarinic antagonists
Ipratropium bromide is a SAMA that nonselectively blocks M1, M2, and M3 muscarinic receptors in the airwaysCitation44 and exerts a bronchodilator effect in patients with asthma.Citation45 Currently, ipratropium is recommended as an alternative reliever agent for adult patients who are unable to tolerate treatment with SABAs.Citation46 Significant improvements in bronchodilation after 4 weeks of treatment with ipratropium bromide/albuterol combination therapy compared with albuterol alone were observed.Citation37
Ipratropium is often used in conjunction with a SABA for the emergency treatment of exacerbations in adults and children; this approach has been associated with fewer hospitalizations and greater improvement in peak expiratory flow (PEF) and forced expiratory volume in 1 second (FEV1) compared with SABA alone.Citation8,Citation47,Citation48 For children experiencing a severe asthma exacerbation, triple therapy with ipratropium, albuterol, and an OCS led to significant improvement in lung function compared with standard therapy.Citation45
Ipratropium has been shown to be more effective in adults with nonatopic asthma and a longer duration of the disease.Citation49 However, Cochrane reviews of the effectiveness of SAMAs in adults and children reveal no advantages to justify their use as maintenance asthma treatments.Citation50,Citation51
Long-acting muscarinic antagonists
Tiotropium bromide
Data from several clinical trials in patients aged 1–75 years with asthma show that the efficacy of tiotropium is consistent in both adults and children, irrespective of disease severity and phenotype. The trials in the tiotropium clinical development program will be briefly summarized here but are discussed in greater detail in other articles in this supplement.
The efficacy and safety of tiotropium in asthma has been demonstrated in five adultCitation52–Citation56 and two pediatricCitation57,Citation58 Phase II studies comparing tiotropium with placebo. Tiotropium’s safety and safety profile (investigated at doses of 5 and 2.5 µg, delivered as two puffs once daily via the Respimat® device) vs placebo was further established with a series of four Phase III trials in adults with symptomatic mild,Citation57 moderate,Citation14,Citation59 or severeCitation13 asthma as add-on to ICS ± LABA (). Phase II trials in both adults and children were performed using different tiotropium dosages: 5, 2.5, and 1.25 µg, each delivered as two puffs once daily via the Respimat® device. Across Phase II and III trials, the results of the 2.5 and 5 µg doses were predominantly similar, and both were superior to placebo for most endpoints tested. Therefore, based on the Phase II and III trials mentioned above, the approved and recommended once-daily tiotropium Respimat® dose for asthma in adults and children 6 years and above is 2.5 µg in the United States and 5 µg in almost all other countries. Of note, the 5 µg dose was superior to the 2.5 µg dose for some endpoints in both the Phase II and III trials in children with moderate and severe asthma, without any differences regarding safety compared with placebo or the 2.5 µg dose. Both doses of tiotropium significantly improved lung function and asthma control. Results from three Phase III studies in children and two in adolescents with symptomatic moderate or severe asthma despite medium- or high-dose ICS also showed that tiotropium Respimat® resulted in a greater improvement in FEV1 within 3 hours postdose (FEV1(0–3h)) response at 12 and 24 weeks compared with placebo ().Citation15–Citation19 Furthermore, in some cases, patient-assessed asthma control and quality of life did not reach statistical significance or cross the threshold for minimal clinical important difference. In addition to data from company-sponsored clinical trials, data from an independent study by Peters et al support the use of tiotropium for the treatment of asthma in adult patients at GINA Step 3 and 4.Citation60 Their findings showed that the use of tiotropium was superior to doubling the ICS dose, with improvements in symptoms and lung function; tiotropium as add-on to medium-dose ICS was also shown to be noninferior to adding salmeterol.Citation60
Table 1 Key results of Phase III studies with 2.5 and 5 µg tiotropium in adult and pediatric patients with asthma
In our experience, some patients believe tiotropium is less effective than LABA therapy due to its slower onset of action. Lung function assessment, however, has demonstrated that tiotropium, as an add-on therapy to LABA, is as effective as size on lung function in terms of timing and effect.Citation53,Citation61 Patient compliance, as we have observed it, increases if patients are informed of the difference in onset of action.
Asthma is a complex heterogeneous disease that comprises different phenotypes and endotypes; as such, patients may respond differently to therapies. However, evidence from subgroup analyses of data from clinical studies in patients aged 18–75 years with symptomatic moderate or severe asthma have demonstrated that tiotropium is effective in asthma, irrespective of baseline characteristics and phenotypes.Citation62 Similarly, data from subgroup analyses of the two replicate MezzoTinA-asthma® studies have demonstrated that tiotropium’s efficacy is independent of baseline characteristics in adults with symptomatic moderate asthma.Citation63 Data from studies with tiotropium in children and adolescents with symptomatic moderate or severe asthma also support these findings, and demonstrate treatment efficacy irrespective of eosinophil numbers in peripheral blood or IgE serum levelsCitation64–Citation66 without the need to phenotype.
