Abstract
Background:
The purpose of this study was to evaluate the release of chlorhexidine as an antifungal drug from doped self-cured poly (methyl methacrylate) (PMMA) acrylic resin and the effect of the drug released on the growth of Candida albicans.
Methods:
Release of chlorhexidine was evaluated using liquid chromatography, and the effect of the drug on the growth of C. albicans was investigated microbiologically using a “well” technique on Saboraud culture medium inoculated with a resistant strain of C. albicans.
Results:
Chlorhexidine leached steadily out of the acrylic resin into distilled water at mouth temperature, and the sustained drug release continued throughout the 28-day test period. The drug released also demonstrated antifungal activity against the resistant strain of C. albicans.
Conclusion:
The findings of this study support the use of chlorhexidine-impregnated self-cured PMMA chair-side resin as a new dosage form for the treatment of denture-induced stomatitis.
Introduction
Candida-induced denture stomatitis is a common form of oral candidosis that manifests as diffuse inflammation of the denture-bearing areas. Oral candidosis appears to be caused by a multiplicity of predisposing factors.Citation1–Citation3 Adherence of Candida albicans has been implicated as the first step in the pathogenesis of oral candidosis,Citation4 and its relative cell hydrophobicity is also a contributory factor.Citation5
Chlorhexidine gluconate is widely prescribed as an antiseptic mouthwash in dentistry due to its broad-spectrum antimicrobial activity, including C. albicans.Citation6 The antifungal effect of chlorhexidine has been shown in many studies, and it has been demonstrated that exposure of C. albicans to chlorhexidine suppresses the ability of candida to adhere to buccal epithelial cells. Immersion of acrylic dentures in chlorhexidine suppresses adhesion of candida to the prosthesis.Citation7 For these reasons, chlorhexidine may be considered as an appropriate alternative to conventional antimycotic drugs in the management of oral candidosis.Citation8 It has been reported that the duration of inhibition of adherence of candida was longer-lasting when chlorhexidine was used rather than amphotericin B and nystatin.Citation9
There are many oral delivery modes for chlorhexidine. It is used principally as a 0.2% mouthwash with a topical mode of action. However, most of the agent is removed from the oral cavity during the first hour due to the diluent effect of saliva and the cleansing effect of the oral musculature, possibly reducing its therapeutic efficacy.Citation10 Biofilms of candida on mucosal and inert surfaces, such as dentures, may also contribute to therapeutic failure by modifying the susceptibility to antifungal agents.Citation11 Poor patient compliance due to the need for frequent drug application and associated adverse effects could also result in recurrence of disease.Citation12 A sustained-release delivery system for treatment of denture stomatitis using chlorhexidine incorporated into a tissue conditioner has been investigated, and it has been confirmed that there was a gradual release of the drug from the tissue conditioner and inhibition of candida growth in vitro.Citation13 A topical sustained-release dosage form could help overcome the side effects of mouth rinsing with chlorhexidine and guarantee availability of the agent in the target area at a therapeutic dosage. Such a dosage form of chlorhexidine would be able to release the drug at a low therapeutic level over a long period of time, and might thus prevent tooth staining and the bitter taste.Citation14 The emergence of chlorhexidine-resistant strains has not been observed clinically compared with the conventional antimycotic drugs, such as nystatin, amphotericin B, and recently mycostatin.Citation15
Methods for detecting and estimating the eluates from poly (methyl methacrylate) (PMMA) acrylic denture bases have progressed over the past decades. The earlier methods were either physical or chemical in nature,Citation16,Citation17 but their poor detection limits rendered them unsuitable for detecting small amounts of leachate concentration. Therefore, these methods failed to provide an accurate estimation of leachates in acrylic resin bases. That was the situation until the advent of gas chromatography as a method of detecting residual monomers and other leachates in acrylic resin bases.Citation18 The ease of this technique has made the procedure suitable for clinical application. The technique has been used in the detection of residual monomer and other leachates in whole saliva of human subjects wearing acrylic palatal appliances.Citation19,Citation20
More recently, a novel method of acquiring the test specimen for investigation by gas chromatography was introduced,Citation21 whereby the eluates were extracted out of the polymerized acrylic resin plate using solvents. The resulting solution was then centrifuged, and the yield was subjected to gas chromatographic analysis for estimation of eluate concentrations. This method of investigation has been praised for its accuracy and precision,Citation22,Citation23 as well as detection sensitivity for extremely small amounts, ie, 1 ppm and less, of leachates.
