Abstract
Purpose
This study investigated the perioperative risk factors of postoperative pulmonary complications (PPCs) after minimally invasive anatomic resection for lung cancer.
Patients and methods
We retrospectively reviewed the data from medical records of 729 lung cancer patients undergoing minimally invasive anatomic lung resections between January 2017 and December 2017. Univariate and binary logistic regression analyses were performed to select the independent risk factors for PPCs during the patient’s postoperative hospitalization after surgery.
Results
The incidence of PPCs was 24.8% (n=181/729). No patient died during the period of hospitalization. Logistic regression analysis revealed that body mass index (BMI) ≥24.0 kg/m2 (vs <24.0 kg/m2: OR 1.514, 95% CI 1.057–2.167, P=0.024), single segmentectomy (vs single lobectomy: OR 2.115, 95% CI 1.150–3.891, P=0.016), bilobectomy or combined lobectomy and segmentectomy (vs single lobectomy: OR 2.731, 95% CI 1.013–7.361, P=0.047), and right lung lobe surgery (vs left lung lobe surgery: OR 1.519, 95% CI 1.046–2.205, P=0.028) were independent risk factors for PPCs in lung cancer patients who received minimally invasive anatomic lung resections.
Conclusion
Individual factors such as BMI ≥24.0 kg/m2, single segmentectomy, bilobectomy or combined lobectomy and segmentectomy, and right lung lobe surgery were independent risk factors of PPCs, which should be helpful for risk stratification, patient counseling, and perioperative care for lung cancer patients.
Introduction
Lung cancer is the leading cause of cancer death worldwide for both genders, and anatomic lung resection is widely considered the optimal therapy for early-stage lung cancer.Citation1 Video-assisted thoracoscopic surgery or robotic-assisted thoracoscopic surgery is being increasingly performed for early-stage lung cancer instead of open thoracotomy because of its minimally invasive nature.Citation2 Despite the improvement in surgical techniques, postoperative pulmonary complications (PPCs) still occur in 12%–40% of patients after surgical lung resection, and increase hospital mortality, postoperative length of stay (PLOS), and total hospital care costs.Citation1 Several previous studies have suggested that the patient’s age, gender, and body mass index (BMI), as well as smoking and COPD, are risk factors of PPCs after lung resection.Citation3–Citation5 However, data regarding the risk factors of PPCs after lung resection are still limited and often from small sample sizes.Citation3–Citation6 Moreover, there are too many perioperative associated factors for PPCs after lung resection, many of which are still are not being studied and understood. Therefore, further research is needed to address this knowledge gap.
In this study, we retrospectively reviewed patients with lung cancer undergoing minimally invasive anatomic resection and compared the perioperative factors between patients with PPCs and patients with no PPCs. The study aimed to identify perioperative risk factors for the development of PPCs after minimally invasive anatomic resection for lung cancer. Knowledge about these factors might help to identify patients at elevated risk for the development of PPCs and, therefore, contribute to a decrease in such complications.
Patients and methods
The Medical Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, China, approved the study. Because the data were recorded retrospectively and without any specific intervention, the Medical Ethics Committee agreed to waive the need for informed consent. Data were deidentified to protect the privacy and maintain confidentiality of patient information. It was conducted in accordance with the 1964 Declaration of Helsinki and its later amendments covering patient data confidentiality.