Preclinical studies also indicate that tiotropium may have other benefits, such as preventing airway remodelingCitation67 and reducing airway inflammation.Citation68,Citation69
Other long-acting muscarinic antagonists
To date, the tiotropium clinical trial program in asthma across all age groups is by far the most advanced of the studies evaluating LAMAs. A number of studies assessing the LAMAs umeclidinium bromide (GlaxoSmithKline plc, Middlesex, UK) and glycopyrronium in adults with symptomatic and uncontrolled asthma have been completed or are ongoing.Citation70–Citation75 Although both therapies are approved for COPD, neither has been approved for an asthma indication. A Phase II trial investigating the efficacy of umeclidinium monotherapy in adults with asthma showed no conclusive therapeutic benefit.Citation70,Citation71 However, a different study in asthma showed that it had potential as a combination therapy with ICS, demonstrating improvements in trough FEV1, morning PEF, and evening PEF.Citation71,Citation72 A Phase III study assessing the safety and efficacy of adding umeclidinium to ICS + LABA in adults with symptomatic uncontrolled asthma is also ongoing.Citation73 Results from a comparative study showed that both tiotropium and glycopyrronium provided clinically significant bronchoprotection against methacholine-induced bronchoconstriction for up to 96 hours, but at 168 hours, this effect was only significant with tiotropium.Citation74 A small crossover study investigating the potential utility of glycopyrronium in asthma showed that it significantly prolonged bronchoprotection and bronchodilation compared with ipratropium and placebo.Citation75 Another LAMA approved for COPD – aclidinium – is currently under investigation in an acute model of asthma.Citation76 Data from a murine model of asthma indicate that aclidinium reduces allergen-induced airway hyperresponsiveness and eosinophilic airway inflammation.Citation77 However, there are no clinical trials currently investigating the effect of aclidinium in patients with asthma.
Overall, studies involving patients with asthma investigating the effects of LAMAs other than tiotropium are few in adults and have yet to be commenced in adolescents or children. Therefore, there is limited information to allow conclusions to be drawn about the potential future use of umeclidinium bromide, glycopyrronium, or aclidinium in the management of asthma.
Future perspectives in asthma management
Asthma in adults
Prior to the approval of tiotropium for the treatment of asthma, alternative options for adults with severe asthma uncontrolled with ICS/LABA were mainly biologic therapies or OCS.Citation8 The use of biologics, while efficacious, requires specific biomarker analysis to phenotype patients,Citation32 and long-term use of OCS is associated with a high potential for side effects.Citation8,Citation33,Citation34 The large body of data demonstrating the efficacy of tiotropium in the treatment of symptomatic asthma regardless of baseline characteristics addresses a previously unmet need in uncontrolled asthma. Its administration without the need for patient phenotyping makes tiotropium relatively easy to incorporate into routine clinical practice. Of note, GINA strategy now recommends the use of tiotropium as preferred option in adults uncontrolled on ICS/LABA at Step 4 and 5, before the addition of anti-IgE, anti-IL-5, or OCS.Citation8
Tiotropium may also provide an alternative option to the use of LABAs in patients with uncontrolled asthma and is currently being investigated as such. In the large Gaining Optimal Asthma ControL study with over 3,000 patients aged 12 to <80 years with uncontrolled asthma, around one-third of participants were not able to gain sufficient control despite ICS/LABA treatment.Citation78 Additionally, studies in adults have shown that some patients may develop a tolerance to long-term use of LABAs,Citation79,Citation80 impairing their efficacy in bronchospasm prevention. There are currently limited treatment options for this group of patients, and evidence suggests that tiotropium has the potential to fill this gap.Citation81,Citation82 Findings from a Cochrane meta-analysis of four double-blind, double-dummy studies with ~2,000 adults, comparing the use of tiotropium vs LABA add-on to medium-dose ICS, show that differences between the two controller medications were small. Tiotropium showed slight benefits over LABA on some measures of lung function, whereas LABA showed benefit in terms of quality of life.Citation82 In a second trial, Kerstjens et al confirmed the aforementioned efficacy and safety of tiotropium add-on therapy compared with salmeterol.Citation14 Data from the Tiotropium Bromide as an Alternative to Increased Inhaled Glucocorticoid in Patients Inadequately Controlled on a Lower Dose of Inhaled Corticosteroid study in adults with inadequately controlled asthma also show that some patients respond to tiotropium but not salmeterol, and vice versa.Citation83 Furthermore, Peters et al have shown that tiotropium is noninferior to the LABA salmeterol in adults with asthma. In that study, the addition of tiotropium to ICS resulted in a greater improvement in FEV1 and PEF compared with doubling the ICS dose.Citation60 As the evidence increases, tiotropium may emerge as the key choice in adults for add-on to ICS/LABA, and in those unsuitable or intolerant to treatment with a LABA. The use of LABAs as monotherapy has been associated with increased rates of hospitalizationCitation84 and asthma-related deathsCitation85 and has been linked to the occurrence of adverse cardiac events such as tachycardia.Citation86
Pediatric asthma
The indication for tiotropium add-on treatment has recently been expanded to patients in the European Union aged ≥6 years with severe asthma who experienced ≥1 severe asthma exacerbation in the past year, and to patients in the United States aged ≥6 years with asthma.Citation11,Citation12 The approval of tiotropium has addressed an important unmet clinical need for children with uncontrolled asthma on ICS/LABA; these patients previously had very limited choices for add-on therapy, with less clear evidence for efficacy and safety, and have thus required specialist referral. Importantly, tiotropium has been demonstrated to be efficacious in pediatric asthma regardless of allergic subtype. Asthma in childhood is often associated with atopy – a condition that predisposes to production of IgE against specific allergensCitation87 – and studies have therefore been performed to determine whether baseline IgE levels or blood eosinophil counts will influence response to tiotropium. Analysis of pooled data from symptomatic moderate patients aged 6–17 years in the CanoTinA-asthma® and RubaTinA-asthma® trials has shown that tiotropium improves lung function irrespective of these markers of an allergic status.Citation64–Citation66 These results demonstrate the effectiveness of tiotropium in pediatric asthma, primarily on lung function, without the need for phenotyping according to IgE levels or blood eosinophil counts, again conferring time- and cost-related benefits in the incorporation of tiotropium into clinical practice. However, while there was a consistent pattern for improved lung function, some improvements at different ages with specific doses did not reach statistical significance. Therefore, further studies investigating the relationship between improvement in pulmonary function, decreased exacerbations and increased quality of life are warranted.