The aim of the present investigation was to:
Establish whether a chlorhexidine antifungal drug can be incorporated in a conventional autopolymerizing PMMA formulation
Use the doped polymer for sustained intraoral delivery of chlorhexidine
Investigate whether intraorally delivered chlorhexidine is of adequate concentration to have fungicidal activity against C. albicans and be a potential treatment for denture-induced oral candidiasis.
Materials and methods
Sample preparation
A polymerized PMMA acrylic resin (Paladur®, Heraeus Kulzer, Wehrheim, Germany) at room temperature was used in this study. The mixing ratio and conditions for processing and polymerization recommended by the manufacturer were followed strictly. The polymer powder/monomer liquid ratio for mixing was 5 g/3 mL. Two groups of disc-shaped PMMA specimens were prepared; samples for the first group were impregnated with chlorhexidine powder 10% w/w and samples for the second group (controls) were not ().
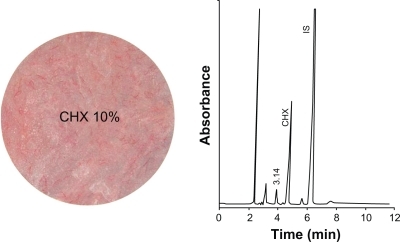
Figure 1 Discs of the two specimen groups showing the drug release device (DRD) impregnated with chlorhexidine (CHX) 10% and the control poly (methyl methacrylate) (PMMA) disc.
Incorporation of chlorhexidine 10% w/w within the polymerized PMMA denture base polymer was indicated by mechanical tests carried out in a pilot study on the polymer prior to the present investigation. The results of these tests indicated that chlorhexidine 10% was the maximum dose that could be safely incorporated in PMMA without having any undue effect on the mechanical properties of the polymer.
The antifungal drug was added in the specified ratio to the acrylic resin powder. The mixture of powders and liquid monomer was then stirred for 15 seconds and left standing for 4 minutes until a plastic dough was formed. The dough was then packed into a specially constructed disc-shaped steel mould to produce a disc specimen (3.8 mm diameter and 1.0 mm thickness). After packing, the mould was allowed to stand for 13 minutes, then placed in a pressure curing unit and cured at 55°C and 2 bar pressure for 15 minutes.
High performance liquid chromatography
Standard chlorhexidine was purchased from Cadilla Pharmaceuticals (Ahmad-Abad, India). The internal standard, p-methyl phenol, was purchased from Acros Organics (Geel, Belgium). The analysis was carried out on isocratic high-performance liquid chromatography apparatus consisting of the following components: a high-performance liquid chromatography pump (LC 1110; GBC Scientific Equipment, Melbourne, Australia), a high-performance liquid chromatography injector (Rheodyne 7125; PerkinElmer, Akron, OH), a high-performance liquid chromatography ultraviolet visible detector (LC 1205; GBC Scientific Equipment), and an integrator (LC 4290; Spectra Physics, Mountain View, CA). The high-performance liquid chromatography apparatus was operated under the following working conditions: acetonitrile/phosphate buffer 0.0 1M (25%:75%) eluent, an eluent flow rate 1.0 mL/min, injection volume 20 μL, a BDS-C18 column (25 cm × 4.6 mm, particle size 5 μm), ultraviolet visible spectrophotometer (λ 210 nm, range 1.0) detector, attenuation 8, and integrator chart speed 0.5 cm/minute.