Study population
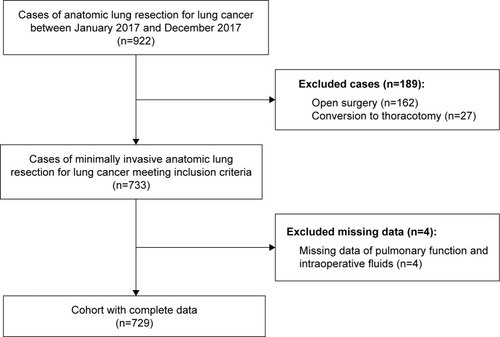
We retrospectively collected the records of consecutive lung cancer patients who had undergone anatomic lung resections between January 2017 and December 2017, at the First Affiliated Hospital, College of Medicine, Zhejiang University. The inclusion criteria were 1) patients aged over 18 years, 2) patients who had undergone minimally invasive anatomic lung resections under general anesthesia with double-lumen intubation, and 3) pathological confirmation of lung cancer. Minimally invasive anatomic lung resection was defined as lobectomy or segmentectomy under video-assisted thoracoscopic surgery or robotic-assisted thoracoscopic surgery. Exclusion criteria were patients who had undergone 1) open surgery and 2) conversion to thoracotomy. A total of 922 patient records were evaluated, but 193 were excluded because of open surgery (n=162), conversion to thoracotomy (n=27), or incomplete data (n=4). Finally, 729 valid cases were included ().
Detailed patient data of the whole cohort are provided in and . A low-tidal-volume ventilation strategy was implemented during single lung ventilation. All patients received prophylactic first/second-generation cephalosporins until removal of chest tubes. However, if the patient was diagnosed with a postoperative infection, the antibiotic was adjusted to sulbactam, ampicillin, and ciprofloxacin. If there was no risk of aspiration, the patients were started on oral feedings in the sixth to eighth postoperative hour.
Table 1 Preoperative factors of patients with or without postoperative pulmonary complications by univariate analysis (n=729)
Table 2 Intraoperative and postoperative factors of patients with or without postoperative pulmonary complications by univariate analysis (n=729)
Variables and outcomes of interest
The variables comprised preoperative, intraoperative, and postoperative factors, extracted from medical records, as described in and . According to the Health Industry Standard of China: Adult Weight Determination (WS/T428-2013), nutritional status is divided into four levels: underweight (BMI <18.5 kg/m2), normal weight (18.5≤ BMI <24.0 kg/m2), pre-obesity (24.0≤ BMI <28.0 kg/m2), and obesity (BMI ≥28.0 kg/m2). Therefore, we used BMI of 24 kg/m2 as the boundary for BMI grading. Preoperative renal insufficiency was defined as creatinine >50% the upper limit of the reference range, which is 1.3 and 1.1 mg/dL for men and women, respectively.Citation7 The amount of total intra-operative fluids was defined as the volumes of crystalloid, colloid, and blood products administered between initiation of anesthesia care and arrival in the postanesthesia care unit.Citation7 The colloid was hydroxyethyl starch. The net intraoperative total fluid was equal to the total intraoperative fluids minus intraoperative bleeding and urine output. Postoperative pathology of lung cancer included adenocarcinoma, squamous cell carcinoma, adenosquamous carcinoma, and small-cell lung cancer.
The PPCs included acute respiratory distress syndrome, reintubation, pneumonia, the need for bedside bronchoscopy, atelectasis, pulmonary embolism, prolonged air leak, and failure to expand during the period of postoperative hospitalization, as reported elsewhere.Citation8 The three criteria used to diagnose postoperative pneumonia were a new pulmonary infiltrate on chest X-ray, leukocytosis, and fever (ear temperature ≥38.0°C).Citation8,Citation9 Bedside bronchoscopy was used to clear lung secretions in the ward after the operation. Atelectasis was diagnosed by chest X-ray or computed tomography (CT), and pulmonary embolism was diagnosed by pulmonary artery CT angiography. A prolonged air leak was defined as leak >7 days.Citation8 Failure to expand was accepted as the inability of the remaining lung to completely fill the pleural cavity with or without air leak, which was diagnosed by chest X-ray.Citation8 The PLOS was defined as the number of hospitalized days after surgery. Hospital costs were the total hospital care costs. Among the 729 patients, 181 (24.8%) had PPCs and were classified into a PPCs group. The remaining 548 (75.2%) patients without PPCs were classified into the no PPCs group.
