Abstract
Background
This 3-year retrospective study compared the outcomes of bisphosphonate-pretreated denosumab therapy with or without vitamin D and calcium supplementation in postmenopausal osteoporosis (OP) patients with rheumatoid arthritis (RA).
Materials and methods
Fifty-eight patients under long-term denosumab treatment were divided into groups without (denosumab group; 31 cases) or with (combination group; 27 cases) vitamin D and calcium supplementation. The bone markers of BAP, TRACP-5b, and urinary NTX were measured at baseline and every year for 3 years. We also evaluated bone mineral density (BMD) of the lumbar 1–4 vertebrae (L-BMD) and bilateral total hips (H-BMD) at the same time points.
Results
There were no significant differences in the percent changes of serum albumin-corrected calcium between the groups. The percent change in TRACP-5b was significantly higher in the combination group at 2 years. Serum 25-hydroxyvitamin D status was persistently high during therapy in both groups, with significant percent increases over baseline at 2 and 6 months in both groups and at 24 months in the combination group. The percent increase from baseline of serum zinc was significantly higher at 3 years in the combination group over the denosumab group. L-BMD and H-BMD were significantly increased at every time point for 3 years vs pretreatment levels in both groups and were significantly higher in the combination group at all time points.
Conclusion
Compared with denosumab monotherapy, the combination group displayed significantly increased serum zinc, L-BMD, and H-BMD at 3 years in OP patients with RA. Thus, calcium and vitamin D supplementation may be beneficial to enhance BMD gains, but not necessarily 25-hydroxyvitamin D status, in patients with OP and RA under denosumab.
Introduction
Osteoporosis (OP) is a widespread condition in which diminished bone quality is caused by losses in bone microarchitecture and bone mass.Citation1,Citation2 Reduced bone strength in OP patients may lead to increased fracture risk, which constitutes the major cause of mortality and morbidity in OP.Citation3
Rheumatoid arthritis (RA) is an autoimmune disease with hallmark joint inflammation and destruction. OP is a major complication of RA,Citation4 with 15%–20% of RA patients having afflictions of the hip and spine.Citation5,Citation6 Inflammation plays key roles in RA activity, bone resorption, and OP progression.Citation7
Vitamin D figures prominently in bone formation and remodeling.Citation8,Citation9 Although maintaining adequate levels of serum vitamin D is necessary to protect against bone fracture,Citation10,Citation11 vitamin D deficiency is a worldwide health concern.Citation8 Tan et al very recently reported that reduced levels of 25-hydroxyvitamin D (25(OH)D) might indicate a high risk of secondary OP in RA patients.Citation12 However, the status and changes of 25(OH)D during osteoporotic treatment for OP with RA are largely unknown, with no data available on denosumab therapy with or without vitamin D and calcium supplementation.
Regarding OP treatment, bisphosphonates (BPs) and denosumab, a fully human monoclonal antibody against receptor activator of nuclear factor κB ligand, are common therapeutic agents. Although BP therapy is generally the standard of care for OP by inhibiting osteoclast activity,Citation13 denosumab is also considered useful for primary as well as secondary OP. Denosumab treatment for 10 years has been associated with low rates of adverse events, low fracture incidence, and sustained increases in bone mineral density (BMD).Citation14
We earlier demonstrated that calcium and vitamin D supplementation of denosumab represented a useful treatment option for OP with RA, imparting additive effects on increases in bilateral total hip BMD (H-BMD) over 12 months in the absence of fracture and hypocalcemia.Citation15 Thus, we have hypothesized that long-term denosumab treatment can also enhance BMD and bone turnover markers and prevent fracture in OP with RA, especially in cases of prolonged BP pretreatment.
This 3-year retrospective study compared the differences in outcomes with or without calcium and vitamin D supplementation in OP patients with RA under denosumab.
