Abstract
Purpose
The aim of this study was to evaluate the risk of immune-related adverse events (irAEs) among cancer patients receiving nivolumab-plus-ipilimumab therapy and nivolumab monotherapy.
Patients and methods
PubMed and Web of Science were searched for related studies from inception to June 2018. Eligible studies included randomized controlled trials comparing nivolumab-plus-ipilimumab with nivolumab alone in cancer patients reporting on all-grade (grade 1–4) and high-grade (grade 3/4) irAEs. Paired reviewers selected studies for inclusion and extracted data. The odds risk and 95% CI were calculated.
Results
A total of 2,946 patients from four studies were included in the meta-analysis. The underlying malignancies included lung cancer (two trials) and melanoma (two trials). Compared with nivolumab monotherapy, the nivolumab-plus-ipilimumab therapy was associated with a significantly higher risk of all- and high-grade irAEs such as pruritus, rash, diarrhea, colitis, alanine aminotransferase elevation, and pneumonitis.
Conclusion
The combination therapy of nivolumab and ipilimumab increased the incidence of irAEs in patients with advanced cancer.
Introduction
The recent advancement regarding immune checkpoint inhibitors (ICIs) represents a major breakthrough in the management of cancer.Citation1 Furthermore, immunotherapy has made great progress in cancer treatment recently, besides the advancements in surgery, chemotherapy, molecularly targeted therapy, and radiation. On certain aberrant circumstances, it is understood that T-cell activation plays a significant role in adaptive immunity resulting in autoimmunity.Citation2 Cytotoxic T-lymphocyte antigen-4 (CTLA-4), which was represented as the first immune checkpoint receptor, was introduced for the immune-associated targeted therapy. CTLA-4 is recruited on the surface of regulatory T cells and interacts with B7 receptors present on antigen-presenting cells, resulting in the downregulation of any further T-cell activation and immune response expansion.Citation3 The abovementioned mechanism shows the significant role played by CTLA-4 in maintaining normal immunologic homeostasis, which was further proven by the death of mice deficient in CTLA-4 due to fatal lymphoproliferation.Citation4–Citation7 The CTLA-4 inhibitor (ipilimumab) was the first agent to be associated with an obvious improvement in overall survival (OS) in a Phase III study (MDX 010–020) that enrolled 676 patients pretreated for metastatic melanoma.Citation8 As a result, ipilimumab was approved in 2011 for the management of advanced melanoma. Programmed death 1 (PD-1), a well-known immune checkpoint molecule, is expressed on a variety of immune cells.Citation9 PD-1 is an inhibitory receptor expressed on activated lymphocytes and is associated with regulation of immune tolerance and autoimmunity. The ligands of PD1, which can be divided into PD-L1 and PD-L2, have distinct patterns of expression and can be induced, or essentially expressed, on an array of cells including a number of tumor cells.Citation10 Eventually, in December 2014, nivolumab was approved for the management of unresectable melanoma that was unresponsive to other drugs.Citation11
The disordered expression of CTLA-4 and PD-1 is suspected to play an important role in tumor immune evasion and has become an appealing target for intervention in therapy.Citation12 Therefore, application of immune checkpoint blockade (ICB) with anti-CTLA4 and anti-PD1 has gained significant attention in tumor immunology. In patients diagnosed with metastatic melanoma, the combination of ipilimumab and nivolumab showed an enhanced activity relative to either monotherapy, although the median OS was not reached after conducting a follow-up study for a minimum of 2 years. Among advanced stage lung cancer patients, tumor mutational burden or 3 years of OS was strikingly higher among patients receiving combination therapy as compared with nivolumab alone. Now, the combination treatment has been approved in the Europe and the US for patients with melanoma.Citation13,Citation14
Immunotherapy, which involves reactivation of the immune system, has led to the occurrence of new toxicity profiles, also called immune-related adverse events (irAEs), which can be fatal in some cases.Citation15 Most frequently, these irAEs affect a wide range of organs like skin, colon, liver, pituitary, thyroid, and lungs, although uncommon events involving the heart, nervous system, and other organs do occur.Citation16,Citation17 Previous research revealed that ipilimumab could increase the risk of mortality by 130% in cancer patients, with an overall incidence of fatal adverse events of 1.13%.Citation18 The combination of nivolumab and ipilimumab was superior as compared to the single agents alone for the treatment of metastatic melanoma.Citation19,Citation20 However, combined PD-1 plus CTLA-4 blockade substantially triggered more toxic events as compared with anti-PD-1 alone (55%–60% vs 10%–20% high-grade events).Citation21 These irAEs remain a major challenge in clinical care and are significant barriers for developing more effective combination therapies.