Standard stock and working solutions
A standard stock solution of 1000 μg/mL was prepared by dissolving 10 mg of the drug in 10.0 mL of high-performance liquid chromatography water. The solutions prepared were kept in a refrigerator. The standard working solution of chlorhexidine was prepared by mixing 20 μg/mL of the drug with 50 μg/mL of the p-methyl phenol internal standard in high-performance liquid chromatography water.
Leaching behavior
In the first group of samples, five discs, weighing 6.32 g each and containing chlorhexidine 10% w/w were each placed separately in a 10 mL screw-capped vial and then covered with 5 mL of double-distilled water. The vials were left in a water bath at 37°C with continuous gentle shaking. After 1 hour, 150 μL of aqueous solution of the corresponding internal standard (160 nmol/mL) was added and mixed. Finally, 20 μL of this mixture were injected onto the high-performance liquid chromatography column according to the abovementioned conditions. This procedure was repeated at 24 hours, at days 2, 3, 4, 5, 6, and 7, and every week for the next 4 weeks. The same procedure was repeated for the five blank PMMA control specimens.
Qualitative and quantitative analysis
A representative chromatogram of a standard mixture of chlorhexidine and the internal standard is shown in . The qualitative identification of the drug peak was performed by comparing the relative retention time of the drug with respect to the internal standard in the real sample with that in the chromatogram for the standard drug mixture, which in this case was 4.860 minutes for chlorhexidine. The quantitative determination was performed using the relative peak areas and relative concentrations.
Microbiological investigation
A resistant strain of C. albicans was isolated from an inpatient at Jordan University Hospital and confirmed by biochemical tests. A reference strain of C. albicans (ATCC90028) was used as a control. Until testing, the yeasts were kept frozen in brain-heart broth (Difco Laboratories, Detroit, MI) with glycerol 5%. For each experiment, the strains were subcultured twice on Saboraud agar (Difco Laboratories) for 24 hours at 35°C to ensure viability and purity. The inoculum suspension was prepared by picking five colonies of at least 1 mm in diameter and suspending them in 5 mL of sterile saline solution 0.85%.
Well diffusion test
The inoculum used was prepared using yeasts from a 24-hour culture on Saboraud agar, with a suspension made in sterile saline solution 0.85%. The turbidity of the suspension was adjusted using a spectrophotometer at 530 nm to obtain a final concentration matching that of a 0.5 McFarland standard (0.5–2.5 × 103). The inoculated agar was poured into the assay plate (9 cm in diameter) and allowed to cool down on a level surface. Once the medium had solidified, “wells” 4 mm in diameter were cut from the agar, and 20 μL of the chlorhexidine 10% eluates were placed into a well in separate assay plates. Control cups containing the drug-release device only were also included in each assay plate. The plates were incubated at 35°C for 72 hours.
The absence of growth of C. albicans, demonstrated by the occurrence of a growth inhibition zone around the wells containing the chlorhexidine-impregnated drug-release device was interpreted as antifungal drug activity, which was expressed by measurement of the diameter of the inhibition zone present around the well accommodating the drug-release device, using a PBI Readbiotic (PBI International, Milano, Italy). Each experiment was carried out five times for the antifungal drug-impregnated release device and controls.
Results
Drug-release device
At the outset, incorporation of chlorhexidine into the polymerized PMMA acrylic resin at room temperature did not have any untoward effect on the polymerization reaction of PMMA or on preparation of the test sample.
Drug release
The antifungal drug, ie, chlorhexidine 10%, contained in the disc specimen demonstrated a high initial rate of elution from the PMMA drug-release reservoir during the first 2–7 days, followed by a controlled sustained elution process that continued throughout the 28-day test period ( and ). In general, chlorhexidine initially displayed a substantially high rate of release, reaching a concentration of up to 606 μg/mL, which was equivalent to 36.4 mg of drug released after 1 day. The rate of release thereafter became steadier until the end of the 28-day test period.