Materials and methods
The inclusion criteria of this 3-year retrospective investigation were OP patients with low (ie, less than −2.5 SD) H-BMD and/or lumbar 1–4 BMD (L-BMD) with RA. The exclusion criteria were chronic renal failure (estimated glomerular filtration rate <40 mL/min/1.73 m2), disorders of bone metabolism or diabetes mellitus that could affect OP, and fracture within 12 months before the study. OP was diagnosed based on the revised criteria established by the Japanese Society of Bone and Mineral Research.Citation16 RA was diagnosed and treated following the 2010 ACR/European League Against Rheumatism (EULAR) classification system.Citation17
We recruited 58 Japanese female OP patients with RA of low-to-moderate disease activity (2.6< disease activity score 28 [DAS28]-C-reactive protein [CRP] ≤5.1) at our hospital between 2014 and 2018 (). All subjects had taken BPs for at least 5 years prior to the start of this study. The patients were retrospectively divided into those with (combination group; 27 cases) or without (denosumab group; 31 cases) vitamin D and calcium supplementation and were matched according to age, gender, body mass index, BP pretreatment period, and RA duration and activity. Alendronate (ALN), risedronate, and minodronate had been prescribed in various regimens as long-term BP pretreatment. The effects of individual BP drugs were not addressed since they were routinely switched if deemed ineffective.
Table 1 Patient characteristics at baseline
Each patient received denosumab (60 mg, subcutaneously) once every 6 months in both groups. Subjects in the combination group took vitamin D and calcium supplementation tablets (precipitated calcium carbonate: 762.5 mg, cholecalciferol: 200 IU, magnesium carbonate: 59.2 mg) twice daily during denosumab administration. All treatments were substituted from BPs to denosumab prior to study commencement for baseline measurements.
All serologic testings were conducted just before denosumab (baseline) and at 1, 2, and 3 years of treatment from frozen samples by commercially available kits, including that for matrix metalloproteinase-3 (MMP-3) (Kyowa Pharma Chemicals, Toyama, Japan). We also examined changes in DAS28-CRP, simplified disease activity index (SDAI), and health assessment questionnaire disability index (HAQ-DI) to determine the RA status of all patients prior to the start of the study. All data were expressed as the mean ± standard error (SE).
Serum bone alkaline phosphatase (BAP) was examined as a bone formation marker using a chemiluminescent enzyme immunoassay. Serum tartrate-resistant acid phosphatase (TRACP)-5b and urinary N-terminal telopeptide of type I collagen (NTX) (Osteomark, Ostex International, Seattle, WA, USA) were used as markers of bone resorption using ELISA. Serum whole parathyroid hormone 1–84 (PTH) was measured by immunoradiometric assays. Serum 1,25(OH)2D3 was determined by immunoradiometric assays. Serum 25(OH)D was determined by solid-phase radioimmunoassays. Serum and urine were collected in a fasting state after omitting the first morning samples between 8:30 am and 11:00 am. Immunoassays were carried out by SRL, Inc. (Tokyo, Japan). Serum samples collected before and at 1, 2, and 3 years of denosumab treatment were maintained at −80°C until bone turnover marker assessment at the end of the study.
BMD was determined using a dual-energy X-ray absorption fan-beam bone densitometer (Lunar Prodigy; GE Healthcare Bio-Sciences Corp., Piscataway, NJ, USA) at the L1–4 levels of the posteroanterior spine and bilateral total hips before treatment commencement and at 1, 2, and 3 years.
Bone turnover markers, whole PTH, 1,25(OH)2D3, serum albumin-corrected calcium (Ca), phosphorus, zinc, iron, magnesium, and BMD were all measured at set time points for statistical comparisons. 25(OH)D were measured before treatment commencement and at 2, 6, 12, 18, 24, 30, and 36 months of therapy for statistical comparisons. Some factors, such as diet habits and ultraviolet exposure that could have influenced vitamin D status in both groups, were excluded during the study period. Differences in percent changes from baseline were calculated using Bonferroni correction with repeated ANOVA for multiple comparisons. Comparisons of markers between the groups were performed by Welch’s t-test.Citation18 The critical value for rejecting the null hypothesis was P<0.05. Data analyses were conducted with the BellCurve for Excel (Social Survey Research Information Co., Ltd., Japan).
This study was approved by the Institutional Ethical Review Board of Shinshu University School of Medicine, Japan, and conducted in adherence to the tenets of the Declaration of Helsinki (2014 revision). All participants provided written informed consent.
Results
Patient backgrounds were comparable between the denosumab and combination groups ().