Currently, the combination of ipilimumab and nivolumab has been proven to enhance objective response rate and progression-free survival as compared with single agent (monotherapy) among patients with advanced tumor. However, there are no evident studies evaluating the risk of irAEs in nivolumab-plus ipilimumab as compared with nivolumab group alone.Citation22 This meta-analysis was performed to evaluate the incidence of irAEs in patients receiving nivolumab-plus-ipilimumab therapy and nivolumab monotherapy. We expect that the pooled studies would be more helpful in detecting significant association than single study alone.
Patients and methods
This meta-analysis was reported according to the PRISMA statement.Citation23
Search strategy
A literature search was carried out using PubMed and Web of Science to identify clinical study from inception to June 2018. The keywords included CTLA-4, PD-1, nivolumab, ipilimumab, clinical trials, immune checkpoint, and immune-related adverse events. The search was conducted in June 2018. Only those studies published in English were included. The retrieved studies were scrutinized and examined for title and abstracts by two reviewers. Further exploration of full texts was conducted in order to check the studies’ eligibility for inclusion in accordance with the inclusion criteria. The literature search was supplemented with manual searches for references of the included studies and for related citations.
Study selection
We included all published studies comparing the irAEs of nivolumab-plus-ipilimumab to nivolumab in patients with advanced malignancy. Case and animal-based studies were excluded. Any trial with incomplete data was excluded from this meta-analysis. Any disagreements with regard to the protocol were to be resolved by the third author, but none occurred.
Outcome measures
The irAEs that were commonly observed were pruritus, rash, diarrhea, colitis, alanine aminotransferase (ALT) elevation, aspartate aminotransferase elevation, hypothyroidism, hyperthyroidism, hypophysitis, hepatitis, and pneumonitis. The immune-related toxicity estimated by adverse events was categorized into two gradings (grade 1–4 and grade 3/4). The severity of adverse events was graded and evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0.Citation24
Data extraction
Two reviewers (SZ and SK) collected the data independently using a predefined data extraction form. We compiled the following information from each study: 1) year of publication, 2) first author’s name, 3) participant characteristics, 4) therapies and number of groups, and 5) number of adverse effects.
Quality assessment
Studies fulfilling the review inclusion criteria were assessed for methodological quality by two reviewers. The Cochrane Collaboration Tool was used to evaluate the risk of bias in the included studies.Citation25
Statistical methods
The data extracted from the included studies were entered into Review Manager software version 5.3 (http://ims.cochrane. org/revman/download) for statistical analysis. The odds ratio (OR) was applied for comparing dichotomous variables. All the results were reported with 95% CI. For dichotomous data, Mantel–Haenszel method was applied for pooled OR and 95% CI estimation. I2 test was used to assess the impact of study heterogeneity. According to the Cochrane review guidelines, if the heterogeneity (I2) <50%, then pooled OR is calculated by the fixed-effect model (Mantel–Haenszel), otherwise the OR is calculated by random-effect model (Der Simonian-Laird). The significance of OR is determined by Z-test.
Results
Search results and patient characteristics
A total of four reports were included in this review. The search provided a total of 1,207 publications at the beginning. Of these, 1,118 studies were discarded after reading the title, abstract, and, if necessary, after reading the manuscript. Eighty-five reports were further excluded after assessing the full text of articles. More detailed information is given in .Citation26–Citation29 A total of 2,946 patients (nivolumab:1,589; nivolumab-plus-ipilimumab:1,357) were included in the analysis from the four included studies. The underlying malignancies were lung cancer (two trials) and melanoma (two trials). The baseline characteristics of each study are presented in .