Figure 3 A plot of drug release concentration against time. The bottom part of the graph demonstrates the concentration of the released drug into distilled water daily. The immersion water was changed at every test interval. The top part of the graph demonstrates a hypothetical simulation of the clinical situation displaying what would be the cumulative concentration of chlorhexidine released at the denture/tissue interface and eventually absorbed by the tissues provided that the patient would wear the upper denture continually for 4 weeks. Error bars represent the distribution of data around the mean value of three repeats.
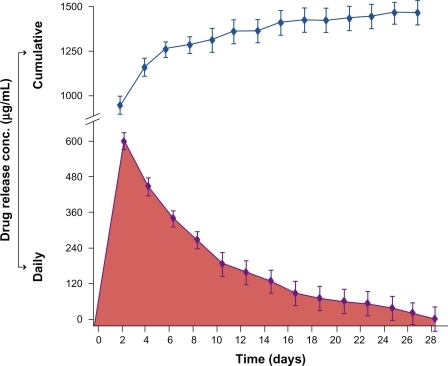
Table 1 Mean concentration (μg/mL) and amount (mg) values (n = 5) of the antifungal drugs released from the autopolymerized acrylic discs into distilled water at weekly intervals for the 28-day test period
Microbiology
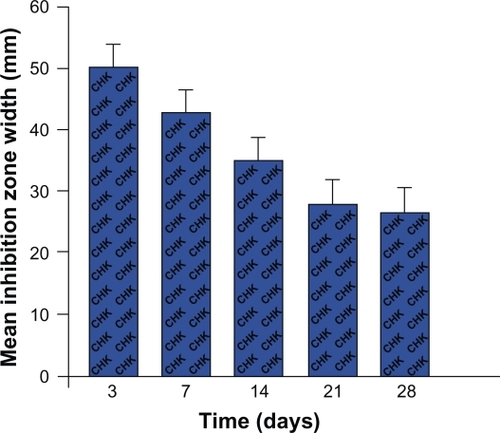
The percolate from the drug-release device containing chlorhexidine 10% showed clear antifungal activity. This was demonstrated by comparing the zones of inhibition of C. albicans growth around the wells containing the chlorhexidine-impregnated drug-release device with those containing the drug-release device alone (). Chlorhexidine continued to demonstrate antifungal potential throughout the 28-day test period, as shown by the width of the inhibition zone of C. albicans growth vs time ().
Discussion
The release of chlorhexidine from the PMMA drug-release device into distilled water indicates that polymerization of the PMMA acrylic resin did not adversely affect the antifungal drug nor did impregnation of the PMMA acrylic resin with chlorhexidine alter the diffusion characteristics of the resin. This finding is in agreement with that of previous studies using polymers for delivering chlorhexidine.Citation24
The elution behavior of chlorhexidine from the autopolymerized acrylic resin showed relatively high initial release into distilled water during the first 4 days, during which the diffusion gradient of chlorhexidine was significantly higher than that demonstrated during the rest of the 28-day exposure to distilled water at mouth temperature.