Further consideration should also be paid to the personalization and monitoring of response to step-up therapy in pediatric asthma that is uncontrolled with ICS monotherapy. Differential responses to step-up therapy with ICS, LABA, or LTRA in ICS-treated children aged 6–17 years with uncontrolled mild-to-moderate asthma were demonstrated in the Best Add-on Therapy Giving Effective Responses trial. In this randomized, double-blind, three-way crossover trial, LABA add-on was significantly more likely to provide the best response vs either increased ICS dose or LTRA as add-on.Citation88 Importantly, a differential response to the three step-up therapies occurred in 98% of patients, demonstrating that a single effective combination treatment for all patients does not currently exist in uncontrolled pediatric asthma. Findings from a meta-analysis of 18 randomized trials witĥ3,750 patients aged ≤18 years, comparing the use of LTRA and ICS alone or in combination in mild-to-moderate asthma, show that patients treated with ICS alone had fewer asthma exacerbations and better lung function and asthma control than with LTRA alone.Citation89 Add-on LTRA to ICS conferred no significant benefit in this analysis. Furthermore, data from a Cochrane meta-analysis of four randomized trials in almost 560 patients aged 6–18 years with mild-to-moderate asthma demonstrated no significant benefit of adding LTRA to ICS in the three trials that evaluated this treatment.Citation90 These data do not support LTRA as a Step 3 add-on in children and adolescents. Collectively, these findings, when considered with the adverse effects on linear growth associated with ICS use in these age groups,Citation91,Citation92 favor the use of add-on LABA to ICS as step-up therapy over add-on LTRA or increased ICS use. Tiotropium is an equally effective add-on to ICS and the only LAMA approved for use in children with asthma. It may provide an important alternative for pediatric patients, particularly those who have inadequate response to ICS + LABA. Thus, further responder analyses by age for different therapeutic options are required and should include tiotropium with ICS ± LABA as a triple therapy.
Tiotropium is a bronchodilator with no anti-inflammatory activities, and this may result in a limited effect size. The effect on future risk, such as exacerbation frequency and severity, was expectedly lower compared with the observed effect on disease control and, more importantly, lung function. Due to this mode of action, tiotropium is to be used as add-on to ICS and not as a single maintenance treatment. The addition of tiotropium to moderate- or high-dose ICS with or without LABA can improve lung function and enhance symptom control, and potentially aid in reducing future risk.
Inhaler considerations
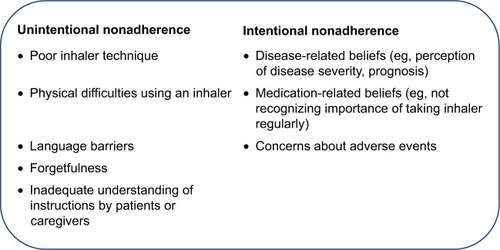
It is notable that, despite the availability of efficacious therapies and international treatment guidelines, achieving asthma control remains an elusive goal for a high proportion of patients of all ages worldwide.Citation93,Citation94 One reason for suboptimal asthma control is nonadherence to prescribed controller therapy,Citation95,Citation96 which could be either unintentional or intentional (). Unintentional reasons include poor inhaler technique, forgetfulness, or inadequate understanding of instructions by patients or caregivers.Citation97,Citation98 It has been shown that control worsens as the number of mistakes in technique increases.Citation99 Additionally, Hodder et al have reported that ease and convenience of use are some of the characteristics that affect patients’ perceptions of their inhaler devices.Citation100 Intentional reasons could be driven by factors such as disease- or medication-related beliefs, or concerns about adverse effects.Citation101 Here, effective patient/caregiver–clinician communication, as advocated by the GINA report, can play an important role.Citation8 Eliciting a patient’s or caregiver’s views on a treatment could help to highlight and guide discussion when addressing possible perceptual barriers, reasons for nonadherence, and practical barriers such as poor inhaler technique. Improved treatment adherence may also be conferred by shared decision-making regarding treatment. Findings from a randomized study by the Better Outcomes of Asthma Treatment group, assessing the effect of shared decision-making on treatment adherence and clinical outcomes in over 600 adults with poorly controlled asthma, demonstrate that conciliation of patient-preferred therapy choices significantly improves treatment adherence and clinical outcomes.Citation102
Another factor that may influence therapy choice is device preference. Currently available inhalers include metered-dose inhalers (MDIs), which can be pressurized (pMDI) or breath-actuated, and dry powder inhalers (DPIs), each of which requires a different pattern of inhalation for optimal drug delivery to the lungs.Citation98 MDIs are often used incorrectly,Citation99 with a study finding that between 28% and 68% of patients following the correct technique do not use them well enough to benefit from the medication.Citation103 Fink and RubinCitation103 and McFaddenCitation104 have also shown that many patients lack coordination for the split-second timing required between beginning a slow inhalation and activation of a pMDI. However, aerosols with a smaller particle size exiting at a low velocity may help to overcome problems with poor coordination.Citation105