Figure 1 The flow diagram of literature search and selection.
Table 1 Characteristics of studies including nivolumab-plus-ipilimumab and nivolumab therapy
Immunotherapy-related adverse events
As compared with nivolumab monotherapy, the combined therapy was associated with a significantly higher risk of all- and high-grade irAEs such as pruritus, rash, diarrhea, colitis, ALT elevation, hyperthyroidism, hypophysitis, and pneumonitis. However, there was no statistical difference with regard to the risk of vitiligo (P<0.05). The analysis results are summarized in .
Table 2 Incidence and OR of irAEs, including 95% CI and number of trials in each analysis
Meta-analysis of the risk of pruritus and rash
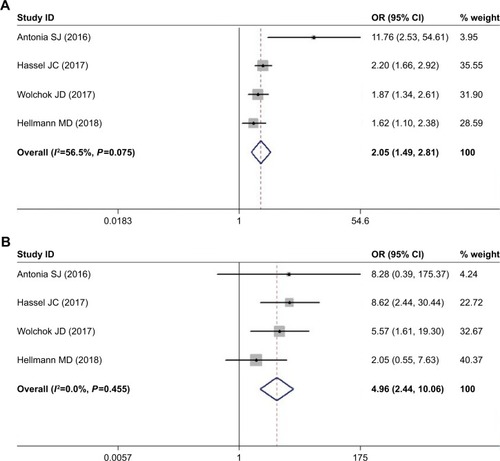
The incidence of pruritus was reported among four studies where patients were divided into nivolumab-plus-ipilimumab group and nivolumab group. The selected studies included cases of melanoma, small-cell lung cancer (SCLC), and non-small-cell lung cancer (NSCLC). The pooled ORs of all-grade (grade 1–4) pruritus and high-grade (grade 3/4) pruritus were 2.104 (95% CI: 1.739–2.546, P<0.05, I2=0.0%) and 7.585 (95% CI: 2.220–25.919, P<0.05, I2=0.0%), respectively ().
Figure 2 Meta-analysis of the risk of pruritus.
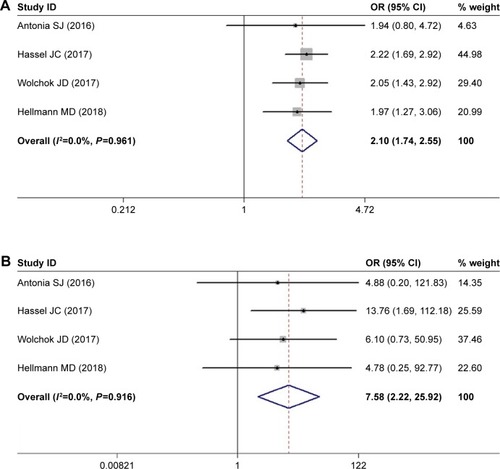
Similarly, the result for rash also showed significantly higher risk among nivolumab-plus-ipilimumab group than the nivolumab group. The pooled ORs for all-grade rash and high-grade rash were 2.046 (95% CI: 1.488–2.814, P<0.05, I2=56.5%) and 4.957 (95% CI: 2.443–10.061, P<0.05, I2=0.0%), respectively ().
Meta-analysis of the risk of diarrhea and colitis
Among the four included studies, patients treated with nivolumab-plus-ipilimumab group had a higher risk of diarrhea. The pooled ORs of all-grade rash and high-grade rash were 2.715 (95% CI: 1.759–4.192, P<0.05, I2=77.7%) and 4.738 (95% CI: 2.943–7.628, P<0.05, I2=28.9%), respectively ().
Figure 4 Meta-analysis of the risk of diarrhea.