Mean ± SE age was 68.7±1.2 years in the denosumab group and 68.3±1.7 years in the combination group. The enrolled patients had undergone pretreatment with BPs for a mean duration of 7.3±0.9 years in the denosumab group and 6.4±0.7 years in the combination group. The mean doses of methotrexate and prednisolone in the denosumab and combination groups were 7.5±0.7 and 4.7±0.2 mg/week and 6.8±0.6 and 5.0±0.0 mg/day, respectively ().
All 58 subjects attended all scheduled visits over the 3-year observational period ().
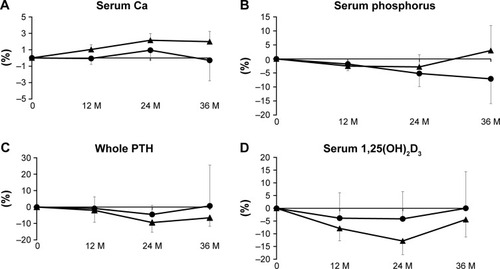
Ca and phosphorus levels
There were no significant differences in the percent changes of Ca between the groups (). No remarkable differences in percent change from baseline were seen for either group or between groups for serum phosphorous ().
Serum whole PTH and 1,25(OH)2D3
The percent change of serum whole PTH was seen to decrease and return to the baseline in denosumab patients and was comparable between the groups ().
The percent change of serum 1,25(OH)2D3 tended to be decreased at 1 and 2 years and return to baseline at 3 years in both groups in a similar manner ().
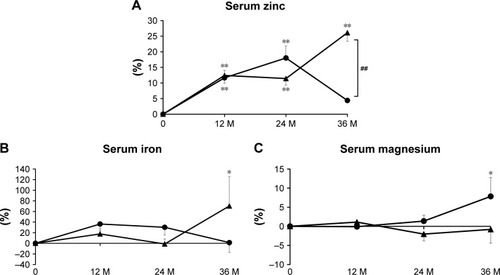
Serum bone-related minerals
The percent change of serum zinc was significantly greater in the combination group than in the denosumab group at 3 years, with significant differences at each time point in the combination group and at 1 and 2 years in the denosumab group vs baseline (). There were no remarkable differences in serum iron between the groups, although a significant increase over baseline was observed at 3 years in the combination group (). The percent change of serum magnesium was comparable between the groups, with a significant difference at 3 years in the denosumab group compared with baseline ().
Figure 2 Percent changes of serum zinc (A), serum iron (B), and serum magnesium (C) at 12, 24, and 36 months (M).
Bone resorption markers
The percent change of serum TRACP-5b was significantly lower than baseline for both groups during treatment. There was a significant difference at 2 years between the groups (). Regarding urinary NTX, no remarkable differences in percent change were seen with baseline for either group or between the groups ().
Figure 3 Percent changes of serum tartrate-resistant acid phosphatase (TRACP)-5b (A), urinary cross-linked N-terminal telopeptide of type I collagen (NTX) (B), serum bone alkaline phosphatase (BAP) (C), and serum 25-hydroxyvitamin D (25(OH)D) (D) at 12, 24, and 36 months (M). Circles show the denosumab group and triangles show the combination group.
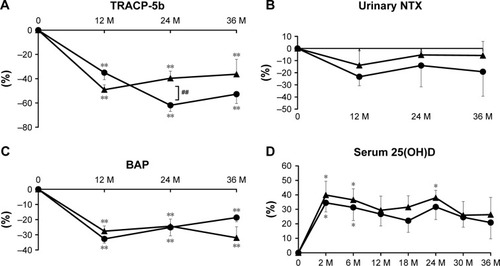
Bone formation marker
The percent change of serum BAP was comparably and significantly lower than baseline throughout the treatment period in both groups ().
Serum 25(OH)D
The percent change of serum 25(OH)D was persistently high and significantly greater than baseline at 2 and 6 months in both groups and at 24 months in the combination group. There were no notable differences between the groups ().
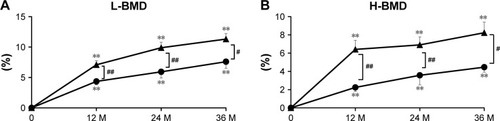
BMD
L-BMD and H-BMD
The percent change of L-BMD increased steadily during treatment in the denosumab (7.6% increase at 3 years) and combination (11.3% increase at 3 years) groups, with significant differences over baseline at every time point for both groups. L-BMD gains were significantly greater in the combination group at years 1, 2, and 3 (). Similarly, the percent change of H-BMD increased gradually in the denosumab (4.5% increase at 3 years) and combination (8.2% increase at 3 years) groups, with significant differences over baseline at every time point. The percent increase in H-BMD was significantly greater in the combination group at years 1, 2, and 3 ().