Tiotropium Respimat® is approved for once-daily administration, most likely supporting adherence to a medication plan rather than a twice-daily regimen. The aerosol cloud generated by the Respimat® Soft Mist™ inhaler contains a higher fraction of fine particles that exit the inhaler more slowly and last for a longer time than most pMDIs or DPIs.Citation106,Citation107 These properties translate into higher lung deposition and may mean that drug delivery and efficacy may be improved with Respimat® in patients who have difficulties actuating and coordinating inhalation when using a pMDI.Citation107 Reported adherence to inhalers in children and adolescents can be as low as 50%, dropping to less than 30% in those aged 16–17 years.Citation108 Characteristics of the inhalers such as mechanism of action were one of the factors affecting adherence in adolescents.Citation108 Involving the adolescent patients in selecting their inhaler device has been suggested as a possible option to facilitate adherence. Findings from a patient questionnaire designed to examine preference for, and satisfaction with, inhaler devices in COPD and asthma showed that patients were more willing to continue to use Respimat® vs a hydrofluoroalkane pMDI. Respimat® also had significantly higher satisfaction ratings than other inhalers.Citation109 The inhaler device was easier to use, and inhalation of a single dose was shown to provide bronchodilator efficacy over a 24-hour period in patients with symptomatic asthma in a similar manner to twice-daily dosing.Citation56 The Soft Mist™ inhaler is suitable for use by all age groups, as demonstrated in the tiotropium clinical trial program. In addition, a handling study to assess the use of this inhaler in children aged ≤5 years concluded its suitability for this age group, although those younger than 5 years are advised to add a valved holding chamber to complement its use.Citation110
Shared decision-making in asthma
A survey of adult asthma patients in a large health maintenance organization reported that 30% of patients were dissatisfied with their current treatment. Dissatisfaction was significantly associated with poor asthma control, patient– provider communication problems, and the patients’ belief in their medication.Citation111,Citation112 For example, 16% of those who had good asthma control were dissatisfied compared with 70% of those who had poor control. Furthermore, the odds of treatment dissatisfaction were up to eight times higher among patients who reported uncertainty about the efficacy of their medication or their ability to take the medication as directed than those without these management problems. Of those with patient–provider issues, half (49.8%) were observed among those who reported that their physician did not involve them in treatment decisions. These findings further highlight the importance of patient/caregiver–clinician communication and shared decision-making in the treatment of asthma.
Cost-effectiveness
Uncontrolled asthma has a negative impact on health status and work productivity and is associated with increased health care costs.Citation113 Asthma costs directly increase with increasing lack of disease control. Between 80% and 90% of patients had uncontrolled asthma, as determined by The Epidemiology and Natural History of Asthma: Outcomes and Treatment Regimens study, and their costs were more than double those of patients with controlled asthma.Citation114 Furthermore, pediatric asthma confers a considerable socioeconomic burden in terms of increased school absences, reduced performance of patients, and missed workdays of caregivers.Citation5 Cost-effectiveness is an important aspect when considering new treatment options and their positioning in therapeutic management. Willson et al developed a Markov model to estimate the cost-effectiveness of tiotropium add-on therapy in patients with uncontrolled asthma despite treatment with ICS/LABA from the perspective of the UK National Health Service.Citation115,Citation116 Using data from two clinical trials in adult patients with severe asthma, the model considered asthma control and exacerbations, and analyzed cost and quality-adjusted life-years (QALYs).Citation115,Citation116 The results showed that tiotropium was cost-effective when added to usual care, generating an incremental 0.19 QALYs and £5,389 in costs over a lifetime horizon, resulting in an incremental cost-effectiveness ratio of £28,383 per QALY gained.Citation116 Other studies conducted in Poland and Portugal also confirmed the cost-effectiveness of tiotropium add-on therapy in treating adults with uncontrolled asthma.Citation117,Citation118 A more recent study compared the cost-effectiveness of tiotropium against another add-on therapy option, omalizumab, in patients with uncontrolled severe allergic asthma.Citation119 The authors reported lower total discounted costs with tiotropium compared with omalizumab, as well as lower expected values of model outcomes over 10 years in relation to number of nonsevere exacerbation weeks.Citation119 Further cost-effectiveness analyses, particularly with biologic therapies, will help to clarify the true cost-effectiveness of tiotropium add-on therapy. With the emergence of novel therapies for asthma that present exciting possibilities for achieving disease control, it is important to critically assess the cost-effectiveness of these options.
Conclusion
Asthma is a chronic disease that confers a considerable burden on individual patient quality of life, as well as on global health and socioeconomics. Great advances have been made in asthma treatment and management; however, there is still a population of patients who remain difficult to control. LAMAs as add-on therapy are proving effective in achieving asthma control in patients with asthma. The extensive study of tiotropium, as part of a structured program of large-scale trials, demonstrated its efficacy and safety in improving lung function as add-on therapy to ICS ± LABA in patients of all ages across a broad spectrum of severities. Tiotropium is cost-effective and, unlike biologics, has been shown to be efficacious in all asthma phenotypes, irrespective of patients’ baseline characteristics and allergic status. Therefore, in adults, adolescents, and children with severe asthma who experienced one or more exacerbations in the previous year, tiotropium is a suitable alternative add-on treatment to ICS, which should be considered ahead of interventions that require phenotyping. Tiotropium may also provide a much-needed alternative for children who are uncontrolled with ICS ± LABA and who would otherwise require higher ICS doses and specialist referral.
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
Medical writing assistance, in the form of the preparation and revision of the draft manuscript, was supported financially by Boehringer Ingelheim and provided by Martina Stagno d’Alcontres, PhD, of MediTech Media, under the authors’ conceptual direction and based on feedback from the authors. Boehringer Ingelheim was given the opportunity to review the manuscript for factual accuracy only. The authors would like to thank Kjeld Hansen, a member of the Patient Ambassador Group for the European Lung Foundation, for his input to the video summary for this manuscript.