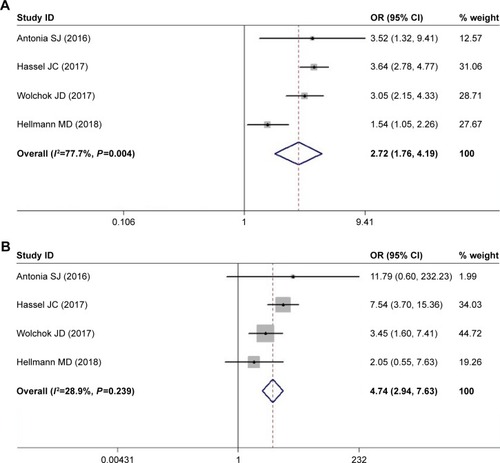
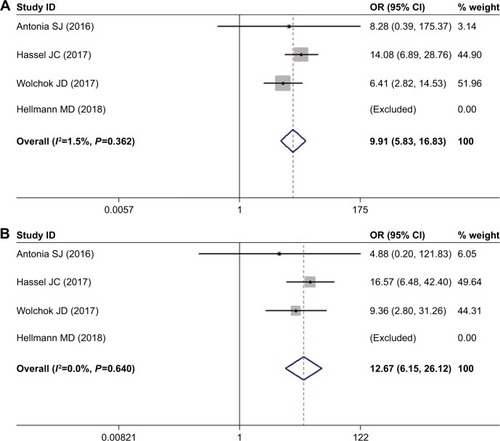
Three studies reporting the incidence of colitis included patients with melanoma and SCLC. The observed pooled OR for all-grade colitis was 9.909 (95% CI 5.833–16.833, P<0.05) with a low heterogeneity (I2=1.5%). The pooled OR for high-grade colitis was 12.671 (95% CI: 6.148–26.118, P<0.05) with a low heterogeneity (I2=0.0%) ().
Meta-analysis of the risk of ALT elevation
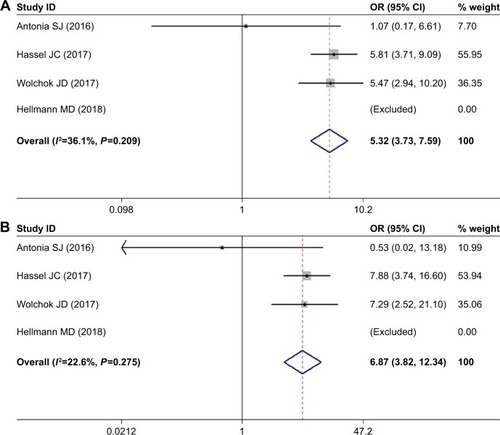
Among the four studies included, a higher risk of ALT elevation was observed for nivolumab-plus-ipilimumab group than the nivolumab group. The pooled OR for all-grade increase in ALT was 5.322 (95% CI: 3.732–7.589, P<0.05, I2=36.1%). The pooled OR for high-grade increase in ALT was 6.866 (95% CI: 3.819–12.344, P<0.05, I2=22.6%) ().
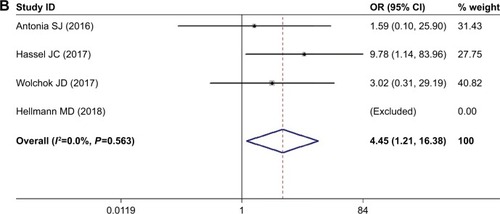
Meta-analysis of the risk of pneumonitis
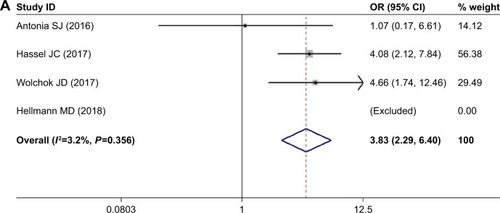
When nivolumab-plus-ipilimumab group was compared with nivolumab alone group, the risk of pneumonitis was higher with combined treatment over nivolumab monotherapy. The pooled ORs of all-grade pneumonitis and high-grade pneumonitis were 3.825 (95% CI: 2.286–6.399, P<0.05, I2=3.2%) and 4.445 (95% CI: 1.206–16.379, P<0.05, I2=0.0%), respectively ().
Study quality and publication bias
Funnel plots were used to evaluate the potential publication bias. We used Begg’s and Egger’s tests to evaluate the asymmetry of the funnel plot.Citation30 Furthermore, the forest plots of irAEs (–), and Begg’s and Egger’s tests (P<0.05) show that there was no publication bias.