Figure 4 Percent changes in lumbar bone mineral density (L-BMD) (A) and bilateral total hip BMD (H-BMD) (B) at 12, 24, and 36 months (M).
Indicators of RA state
MMP-3, DAS28-CRP, SDAI, and HAQ-DI
Pretreatment RA state was comparable between the groups, with no remarkable differences in the values of MMP-3, DAS28-CRP, SDAI, or HAQ-DI prior to treatment commencement ().
Adverse events
No hypocalcemia, fracture, or other serious adverse events were seen during the 3-year study period.
Discussion
This study is the first to compare denosumab therapy for 3 years with or without vitamin D and calcium supplementation in patients with OP and RA. The status of serum 25(OH)D showed persistently high levels throughout the study period in both groups. L-BMD and H-BMD values increased significantly over 3 years in the groups, with those in the combination group rising significantly more. Since no fracture or hypocalcemia occurred in any patient, vitamin D and calcium addition appears to enhance BMD gains, but not necessarily 25(OH)D status, during long-term denosumab therapy for OP with RA.
There have been no reports describing serum 25(OH)D changes during 3 years of denosumab therapy with or without vitamin D and calcium supplementation in patients with OP and RA. Several investigations have addressed the correlation between serum 25(OH)D status and BMD.Citation19–Citation24 Roux et al described a positive association between 25(OH)D and L-BMD or femoral neck BMD in postmenopausal women treated with ALN.Citation19 In Malaysian RA female patients, serum 25(OH)D levels were low compared with those in healthy controls, with no associations between 25(OH)D and BMD,Citation20 while a negative relationship was identified between 25(OH)D levels and BMD in both sexes in a healthy Iranian population.Citation21 In Palestinian postmenopausal OP patients, a positive correlation was reported between 25(OH)D and L-BMD.Citation22 It was noteworthy that all of the OP patients in those studies had taken vitamin D supplementation.Citation19–Citation22 We previously found that serum 25(OH)D levels were significantly increased after 3-year BP therapy without additional vitamin D in Japanese postmenopausal OP patients.Citation23 On the other hand, serum 25(OH) D had decreased significantly after 4 months of BPs in a Japanese postmenopausal OP cohort.Citation24 Thus, it is conceivable that vitamin D addition may be optional in prolonged BP therapy. In the present denosumab study, serum 25(OH) D was persistently increased during 3 years regardless of vitamin D and calcium supplementation, with significant gains in both L-BMD and H-BMD. On the other hand, Augoulea et al reported that an increase in PTH caused by denosumab therapy was not associated with serum 25(OH) D levels.Citation25 Serum 1,25(OH)2D3 levels are strictly regulated by elevations in PTHCitation26 that are initiated by transient changes in calcium levels as reported previously,Citation27 and there is a negative correlation between 25(OH)D and PTH levels in Brazilians.Citation28 Also, Wintermeyer et al have reviewed and reported that 25(OH)D is metabolized to 1,25(OH)2D3 (calcitriol), which is the biologically active form of vitamin D in the kidney.Citation29 Thus, it is considered that serum PTH, 1,25(OH)2D3, and 25(OH)D are tightly regulated to one another. Our results suggested that the percent changes of whole PTH and 1,25(OH)2D3 in both groups were potentially inhibited due to an increase in Ca during the study period and that denosumab could increase and maintain 25(OH)D levels regardless of vitamin D and calcium supplementation from an as yet unknown mechanism.