The change in the rate of drug release is attributed to the fact that leaching of chlorhexidine into water is governed by a concentration-dependent diffusion process.Citation25 A similar elution profile was reported for residual unpolymerized methyl methacrylate monomer from the same autopolymerizing acrylic resin when exposed to both distilled water and artificial saliva at mouth temperature.Citation26,Citation27
The results of the present investigation highlight the remarkable release behavior of an antifungal drug, chlorhexidine 10% w/w, contained within a PMMA drug-release device. It has been clearly established that methacrylate-based polymers absorb up to 30% water depending on the osmolarity of the external solutionCitation16 and the formulation of a particular polymer.Citation24,Citation25 The mechanism of elution seemed to consist of two phases, ie, rapid linear behavior obeying Fick’s law, followed by development of discrete clusters of the immersion liquid of unidentified osmotic activity.Citation25
In the presence of chlorhexidine, the rapid elution phase indicates a surface release process. The subsequent slow sustained release may be the result of complex processes, involving formation of fluid clusters around the drug molecules and the interaction of these clusters with the mechanism of fluid absorption of the acrylic resin. Similar behavior has been reported for the release of another drug from a methacrylate-based polymeric system into distilled water.Citation24 The elution behavior of chlorhexidine may also be enhanced by surface crazing and porosity, formed in the brittle PMMA by osmotic forces consequent to the inclusion of the antifungal drug. This is consistent with findings from a study using the same polymeric system for delivery of hydrocortisone.Citation28
Having established that chlorhexidine-supplemented polymeric devices do release the antifungal drug in controlled concentrations for up to 4 weeks, it was essential to determine whether these concentrations were high enough to have antifungal activity against C. albicans, a common pathogen causing denture-induced stomatitis lesions. In this study, microbiological investigation showed that the antifungal drug was released at concentrations that did have an antifungal effect against C. albicans by inhibiting its growth in Saboraud culture for the 4-week test period. These findings are consistent with those of an earlier studyCitation29 showing that chlorhexidine diffuses out of an autopolymerized acrylic resin in effective fungicidal concentrations over a period of at least 3 weeks.
Our results indicate that a chlorhexidine-supplemented drug-release device has a powerful antifungal effect, demonstrated by its capacity to inhibit growth of C. albicans. This interesting finding should encourage use of antifungal drugs in lower concentrations, thus reducing the chance of the host developing an allergic reaction to the drug, yet possessing a substantially high antifungal potential.
Future laboratory investigations could focus on studying the elution profile of chlorhexidine from an autopolymerized denture-based polymer into artificial saliva at different pH values. Such investigations may yield findings more comparable with the clinical situation. In the clinical context, the cumulative nature of chlorhexidine release into the surrounding fluid media helps saturate the salivary film, bathing the tissue surface of a denture base with continuous-release antifungal drug.
Conclusion
The findings of the present study show that the antifungal drug, chlorhexidine, can be successfully incorporated with autopolymerized PMMA, and that chlorhexidine leaches out of the polymer into the surrounding fluid medium in an environment similar to that in the oral cavity with respect to humidity and temperature. The sustained incremental elution of the antifungal drug from the impregnated-polymeric denture base maintains a gradually increasing concentration of the drug at the exact site where candidal infection lesions in the tissue bed of the maxillary denture are usually found. The results of the microbiological investigation in this study confirm the antifungal efficacy of the drug-supplemented delivery system and favor the use of polymeric systems doped with antifungal drugs in small concentrations. Clinical studies are essential in order to test the efficacy of the suggested drug delivery system, before implementing the new dosage form of antifungal drugs for the treatment of denture-associated oral candidiasis.
Acknowledgements
The authors express their sincere gratitude to Mrs Sana’a Alawi of the Department of Chemistry, Faculty of Sciences, University of Jordan, for her help in conducting the chromatography experiments. The authors would also like to express their thanks to Mr Ameed Al-Taher of the Faculty of Pharmacy, University of Jordan, for his invaluable help with the microbiology experiments.
Disclosure
The authors report no conflicts of interest in this work.