Discussion
ICB therapy is increasingly being recognized in patients with lung cancer and advanced melanoma and is further being evaluated for various other cancers. However, irAEs can limit their use due to serious adverse outcomes including death. It has been reported that combined therapy is more frequently associated with multiorgan involvement, with nearly one-third of all deaths resulting from myocarditis, myositis, and neurologic events.Citation31 In the present study, we performed a meta-analysis to evaluate the incidence of irAEs in patients receiving nivolumab-plus-ipilimumab and nivolumab therapy alone.
Previously, Komaki et al published a meta-analysis of irAEs with CTLA-4 blockade and PD-1 therapy, which included nivolumab, ipilimumab, and tremelimumab (non-approved).Citation32 They pooled 11,144 patients’ data from clinical trials. However, their search strategy was limited and included cases only up to December 2016. Anti-CTLA4 therapy was associated with a higher risk of overall irAEs. Anti-PD1 therapy was associated with a higher risk of pruritus in comparison to other adverse events. In our study, we included studies from inception to June 2018. The majority of irAEs included in our analysis were from recent trials, which could be due to the fact that researchers were more aware and aggressive in their management of irAEs. Furthermore, we evaluated all-grade and high-grade irAEs in patients receiving nivolumab-plus-ipilimumab and nivolumab therapy.
Immune-mediated pneumonitis during treatment with ICIs was rare (considering all grades of severity, <10% of patients).Citation33 A previous report demonstrated that 4% of patients on ipilimumab developed pneumonitis, whereas the percentage was 11% among patients treated with ipilimumab plus nivolumab in a comparative metastatic melanoma study.Citation14 So, there was an increase in the prevalence of pneumonitis on combined therapy with PD-1 and CTLA-4 inhibitors. Moreover, the pooled incidences of all-grade and high-grade pneumonitis in patients treated with PD-1/PD-L1 inhibitors were 3.2% and 1.1%, respectively,Citation34 while our data showed that the risk of pneumonitis was 4.445 times higher in nivolumab-plus-ipilimumab than in nivolumab group. Therefore, clinicians should pay enough attention to patients manifesting pulmonary symptoms including dry cough, hypoxia, and shortness of breath.Citation35 Furthermore, comorbidities such as chronic obstructive airways disease and prior therapies including lung irradiation in patients with NSCLC would have influenced the observed higher risks of pneumonitis when treated with PD-1 inhibitors.Citation15 Given the occurrence of pneumonia, the management of pneumonitis primarily consisted of administering rapid and high-dose corticosteroid treatment.
One of the common irAEs was diarrhea caused by immune-related colitis.Citation36 A previous report showed that the incidence of severe diarrhea/colitis with anti-PD1 therapy was much lower than with anti-CTLA4 therapy (8%–19% vs 23%–33%).Citation37 Combined therapy had a striking 44% increase in the risk of diarrhea and immune-related colitis.Citation38 It is reported that diarrhea and colitis could also be associated with autoimmune diseases, long-term use of nonsteroidal anti-inflammatory drugs, elevated plasma IL-17, and elevated expression of carcinoembryonic antigen-related cellular adhesion molecule 1, but it was noticed that these risk factors could not be detected in most laboratory centers. Therefore, the clinical application of detecting the risk factors associated with diarrhea and colitis is still lagging.
The analysis of nivolumab-plus-ipilimumab and nivolumab monotherapy for the immune-mediated hypophysitis (IMH) revealed that the incidence increased to 8%, with high-grade hypophysitis occurring in 2% of cases.Citation13 The results indicated that the risk of hypophysis was 18.487 times higher in ipilimumab-plus-nivolumab therapy in comparison to nivolumab therapy. These pituitary adverse events often occurred between 6 and 13 weeks, as well as 19 months after the completion of treatment.Citation39 This condition is usually associated with atypical symptoms, such as mild fatigue, joint pain, and decreased sexual interest caused by hormonal changes. Severe symptoms included headache due to gravitational effects caused by glandular edema. Due to these atypical symptoms, pituitary inflammation was easily missed. With no standard management protocol for IMH, it is important to be cognizant of the fact that adrenocortical failure caused by hypophysitis may be life threatening, and further management studies are required on this premise.Citation33
Since ICIs are associated with distinctive toxicity, many oncologists are not acquainted with the optimal principle management of irAEs, which requires early recognition and appropriate treatment. Further, clinicians are required to identify the risk factors and assess the severity of irAEs accurately. Fortunately, it is inspiring to know that most of the irAEs are sensitive to glucocorticoid therapy and can be well controlled within 6–12 weeks. Our study could be important in considering the benefit/risk trade-off by providing the odds risks for irAEs in patients treated with combination therapy.