We witnessed that L-BMD and H-BMD were ameliorated at 3 years in both the denosumab (7.6% and 4.5%, respectively) and combination (11.3% and 8.2%, respectively) groups compared with baseline values. BMD was more significantly increased during the 3 years in the combination group. The mechanism of this phenomenon is unclear since bone metabolism was more greatly suppressed in the denosumab group. However, Ca levels in the combination group were more substantially increased during the study period. Kinoshita et al concluded that Ca was associated with L-BMD gainsCitation30 since bone metabolism was closely related to calcium metabolism due to calcium storage in the bone (>99%).Citation31 Here, baseline Ca was 9.3±0.1 in the denosumab group and 9.2±0.1 in the combination group. At 2 years of treatment, the percent change of Ca was 2.2% in the combination group and 0.9% in the denosumab group. Thus, BMD might have become more significantly increased in the combination group owing to improvements in Ca during therapy. Second, as we have previously reported, denosumab might have augmented zinc and iron metabolism to increase BMD in Japanese postmenopausal OP patients.Citation32 Similarly to other Japanese OP patients with RA, serum zinc was improved by denosumab therapy, which presumably contributed to ameliorations in BMD.Citation33 Here, a more significant increase in serum zinc at 3 years in the combination group might have increased L-BMD and H-BMD more than in the denosumab group, although there were no remarkable differences in serum iron or magnesium between the test groups.
Despite our previous report on percent changes of BAP returning to baseline levels at 2 years of denosumab therapy in OP with RA patients who had received BP pretreatment,Citation34 this study revealed continuously strong BAP inhibition for 3 years. This may be due to 1) the patient background differed considerably and 2) the duration of BP use was different, which was 6–7 years here vs approximately 15 years earlier.Citation34
The present investigation’s main limitation was its sample size and retrospective design. Further assessment of fracture protection will also be necessary to validate our findings.
Conclusion
This study is the first to show that the addition of vitamin D and calcium supplementation to denosumab in patients with OP and RA exhibited higher serum Ca, zinc, and L-BMD and H-BMD gains over 3 years, suggesting the additive benefits of vitamin D and calcium supplementation on the reduction of fracture risk. This study is also the first to directly compare the long-term effects on serum 25(OH)D with or without vitamin D and calcium supplementation, both of which revealed a persistent increase during the study period, suggesting that vitamin D and calcium supplementation might not be necessary for 25(OH)D status in the same population.
Disclosure
The authors report no conflicts of interest in this work.
References
- KanisJADiagnosis of osteoporosisOsteoporosis Int19977S3S108S116
- SimsNAMartinTJCoupling the activities of bone formation and resorption: a multitude of signals within the basic multicellular unitBonekey Rep2014348124466412
- BurgeRDawson-HughesBSolomonDHWongJBKingATostesonAIncidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025J Bone Miner Res200722346547517144789
- KvienTKHaugebergGUhligTData driven attempt to create a clinical algorithm for identification of women with rheumatoid arthritis at high risk of osteoporosisAnn Rheum Dis2000591080581111005782
- LodderMCHaugebergGLemsWFOslo-Truro-Amsterdam (OSTRA) Collaborative StudyRadiographic damage associated with low bone mineral density and vertebral deformities in rheumatoid arthritis: the Oslo-Truro-Amsterdam (OSTRA) collaborative studyArthritis Rheum200349220921512687512
- HaugebergGUhligTFalchJAHalseJIKvienTKBone mineral density and frequency of osteoporosis in female patients with rheumatoid arthritis: results from 394 patients in the Oslo County Rheumatoid Arthritis registerArthritis Rheum200043352253010728744
- DimitroulasTNikasSNTrontzasPKitasGDBiologic therapies and systemic bone loss in rheumatoid arthritisAutoimmun Rev2013121095896623542506
- HolickMFVitamin D deficiencyN Engl J Med2007357326628117634462
- MorrisHAVitamin D activities for health outcomesAnn Lab Med201434318118624790904
- LipsPGielenEvan SchoorNMVitamin D supplements with or without calcium to prevent fracturesBonekey Rep2014351224818004