Disclosure
The authors declare the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: RB has received grants and personal fees from Boehringer Ingelheim, GlaxoSmithKline, Novartis, and Roche, and personal fees from AstraZeneca, Chiesi, and Teva. EH declares that there is no conflict of interest regarding the publication of this article.
References
- World Health OrganizationAsthma fact sheet 307 Available from: http://www.who.int/mediacentre/factsheets/fs307/en/Accessed January 24, 2018
- Global Burden of Disease 2016 Disease and Injury Incidence and Prevalence CollaboratorsGlobal, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016Lancet2017390101001211125928919117
- DemolyPPaggiaroPPlazaVPrevalence of asthma control among adults in France, Germany, Italy, Spain and the UKEur Respir Rev20091811210511220956130
- PriceDMathiesonNMulgirigamaAP17 The burden of ICS/LABA-treated asthma patients in the UK adult populationThorax201368Suppl 3A82
- SchmierJKManjunathRHalpernMTJonesMLThompsonKDietteGBThe impact of inadequately controlled asthma in urban children on quality of life and productivityAnn Allergy Asthma Immunol200798324525117378255
- PriceDFletcherMvan der MolenTAsthma control and management in 8,000 European patients: the REcognise Asthma and LInk to Symptoms and Experience (REALISE) surveyNPJ Prim Care Respir Med20142411400924921985
- CustovicAJohnstonSLPavordIEAACI position statement on asthma exacerbations and severe asthmaAllergy201368121520153124410781
- Global Initiative for AsthmaGINA report: global strategy for asthma management and prevention Available from: http://ginasthma.org/2018-gina-report-global-strategy-for-asthma-management-and-prevention/Accessed October 22, 2018
- JenkinsCTiotropium in adults with asthma: current management strategiesTher Clin Risk Manag2018
- GoldsteinSClinical efficacy and safety of anticholinergic therapies in pediatric patientsTher Clin Risk Manag2018
- Boehringer IngelheimAsthma: Expanded indication for SPIRIVA® Respimat® for people 6 years and older Available from: https://www.boehringer-ingelheim.com/press-release/expanded-asthma-indication-spiriva-respimat-euAccessed October 22, 2018
- U.S. Food and Drug AdministrationPrescribing information for Spiriva® Respimat® (tiotropium bromide) inhalation spray, for oral inhalation Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021936s007lbl.pdfAccessed October 22, 2018
- KerstjensHAEngelMDahlRTiotropium in asthma poorly controlled with standard combination therapyN Engl J Med2012367131198120722938706
- KerstjensHACasaleTBBleeckerERTiotropium or salmeterol as add-on therapy to inhaled corticosteroids for patients with moderate symptomatic asthma: two replicate, double-blind, placebo-controlled, parallel-group, active-comparator, randomised trialsLancet Respir Med20153536737625682232
- HamelmannEBatemanEDVogelbergCTiotropium add-on therapy in adolescents with moderate asthma: a 1-year randomized controlled trialJ Allergy Clin Immunol2016138244145026960245
- HamelmannEBernsteinJAVandewalkerMA randomised controlled trial of tiotropium in adolescents with severe symptomatic asthmaEur Respir J2017491160110027811070
- VogelbergCEngelMLakiITiotropium add-on therapy improves lung function in children with symptomatic moderate asthmaJ Allergy Clin Immunol Pract2018662160216229751157
- SzeflerSJMurphyKHarperTA phase III randomized controlled trial of tiotropium add-on therapy in children with severe symptomatic asthmaJ Allergy Clin Immunol201714051277128728189771
- VrijlandtEEl AzziGVandewalkerMSafety and efficacy of tiotropium in children aged 1–5 years with persistent asthmatic symptoms: a randomised, double-blind, placebo-controlled trialLancet Respir Med20186212713729361462
- RodrigoGJNeffenHEfficacy and safety of tiotropium in school-age children with moderate-to-severe symptomatic asthma: a systematic reviewPediatr Allergy Immunol201728657357828692145
- VogelbergCMoroni-ZentgrafPLeonaviciute-KlimantavicieneMA randomised dose-ranging study of tiotropium Respimat® in children with symptomatic asthma despite inhaled corticosteroidsRespir Res20151612025851298
- U.S. Food and Drug AdministrationPrescribing information for XOLAIR® (omalizumab) Available from: https://www.gene.com/download/pdf/xolair_prescribing.pdfAccessed November 1, 2017
- PharmaTCINQAIR – Summary of Product Characteristics Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/761033lbl.pdfAccessed June 1, 2018
- GlaxoSmithKline UKNUCALA summary of product characteristics Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/761033lbl.pdfAccessed June 1, 2018
- OrtegaHGLiuMCPavordIDMepolizumab treatment in patients with severe eosinophilic asthmaN Engl J Med Overseas Ed20143711311981207
- BjermerLLemiereCMasperoJWeissSZangrilliJGerminaroMReslizumab for inadequately controlled asthma with elevated blood eosinophil levels: a randomized Phase 3 StudyChest2016150478979827056586
- AstraZeneca plcAstraZeneca receives EU approval of Fasenra for severe eosinophilic asthma Available from: https://www.astrazeneca.com/media-centre/press-releases/2018/astrazeneca-receives-eu-approval-of-fasenra-for-severe-eosinophilic-asthma-10012018.htmlAccessed June 1, 2018
- CastroMCorrenJPavordIDDupilumab efficacy and safety in moderate-to-severe uncontrolled asthmaN Engl J Med2018378262486249629782217