There are certain limitations in our meta-analysis, which should be mentioned. First, our results were based on unadjusted analysis, and more accurate outcomes would result from making adjustments for other confounders such as gender, age, dosage of drugs, PD-L1 status, prior systemic therapy, and so on. Second, the small number of included studies would make the outcomes more prone to be influenced by a potential publication bias. As a result, we could not confidently assess the publication bias. Third, language of studies was limited to English, which may result in missing data from studies published in other languages.
Conclusion
In summary, our study indicated that the combination of nivolumab and ipilimumab increased the risk of all- and high-grade irAEs in patients with advanced cancer. In spite of its considerable benefit in patients, ICB can be limited by the occurrence of irAEs, which can be life threatening. Therefore, additional drug intervention to prevent or treat these adverse events should be considered when standardizing an anti-PD-1 combined with anti-CTLA-4 therapy for metastatic tumors.
Acknowledgments
We would like to thank Mr Zhang, Southeast University, for excellent technical assistance.
Disclosure
The authors report no conflicts of interest in this work.
References
- HarrisTJDrakeCGPrimer on tumor immunology and cancer immunotherapyJ Immunother Cancer201311224829749
- KumarPBhattacharyaPPrabhakarBSA comprehensive review on the role of co-signaling receptors and Treg homeostasis in autoimmunity and tumor immunityJ Autoimmun201895779930174217
- MellmanICoukosGDranoffGCancer immunotherapy comes of ageNature2011480737848048922193102
- LinsleyPSBradyWUrnesMGrosmaireLSDamleNKLedbetterJACTLA-4 is a second receptor for the B cell activation antigen B7J Exp Med199117435615691714933
- QureshiOSZhengYNakamuraKTrans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4Science2011332602960060321474713
- RileyJLMaoMKobayashiSModulation of TCR-induced transcriptional profiles by ligation of CD28, ICOS, and CTLA-4 receptorsProc Natl Acad Sci U S A20029918117901179512195015
- SchneiderHDowneyJSmithAReversal of the TCR stop signal by CTLA-4Science200631357951972197516931720
- HodiFSO’DaySJMcdermottDFImproved survival with ipilimumab in patients with metastatic melanomaN Engl J Med2010363871172320525992
- BardhanKAnagnostouTBoussiotisVAThe PD1:PD-L1/2 Pathway from Discovery to Clinical ImplementationFront Immunol20167155028018338
- KeirMELiangSCGuleriaITissue expression of PD-L1 mediates peripheral T cell toleranceJ Exp Med2006203488389516606670
- FaghfuriEFaramarziMANikfarSAbdollahiMNivolumab and pembrolizumab as immune-modulating monoclonal antibodies targeting the PD-1 receptor to treat melanomaExpert Rev Anticancer Ther201515998199326313415
- OkazakiTChikumaSIwaiYFagarasanSHonjoTA rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical applicationNat Immunol201314121212121824240160
- LarkinJChiarion-SileniVGonzalezRCombined nivolumab and ipilimumab or monotherapy in untreated melanomaN Engl J Med20153731233426027431
- PostowMAChesneyJPavlickACNivolumab and ipilimumab versus ipilimumab in untreated melanomaN Engl J Med2015372212006201725891304
- KhojaLDayDWei-Wu ChenTSiuLLHansenARTumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic reviewAnn Oncol201728102377238528945858
- HasselJCHeinzerlingLAberleJCombined immune checkpoint blockade (anti-PD-1/anti-CTLA-4): evaluation and management of adverse drug reactionsCancer Treat Rev201757364928550712
- GirotraMHansenAFarookiATask Force collaborationThe current understanding of the endocrine effects from immune checkpoint inhibitors and recommendations for managementJNCI Cancer Spectr201823pky02130057972