- EbelingPRVitamin D and bone health: Epidemiologic studiesBonekey Rep2014351124818003
- TanLMLongTTGuanXLDiagnostic Value of Vitamin D Status and Bone Turnover Markers in Rheumatoid Arthritis Complicated by OsteoporosisAnn Clin Lab Sci201848219720429678847
- BlackDMBauerDCSchwartzAVCummingsSRRosenCJContinuing bisphosphonate treatment for osteoporosis – for whom and for how long?N Engl J Med2012366222051205322571169
- BoneHGWagmanRBBrandiML10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extensionLancet Diabetes Endocrinol20175751352328546097
- NakamuraYSuzukiTKamimuraMVitamin D and calcium are required at the time of denosumab administration during osteoporosis treatmentBone Res201751702129021920
- SoenSNew Diagnostic Criteria and Guidelines on Osteoporosis. Diagnostic criteria for primary osteoporosis: year 2012 revision. (Article in Japanese)Clin Calcium20142432332924576928
- van der LindenMPKnevelRHuizingaTWvan der Helm-van MilAHClassification of rheumatoid arthritis: comparison of the 1987 American College of Rheumatology criteria and the 2010 American College of Rheumatology/European League Against Rheumatism criteriaArthritis Rheum2011631374220967854
- KamimuraMTaguchiANakamuraYPretreatment of daily teriparatide enhances the increase of bone mineral density in cortical bones by denosumab therapyTher Clin Risk Manag20181463764229662314
- RouxCBinkleyNBoonenSFOCUS-D InvestigatorsVitamin D status and bone mineral density changes during alendronate treatment in postmenopausal osteoporosisCalcif Tissue Int201494215315723912950
- WongTHGuptaEDRadhakrishnanAKGunSCChembalingamGYeapSSEffects of 25-hydroxyvitamin D and vitamin D-binding protein on bone mineral density and disease activity in Malaysian patients with rheumatoid arthritisInt J Rheum Dis2018215992100028217867
- KhashayarPAghaei MeybodiHRRezai HemamiMKeshtkarADimaiHPLarijaniBVitamin D status and its relationship with bone mineral density in a healthy Iranian populationRev Bras Ortop201651445445827517026
- KharroubiASabaESmoomRBaderKDarwishHSerum 25-hydroxyvitamin D and bone turnover markers in Palestinian postmenopausal osteoporosis and normal womenArch Osteoporos20171211328124221
- NakamuraYUchiyamaSKamimuraMIkegamiSKomatsuMKatoHIncreased Serum 25(OH)D3 Levels in Post-Menopausal Japanese Women with Osteoporosis after 3-Year Bisphosphonate TreatmentTohoku J Exp Med2017242324124628740036
- KamimuraMUchiyamaSNakamuraYIkegamiSMukaiyamaKKatoHShort-term bisphosphonate treatment reduces serum 25(OH) vitamin D3 and alters values of parathyroid hormone, pentosidine, and bone metabolic markersTher Clin Risk Manag20171316116828243105
- AugouleaATsakonasETriantafyllopoulosIComparative effects of denosumab or bisphosphonate treatment on bone mineral density and calcium metabolism in postmenopausal womenJ Musculoskelet Neuronal Interact201717144444928250248
- FleetJCThe role of vitamin D in the endocrinology controlling calcium homeostasisMol Cell Endocrinol2017453364528400273
- NakamuraYKamimuraMIkegamiSChanges in serum vitamin D and PTH values using denosumab with or without bisphosphonate pre-treatment in osteoporotic patients: a short-term studyBMC Endocr Disord2015158126666998
- MartinsJSPalharesMOTeixeiraOCGontijo RamosMVitamin D Status and Its Association with Parathyroid Hormone Concentration in BraziliansJ Nutr Metab20172017905647028265467
- WintermeyerEIhleCEhnertSCrucial Role of Vitamin D in the Musculoskeletal SystemNutrients201686E31927258303
- KinoshitaMIshijimaMKanekoHThe increase in bone mineral density by bisphosphonate with active vitamin D analog is associated with the serum calcium level within the reference interval in postmenopausal osteoporosisMod Rheumatol Epub201893
- FavusMJGoltzmanDRegulation of calcium and magnesiumRosenCJPrimer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism8th edHoboken, NJJohn Wiley & Sons2013172179
- SuzukiTNakamuraYKatoHChanges of Bone-Related Minerals during Denosumab Administration in Post-Menopausal Osteoporotic PatientsNutrients201798E87128805705
- SuzukiTNakamuraYKatoHDetermination of serum bone-related minerals during denosumab treatment in osteoporosis patients with rheumatoid arthritisClin Nutr ESPEN201826535629908683
- NakamuraYSuzukiTKatoHDenosumab significantly improves bone mineral density with or without bisphosphonate pre-treatment in osteoporosis with rheumatoid arthritis: Denosumab improves bone mineral density in osteoporosis with rheumatoid arthritisArch Osteoporos20171218028936606