- RabeKFNairPBrusselleGEfficacy and safety of dupilumab in glucocorticoid-dependent severe asthmaN Engl J Med2018378262475248529782224
- WenzelSCastroMCorrenJDupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting β2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trialLancet201638810039314427130691
- CarusoMMorjariaJEmmaRAmaradioMDPolosaRBiologic agents for severe asthma patients: clinical perspectives and implicationsIntern Emerg Med201813215517629238905
- ChangABosséYTargeting single molecules in asthma benefits fewTrends Mol Med2016221193594527692867
- WalshLJWongCAOborneJAdverse effects of oral corticosteroids in relation to dose in patients with lung diseaseThorax200156427928411254818
- LefebvrePDuhMSLafeuilleMHAcute and chronic systemic corticosteroid-related complications in patients with severe asthmaJ Allergy Clin Immunol201513661488149526414880
- GibsonPGYangIAUphamJWEffect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trialLancet20173901009565966828687413
- WahidiMMKraftMBronchial thermoplasty for severe asthmaAm J Respir Crit Care Med2012185770971422077066
- DonohueJFWiseRBusseWWEfficacy and safety of ipratropium bromide/albuterol compared with albuterol in patients with moderate-to-severe asthma: a randomized controlled trialBMC Pulm Med20161616527130202
- CazzolaMOraJRoglianiPMateraMGRole of muscarinic antagonists in asthma therapyExpert Rev Respir Med201711323925328140686
- MeursHOenemaTAKistemakerLEGosensRA new perspective on muscarinic receptor antagonism in obstructive airways diseasesCurr Opin Pharmacol201313331632323643733
- Boehringer Ingelheim International GmbHSpiriva Respimat 2.5 microgram, solution for inhalation – Summary of Product Characteristics, Europe Available from: https://www.boehringer-ingelheim.com/products/prescription_medicines/respiratory/asthma/spiriva/public/images/14-3946-Blatter-SPC-Spiriva-ERS.pdfAccessed June 22, 2014
- HerediaJLTiotropium bromide: an updateOpen Respir Med J200931435219461900
- BarnesPJThe pharmacological properties of tiotropiumChest20001172 Suppl63S66S10673478
- KoumisTSamuelSTiotropium bromide: a new long-acting bronchodilator for the treatment of chronic obstructive pulmonary diseaseClin Ther200527437739215922812
- MoultonBCFryerADMuscarinic receptor antagonists, from folklore to pharmacology; finding drugs that actually work in asthma and COPDBr J Pharmacol20111631445221198547
- WardMJFentemPHSmithWHDaviesDIpratropium bromide in acute asthmaBr Med J (Clin Res Ed)19812826264598600
- LougheedMDLemièreCdellSDCanadian Thoracic Society Asthma Management Continuum – 2010 Consensus Summary for children six years of age and over, and adultsCan Respir J2010171152420186367
- GriffithsBDucharmeFMCombined inhaled anticholinergics and short-acting beta2-agonists for initial treatment of acute asthma in childrenCochrane Database Syst Rev2013218CD000060
- RodrigoGJCastro-RodriguezJAAnticholinergics in the treatment of children and adults with acute asthma: a systematic review with meta-analysisThorax200560974074616055613
- JolobeOMAsthma vs. non-specific reversible airflow obstruction: clinical features and responsiveness to anticholinergic drugsRespiration19844532372426235559
- McdonaldNJBaraAIAnticholinergic therapy for chronic asthma in children over two years of ageCochrane Database Syst Rev20033CD003535
- WestbyMBensonMGibsonPAnticholinergic agents for chronic asthma in adultsCochrane Database Syst Rev20043CD003269
- BeehKMMoroni-ZentgrafPAblingerOTiotropium Respimat® in asthma: a double-blind, randomised, dose-ranging study in adult patients with moderate asthmaRespir Res20141516124890738
- KerstjensHADisseBSchröder-BaboWTiotropium improves lung function in patients with severe uncontrolled asthma: a randomized controlled trialJ Allergy Clin Immunol2011128230831421636120
- BatemanEDKornmannOSchmidtPPivovarovaAEngelMFabbriLMTiotropium is noninferior to salmeterol in maintaining improved lung function in B16-Arg/Arg patients with asthmaJ Allergy Clin Immunol2011128231532221807250
- BeehKMKirstenAMDusserDPharmacodynamics and pharmacokinetics following once-daily and twice-daily dosing of Tiotropium Respimat® in asthma using standardized sample- contamination avoidanceJ Aerosol Med Pulm Drug Deliv201629540641526859538
- TimmerWMoroni-ZentgrafPCornelissenPUnseldAPizzichiniEBuhlROnce-daily tiotropium Respimat® 5 µg is an efficacious 24-h bronchodilator in adults with symptomatic asthmaRespir Med2015109332933825661281
- PaggiaroPHalpinDMBuhlRThe effect of tiotropium in symptomatic asthma despite low- to medium-dose inhaled corticosteroids: a randomized controlled trialJ Allergy Clin Immunol Pract20164110411326563670
- VogelbergCEngelMMoroni-ZentgrafPTiotropium in asthmatic adolescents symptomatic despite inhaled corticosteroids: a randomised dose-ranging studyRespir Med201410891268127625081651
- OhtaKIchinoseMTohdaYLong-term once-daily tiotropium Respimat® is well tolerated and maintains efficacy over 52 weeks in patients with symptomatic asthma in Japan: a randomised, placebo-controlled studyPLoS One2015104e012410925894430
- PetersSPKunselmanSJIcitovicNTiotropium bromide step-up therapy for adults with uncontrolled asthmaN Engl J Med2010363181715172620979471