Statistical analysis
Potential risk factors were detected using single-factor analysis. Continuous data were analyzed using one-way variance analysis. If the variance was not homogeneous, a nonparametric test (Kruskal–Wallis H-test for multiple independent samples) was used. Categorical variables were compared by the R×C chi-squared test or Fisher’s exact test, as appropriate. The potential risk factors with P<0.05 were further assigned, imported into the binary logistic regression equation, and analyzed. Table S1 shows the assignment of variables in binary logistic regression analysis. P<0.05 was considered statistically significant. The OR and 95% CI were calculated. All analyses were performed using SPSS 25.0 (SPSS, Inc., Chicago, IL, USA).Citation7,Citation10
Results
Patient selection and clinical outcomes
A total of 729 patients met our criteria for analysis (). The incidence of PPCs was 24.8% (n=181). The four most common PPCs were pneumonia (n=148, 20.3%), failure to expand (n=22, 3.0%), atelectasis (n=16, 2.2%), and prolonged air leak (n=16, 3.0%). No patient died during the period of hospitalization. The PLOS and total hospital care costs in the PPCs group were significantly higher than those in the no PPCs group ().
Table 3 Clinical outcomes of patients with or without postoperative pulmonary complications
Comparative univariate analysis of the potential risk factors of PPCs
Univariate analysis was performed to determine the potential risk factors for PPCs after minimally invasive anatomic resection for lung cancer. Compared to the no PPCs group, the PPCs group demonstrated significant differences in BMI grading (P=0.009), type of anatomical lung resection (P=0.007), surgical lobe (P=0.020), intraoperative bleeding (P=0.024), intraoperative bleeding grading (P=0.027), and length of anesthesia grading (P=0.040), while there were no discrepancies among the other variables ( and ).
Binary logistic regression analysis of the risk factors of PPCs
We included statistically significant factors in univariate analysis, namely, BMI grading, type of anatomical lung resection, surgical lobe, intraoperative bleeding grading, and length of anesthesia grading, in our binary logistic regression model, to evaluate the perioperative predictors of PPCs.
Binary logistics regression analysis demonstrated that BMI grading (≥24.0 kg/m2 vs <24.0 kg/m2: OR 1.514, 95% CI 1.057–2.167, P=0.024), type of anatomical lung resection (single segmentectomy vs single lobectomy: OR 2.115, 95% CI 1.150–3.891, P=0.016; bilobectomy or combined lobectomy and segmentectomy vs single lobectomy: OR 2.731, 95% CI 1.013–7.361, P=0.047), and surgical lobe (right vs left lung lobe: OR 1.519, 95% CI 1.046–2.205, P=0.028) were independent risk factors of PPCs after minimally invasive anatomic resection for lung cancer ().
Table 4 Logistic model of risk factors for postoperative pulmonary complications after minimally invasive anatomic resection for lung cancer
Discussion
This study has shown a PPC frequency of 24.8% in lung cancer patients undergoing minimally invasive anatomic resection, which is still associated with significantly poor short-term outcomes, including increased PLOS and total hospital care costs (). No patient died during the period of hospitalization. Furthermore, four independent risk factors for PPCs after minimally invasive anatomic resection for lung cancer were identified: BMI ≥24.0 kg/m2, single segmentectomy, bilobectomy or combined lobectomy and segmentectomy, and right lung lobe surgery ().
The incidence of PPCs after lung resection varies. Agostini et alCitation11 reported frequencies of 7.4%, and Lugg et alCitation6 documented a prevalence of 13.0%, whereas Arslantas et alCitation8 noted that PPCs occurred in 54.7% patients after lung resection. This fluctuation is due to the differences in the definition of PPCs. In the current study, PPCs were defined similarly to Arslantas et al,Citation8 which included acute respiratory distress syndrome, reintubation, pneumonia, need for bedside bronchoscopy, atelectasis, pulmonary embolism, prolonged air leak, and failure to expand during the period of postoperative hospitalization. Our incidence of PPCs was 24.8%, which was compatible with the reported frequency.Citation1 In our series, the four most frequent complications were pneumonia (20.3%), failure to expand (3.0%), atelectasis (2.2%), and prolonged air leak (3.0%) (). No reintubation was identified in our series.