References
- SamaranayakeLPOral mycoses in HIV infectionOral Surg Oral Med Oral Pathol19927321711801549312
- KorayMAkGKurkluEFluconazole and/or hexetidine for management of oral candidiasis associated with denture-induced stomatitisOral Dis200511530931316120118
- SamaranayakeLPHost factors and oral candidosisSamaranayakeLPMacFarlaneTWOral CandidosisLondon, UKButterworth & Company Ltd1990
- RuechelRVirulence factors of Candida speciesSamaranayakeLPMacFarlaneTWOral CandidosisLondon, UKButterworth & Company Ltd1990
- KlotzSADrutzDJZajicJEFactors governing adherence of Candida species to plastic surfacesInfect Immun1985501971013899942
- MacNeillSRindlerEWalkerABrownARCobbCMEffects of tetracycline hydrochloride and chlorhexidine gluconate on Candida albicans. An in vitro studyJ Clin Periodontol199724107537609350560
- GiulianaGPizzoGMiliciMEMusottoGCGiangrecoRIn vitro antifungal properties of mouthrinses containing antimicrobial agentsJ Periodontol19976887297339287062
- TobgiRSSamaranayakeLPMcFarlaneTWAdhesion of Candida albicans to buccal epithelial cells exposed to chlorhexidine gluconateJ Med Vet Mycol19872553353383323451
- McCourtieJMcFarlaneTWSamaranayakeLPA comparison of the effects of chlorhexidine gluconate, amphotericin B and nystatin on the adherence of Candida species to denture acrylicJ Antimicrob Chemother19861755755833722033
- BonesvollPLokkenPRollaGRetention of chlorhexidine in the human oral cavity after mouth rinsesArch Oral Biol197419112092124525807
- BaillieGSDouglasLJCandida biofilms and their susceptibility to antifungal agentsMethods Enzymol199931064465610547826
- Budtz-JorgensenEEtiology, pathogenesis, therapy, and prophylaxis of oral yeast infectionsActa Odontol Scand199048161692181812
- AddyMThawMIn vitro studies into the release of chlorhexidine acetate, prednisolone sodium phosphate, and prednisolone alcohol from cold cure denture base acrylicJ Biomed Mater Res19821621451577061533
- FriedmanMGolombGNew sustained release dosage form of chlorhexidine for dental useJ Perio Res1982173323328
- ThurmondJMBrownATSimsREOral Candida albicans in bone marrow transplant patients given chlorhexidine rinses: Occurrences and susceptibilities to the agentOral Surg Oral Med Oral Pathol19917232912951923415
- WolcottRBPaffenbargerGCSchoonoverIRDirect resinous filling materials: Temperature rise during polymerizationJ Am Dent Assoc195142525326314803190
- SmithDCBainsMEDThe detection and estimation of residual monomer in polymethyl methacrylateJ Dent Res1956351162413286381
- CaulHJSweeneyWIPaffenbargerGCRelationship between residual monomer and some properties of self-curing dental resinsJ Am Dent Assoc1956531606313331705
- AndersonJNApplied Dental Materials5th edOxford, UKBlackwell1976
- AustinAIBaskerRMThe level of residual monomer in acrylic denture base materialsBr Dent J1980149102812866934785
- SadamoriSKotaniHHamadaTThe usage period of dentures and their residual monomer contentJ Prosthet Dent19926823743761501194
- UrbanVMCassQBOliveiraRVGiampaoloETMachadoALDevelopment and application of methods for determination of residual monomer in dental acrylic resins using high performance liquid chromatographyBiomed Chromatogr200620436937616177959
- ChaiXSHouQXSchorkFJDetermination of residual monomer in polymer latex by full evaporation headspace gas chromatographyJ Chromatogr A20041040216316715230522
- RiggsPDBradenMPatelMChlorhexidine release from room temperature polymerizing methacrylate systemsBiomaterials200021434535110656315
- PatelMPCruchleyATColemanDCSwaiHBradenMWilliamsDMA polymeric system for intra-oral delivery of an anti fungal agentBiomaterials200122172319232411511028
- AminWMA study of adhesion between soft lining materials and poly (methyl methacrylate) PhD Thesis, University of London, UK1987
- AlawiMAAminWMEffect of aging on monomer elution from poly (methyl methacrylate) resin under simulated intra-oral conditionsFEB2007164408414
- BrookIMvan NoortRDrug release from acrylic polymers via channels and cracks: In vitro studies with hydrocortisoneBiomaterials1985642812854052543
- LambDJMartinMAn in vitro and in vivo study of the effect of incorporation of chlorhexidine into autopolymerizing acrylic resin plates upon the growth ofCandida albicans Biomaterials198343205209