- SharmaASchmidMRappBMoroni-ZentgrafPEngelMPharmacokinetics of tiotropium in asthma patients from three paediatric clinical trialsEur Respir J201648PA316
- KerstjensHAMoroni-ZentgrafPTashkinDPTiotropium improves lung function, exacerbation rate, and asthma control, independent of baseline characteristics including age, degree of airway obstruction, and allergic statusRespir Med201611719820627492532
- CasaleTBBatemanEDAalbersROnce-daily tiotropium Respimat add-on therapy improves lung function and asthma control in moderate symptomatic asthma, independent of baseline characteristicsEur Respir J201750PA647
- VandewalkerMVogelbergCHamelmannETiotropium Respimat® add-on therapy improves lung function in adolescents and children with moderate symptomatic asthma, irrespective of IgE levels and eosinophil countPoster P521 presented at: The American Thoracic Society International ConferenceMay 19–24, 2016Washington, DC
- GoldsteinSVogelbergCHamelmannETiotropium Respimat® add-on therapy is effective in children and adolescents with severe symptomatic asthma, irrespective of immunoglobulin E levels and eosinophil countPoster P520 presented at: The American Thoracic Society International ConferenceMay 19–24, 2016Washington, DC
- HamelmannEVogelbergCVoelkerBTiotropium add-on therapy improves lung function in children and adolescents with moderate and severe symptomatic asthma, independent of markers of allergic statusAllergy201772S1030659
- KangJYRheeCKKimJSEffect of tiotropium bromide on airway remodeling in a chronic asthma modelAnn Allergy Asthma Immunol20121091293522727154
- OhtaSOdaNYokoeTEffect of tiotropium bromide on airway inflammation and remodelling in a mouse model of asthmaClin Exp Allergy20104081266127520337647
- BosnjakBTilpCTomsicCTiotropium bromide inhibits relapsing allergic asthma in BALB/c micePulm Pharmacol Ther2014271445124090641
- LeeLABriggsAEdwardsLDYangSPascoeSA randomized, three-period crossover study of umeclidinium as monotherapy in adult patients with asthmaRespir Med20151091637325464907
- YangSGoyalNBeeraheeMTrivediRLeeLPascoeSDose- response modelling of umeclidinium and fluticasone furoate/umeclidinium in asthmaEur J Clin Pharmacol20157191051105826174114
- LeeLAYangSKerwinETrivediREdwardsLDPascoeSThe effect of fluticasone furoate/umeclidinium in adult patients with asthma: a randomized, dose-ranging studyRespir Med20151091546225452139
- ClinicalTrials.gov (US)A phase III parallel group study, comparing the efficacy, safety and tolerability of the fixed dose combination (FDC) of fluticasone furoate+umeclidinium bromide+vilanterol (FF/UMEC/VI) with the FDC of FF/VI in subjects with inadequately controlled asthma (NCT02924688) Available from: https://clinicaltrials.gov/ct2/show/NCT02924688Accessed October 11, 2017
- BlaisCMDavisBECockcroftDWDuration of bronchoprotection of the long-acting muscarinic antagonists tiotropium & glycopyrronium against methacholine-induced bronchoconstriction in mild asthmaticsRespir Med20161189610127578477
- HanselTTNeighbourHErinEMGlycopyrrolate causes prolonged bronchoprotection and bronchodilatation in patients with asthmaChest200512841974197916236844
- AntoniuSAAclidinium bromide in experimental asthmaExpert Opin Investig Drugs2011206871873
- DameraGJiangMZhaoHAclidinium bromide abrogates allergen-induced hyperresponsiveness and reduces eosinophilia in murine model of airway inflammationEur J Pharmacol20106491–334935320868661
- BatemanEDBousheyHABousquetJCan guideline-defined asthma control be achieved? The Gaining Optimal Asthma Control studyAm J Respir Crit Care Med2004170883684415256389
- TanKSGroveAMcleanAGnosspeliusYHallIPLipworthBJSystemic corticosteriod rapidly reverses bronchodilator subsensitivity induced by formoterol in asthmatic patientsAm J Respir Crit Care Med1997156128359230722
- YatesDHSussmanHSShawMJBarnesPJChungKFRegular formoterol treatment in mild asthma. Effect on bronchial responsiveness during and after treatmentAm J Respir Crit Care Med19951524117011747551366
- EvansDJKewKMAndersonDEBoyterACLong-acting muscarinic antagonists (LAMA) added to inhaled corticosteroids (ICS) versus higher dose ICS for adults with asthmaCochrane Database Syst Rev2015217CD011437
- KewKMEvansDJAllisonDEBoyterACLong-acting muscarinic antagonists (LAMA) added to inhaled corticosteroids (ICS) versus addition of long-acting beta2-agonists (LABA) for adults with asthmaCochrane Database Syst Rev201526CD011438
- PetersSPBleeckerERKunselmanSJPredictors of response to tiotropium versus salmeterol in asthmatic adultsJ Allergy Clin Immunol201313251068107424084072
- BenschGBergerWEBlokhinBMOne-year efficacy and safety of inhaled formoterol dry powder in children with persistent asthmaAnn Allergy Asthma Immunol200289218019012197575
- NelsonHSWeissSTBleeckerERYanceySWDorinskyPMSMART Study GroupThe Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterolChest20061291152616424409
- National Institute for Health and Care Excellence2014Asthma: fluticasone furoate/vilanterol (Relvar Ellipta) combination inhaler Available from: https://www.nice.org.uk/advice/esnm34/chapter/Key-points-from-the-evidenceAccessed June 1, 2018
- HolgateSTBousquetJChungKFSummary of recommendations for the design of clinical trials and the registration of drugs used in the treatment of asthmaRespir Med200498647948715191031