BMI is a well-known risk factor for PPCs.Citation1 Being underweight or overweight has been shown to increase the risk of PPCs after lung resection.Citation12 Our data demonstrated that the risk of PPCs in patients with BMI ≥24.0 kg/m2 was higher than in patients with BMI <24.0 kg/m2 (OR 1.514, 95% CI 1.057–2.167, P=0.024) (). Obese patients often have reduced lung volume, altered ventilation pattern, and comorbid conditions, which are risk factors for intra-and postoperative complications.Citation13,Citation14 Being overweight is an important influencing factor on pre- and postoperative immune function.Citation15 Additionally, an elevated BMI in patients will lead to increased difficulty during surgery and prolonged operative times.Citation16 The prevalence of overweight and obesity is showing an upward trend.Citation17 Thoracic surgeons will encounter more overweight and obese lung cancer patients in the future. Therefore, it was important to find whether BMI ≥24.0 kg/m2 negatively impacts on outcomes after minimally invasive anatomic lung resection. Although a BMI ≥24.0 kg/m2 is not a contradiction for surgery, it should be considered when deciding to perform anatomic lung resection, given its strong association with PPCs.
Lobectomy has been the standard procedure for surgical treatment of non-small-cell lung cancer. In recent years, there has been growing evidence suggesting that segmentectomy can yield results equivalent to lobectomy in patients with early non-small-cell lung cancer.Citation18 However, few reports have investigated postoperative complications exclusively in patients with lung cancer undergoing lobectomy or segmentectomy. In the present investigation, compared with single lobectomy, single segmentectomy and bilobectomy or combined lobectomy and segmentectomy were associated with an increased risk of PPCs (). Why is segmentectomy more prone to PPCs? Patients after segmentectomy had worse mean FEV1 than after lobectomy.Citation19 When performing segmentectomy, surgeons have to divide the intersegmental plane using staplers or electrocautery, which may cause postoperative air leak and development of PPCs.Citation18 Moreover, frequent clamping and pulling of the healthy lung tissue during segmentectomy would lead to postoperative pulmonary edema and development of PPCs.Citation20 Unsurprisingly, bilobectomy or combined lobectomy and segmentectomy were associated with the development of PPCs because they produce greater trauma and more lung surface wound than single lobectomy.
Little information about the effects of lung surgery, performed on different lung lobes, on postoperative outcomes has been published to date. Our multivariate analysis revealed that patients undergoing right lung lobe surgery had an increased risk of PPCs (). The association is possibly related to the difference in lung volume between the left and right lung (ratio of right and left lung volume, 1.22±0.14),Citation21 so the right lung lobe surgery has a greater impact on lung function, resulting in greater trauma and a higher risk of PPCs. In addition, because most of the bilobectomy or combined lobectomy and segmentectomy surgeries involved the right lung (15/17), the surgery of the right lung lobe was associated with an increasing risk of PPCs.
Limitations
There were several limitations to this study. First, it was a single-center retrospective analysis, which may restrict the wide usage of our indications in clinics. A prospective multi-center study would be necessary to validate the risk factors of PPCs identified in our research and to define the best predictors of PPCs more rigorously. Second, numerous perioperative variables can lead to PPCs, but it is unlikely that all of these possible variables will be included in the study. Third, we only followed up our patients during their hospitalization. Hence, longitudinal studies are needed to evaluate the long-term clinical impacts of PPCs.
Conclusion
We identified several perioperative risk factors of PPCs following minimally invasive anatomic resection for lung cancer, including BMI ≥24.0 kg/m2, single segmentectomy, bilobectomy or combined lobectomy and segmentectomy, and right lung lobe surgery. These results should be helpful for risk stratification, patient counseling, and perioperative care of lung cancer patients. Further studies are necessary to validate these findings and create a clinical scoring system to predict PPCs.