- ZhangSLiangFLiWWangQRisk of treatment-related mortality in cancer patients treated with ipilimumab: a systematic review and meta-analysisEur J Cancer201783717928719841
- HryniewickiATWangCShatskyRACoyneCJManagement of immune checkpoint inhibitor toxicities: a review and clinical guideline for emergency physiciansJ Emerg Med201855448950230120013
- GrimaldiAMMarincolaFMAsciertoPASingle versus combination immunotherapy drug treatment in melanomaExpert Opin Biol Ther201616443344126642234
- ShoushtariANFriedmanCFNavid-AzarbaijaniPMeasuring toxic effects and time to treatment failure for nivolumab plus ipilimumab in melanomaJAMA Oncol2018419810128817755
- NagaiHMutoMOptimal management of immune-related adverse events resulting from treatment with immune checkpoint inhibitors: a review and updateInt J Clin Oncol201823341042029516216
- MoherDLiberatiATetzlaffJAltmanDGPRISMA GroupPreferred reporting items for systematic reviews and meta-analyses: the PRISMA statementPLoS Med200967e100009719621072
- National Cancer Institute [webpage on the Internet]Common terminology criteria for adverse events (CTCAE) version 5.02018 Available from: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htmAccessed January 23, 2019
- HigginsJPAltmanDGGøtzschePCCochrane Bias Methods GroupThe Cochrane collaboration’s tool for assessing risk of bias in randomised trialsBMJ2011343d592822008217
- AntoniaSJLópez-MartinJABendellJNivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (Check-Mate 032): a multicentre, open-label, phase 1/2 trialLancet Oncol201617788389527269741
- HellmannMDCiuleanuTEPluzanskiANivolumab plus ipilimumab in lung cancer with a high tumor mutational burdenN Engl J Med2018378222093210429658845
- WolchokJDChiarion-SileniVGonzalezROverall survival with combined nivolumab and ipilimumab in advanced melanomaN Engl J Med2017377141345135628889792
- HasselJCHeinzerlingLAberleJCombined immune checkpoint blockade (anti-PD-1/anti-CTLA-4): evaluation and management of adverse drug reactionsCancer Treat Rev201757364928550712
- SterneJAGavaghanDEggerMPublication and related bias in meta-analysis: power of statistical tests and prevalence in the literatureJ Clin Epidemiol200053111119112911106885
- WangDYSalemJECohenJVFatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysisJAMA Oncol20184121721172830242316
- KomakiYKomakiFYamadaAMicicDIdoASakurabaAMeta-analysis of the risk of immune-related adverse events with Anticytotoxic T-Lymphocyte-Associated antigen 4 and Antiprogrammed death 1 therapiesClin Pharmacol Ther2018103231833128118483
- Abdel-WahabNShahMSuarez-AlmazorMEAdverse events associated with immune checkpoint blockade in patients with cancer: a systematic review of case reportsPLoS One2016117e016022127472273
- ZhangSLiangFZhuJChenQRisk of pneumonitis associated with programmed cell death 1 inhibitors in cancer patients: a meta-analysisMol Cancer Ther20171681588159528446641
- ZhaoBZhaoHZhaoJSerious adverse events and fatal adverse events associated with nivolumab treatment in cancer patients: nivolumab-related serious/fatal adverse eventsJ Immunother Cancer20186110130285872
- WeberJSDummerRde PrilVLebbéCHodiFSMDX010-20 InvestigatorsPatterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: detailed safety analysis from a phase 3 trial in patients with advanced melanomaCancer201311991675168223400564
- VerschurenECvan den EertweghAJWondersJClinical, endoscopic, and histologic characteristics of Ipilimumab-Associated colitisClin Gastroenterol Hepatol201614683684226748223
- EmensLABraitehFSCassierPAbstract 2859: inhibition of PD-L1 by MPDL3280A leads to clinical activity in patients with metastatic triple-negative breast cancer (TNBC)Cancer Res20157515 Supplement2859
- SangroBGomez-MartinCde La MataMA clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis CJ Hepatol2013591818823466307