- LemanskeRFMaugerDTSorknessCAStep-up therapy for children with uncontrolled asthma receiving inhaled corticosteroidsN Engl J Med Overseas Ed201036211975985
- Castro-RodriguezJARodrigoGJThe role of inhaled corticosteroids and montelukast in children with mild-moderate asthma: results of a systematic review with meta-analysisArch Dis Child201095536537019946008
- ChauhanBFBen SalahRDucharmeFMAddition of anti-leukotriene agents to inhaled corticosteroids in children with persistent asthmaCochrane Database Syst Rev2013210CD009585
- ZhangLPrietschSODucharmeFMInhaled corticosteroids in children with persistent asthma: effects on growthEvid Based Child Health20149482993025504972
- PruteanuAIChauhanBFZhangLPrietschSODucharmeFMInhaled corticosteroids in children with persistent asthma: dose-response effects on growthCochrane Database Syst Rev2014177CD009878
- RabeKFAdachiMLaiCKWorldwide severity and control of asthma in children and adults: the global asthma insights and reality surveysJ Allergy Clin Immunol20041141404715241342
- FitzgeraldJMBouletLPMcivorRAZimmermanSChapmanKRAsthma control in Canada remains suboptimal: the Reality of Asthma Control (TRAC) studyCan Respir J200613525325916896426
- SpectorSNoncompliance with asthma therapy – are there solutions?J Asthma200037538138810983615
- SuissaSErnstPBenayounSBaltzanMCaiBLow-dose inhaled corticosteroids and the prevention of death from asthmaN Engl J Med Overseas Ed20003435332336
- Inhaler Error Steering CommitteePriceDBosnic-AnticevichSInhaler competence in asthma: common errors, barriers to use and recommended solutionsRespir Med20131071374623098685
- HaughneyJPriceDKaplanAAchieving asthma control in practice: understanding the reasons for poor controlRespir Med2008102121681169318815019
- GiraudVRocheNMisuse of corticosteroid metered-dose inhaler is associated with decreased asthma stabilityEur Respir J200219224625111866004
- HodderRReesePRSlatonTAsthma patients prefer Respimat Soft Mist Inhaler to TurbuhalerInt J Chron Obstruct Pulmon Dis2009422523219554196
- GadkariASMchorneyCAUnintentional non-adherence to chronic prescription medications: how unintentional is it really?BMC Health Serv Res20121219822510235
- WilsonSRStrubPBuistASShared treatment decision making improves adherence and outcomes in poorly controlled asthmaAm J Respir Crit Care Med2010181656657720019345
- FinkJBRubinBKProblems with inhaler use: a call for improved clinician and patient educationRespir Care2005501013741375
- McfaddenERImproper patient techniques with metered dose inhalers: clinical consequences and solutions to misuseJ Allergy Clin Immunol19959622782837636071
- AndersonPUse of Respimat Soft Mist inhaler in COPD patientsInt J Chron Obstruct Pulmon Dis20061325125918046862
- Moroni-ZentgrafPImpact of patient needs on design and usage of an inhalation device in respiratory medicineRespir Drug Delivery Euro20131141152
- WatchelHKattenbeckKDunneSThe Respimat® development story: patient-centered innovationPulm Ther201731930
- de SimoniAHorneRFlemingLBushAGriffithsCWhat do adolescents with asthma really think about adherence to inhalers? Insights from a qualitative analysis of a UK online forumBMJ Open201776e015245
- SchürmannWSchmidtmannSMoroniPMasseyDQidanMRespimat Soft Mist inhaler versus hydrofluoroalkane metered dose inhaler: patient preference and satisfactionTreat Respir Med200541536115725050
- KaminWFrankMKattenbeckSMoroni-ZentgrafPWachtelHZielenSA Handling study to assess use of the Respimat(®) Soft Mist™ inhaler in children under 5 years oldJ Aerosol Med Pulm Drug Deliv201528537238125844687
- MarksonLEVollmerWMFittermanLInsight into patient dissatisfaction with asthma treatmentArch Intern Med2001161337938411176763
- SmallMAndersonPVickersAKaySFermerSImportance of inhaler-device satisfaction in asthma treatment: real-world observations of physician-observed compliance and clinical/patient-reported outcomesAdv Ther201128320221221331556
- AccordiniSCorsicoAGBraggionMThe cost of persistent asthma in Europe: an international population-based study in adultsInt Arch Allergy Immunol201316019310122948386
- ChippsBEZeigerRSBorishLKey findings and clinical implications from the epidemiology and natural history of asthma: outcomes and treatment regimens (TENOR) studyJ Allergy Clin Immunol20121302e10:332342
- WillsonJBatemanEDPavordILloydAKrivasiTEsserDCost effectiveness of tiotropium in patients with asthma poorly controlled on inhaled glucocorticosteroids and long-acting β-agonistsAppl Health Econ Health Policy201412444745924974107
- WillsonJBatemanEDPavordILloydAKrivasiTEsserDErratum to: cost effectiveness of tiotropium in patients with asthma poorly controlled on inhaled glucocorticosteroids and long-acting β-agonistsAppl Health Econ Health Policy201614111912526816028
- PawlikMWalczakJPieniazekIEconomic evaluation of tiotropium administrated through the Respimat inhaler as add-on therapy in patients with uncontrolled severe asthma in PolandValue Health2015187A502
- Silva MiguelLManaçasMPinheiroBEconomic evaluation of tiotropium for severe persistent asthma in PortugalValue Health2015187A502
- ZafariZSadatsafaviMMarkFitzGerald JCanadian Respiratory Research NetworkCost-effectiveness of tiotropium versus omalizumab for uncontrolled allergic asthma in USCost Eff Resour Alloc2018161329422778