Acknowledgments
This research was funded by the Natural Science Foundation of Zhejiang Province (grant number LQ18H180002) and the National Natural Science Foundation of China (grant number 31700690). In addition, we appreciate the assistance of Large-scale Data Analysis Center of Cancer Precision Medicine-LinkDoc database for clinical and pathological data collected.
Supplementary material
Table S1 Assignment of variables in binary logistics regression analysis
Disclosure
The authors report no conflicts of interest in this work.
References
- KimESKimYTKangCHPrevalence of and risk factors for postoperative pulmonary complications after lung cancer surgery in patients with early-stage COPDInt J Chron Obstruct Pulmon Dis2016111317132627366059
- HowingtonJABlumMGChangACBalekianAAMurthySCTreatment of stage I and II non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of chest physicians evidence-based clinical practice guidelinesChest20131435 Supple278Se313S23649443
- GuptaHRamananBGuptaPKImpact of COPD on postoperative outcomes: results from a national databaseChest201314361599160623287892
- LuggSTAgostiniPJTikkaTLong-term impact of developing a postoperative pulmonary complication after lung surgeryThorax201671217117626769017
- SekineYSuzukiHYamadaYKohEYoshinoISeverity of chronic obstructive pulmonary disease and its relationship to lung cancer prognosis after surgical resectionThorac Cardiovasc Surg201361212413022535670
- LuggSTTikkaTAgostiniPJSmoking and timing of cessation on postoperative pulmonary complications after curative-intent lung cancer surgeryJ Cardiothorac Surg20171215228629433
- ShinCHLongDRMcleanDEffects of intraoperative fluid management on postoperative outcomes: a hospital registry studyAnn Surg201826761084109228288059
- ArslantasMKKaraHVTuncerBBEffect of the amount of intra-operative fluid administration on postoperative pulmonary complications following anatomic lung resectionsJ Thorac Cardiovasc Surg2015149131432125304302
- MommersEHHWegdamJAvan der WolkSNienhuijsSWde Vries ReilinghTSImpact of hernia volume on pulmonary complications following complex hernia repairJ Surg Res201721181328501135
- van LierFvan der GeestPJHoeksSEEpidural analgesia is associated with improved health outcomes of surgical patients with chronic obstructive pulmonary diseaseAnesthesiology2011115231532121796055
- AgostiniPJLuggSTAdamsKRisk factors and short-term outcomes of postoperative pulmonary complications after VATS lobectomyJ Cardiothorac Surg20181312829673386
- FergusonMKImHKWatsonSJohnsonEWigfieldCHVigneswaranWTAssociation of body mass index and outcomes after major lung resectionEur J Cardiothorac Surg2014454e94e9924504655
- SuemitsuRTakeoSHamatakeMMorokumaASuemoriYTanakaHThe results of surgery under general anesthesia in patients with lung cancerSurg Today2011411606621191692
- XiaLTaylorBLGuzzoTJCharacteristics and associated factors of postoperative pulmonary complications in patients undergoing radical cystectomy for bladder cancer: a national surgical quality improvement Program StudyClin Genitourin Cancer201715666166928479282
- LachmannGvon HaefenCKurthJYuerekFWerneckeKDSpiesCSmoking, gender, and overweight are important influencing factors on monocytic HLA-DR before and after major cancer surgeryBiomed Res Int20172017517
- SalemAIThauMRStromTJEffect of body mass index on operative outcome after robotic-assisted Ivor-Lewis esophagectomy: retrospective analysis of 129 cases at a single high-tertiary care centerDis Esophagus201730117
- WangCGuoMZhangNWangGAssociation of body mass index and outcomes following lobectomy for non-small-cell lung cancerWorld J Surg Oncol20181619029751759
- OhtsukaTKamiyamaIAsakuraKKohnoMThirty-day outcomes after lobectomy or segmentectomy for lung cancer surgeryAsian Cardiovasc Thorac Ann201523782883126071452
- ShapiroMWeiserTSWisniveskyJPChinCArustamyanMSwansonSJThoracoscopic segmentectomy compares favorably with thoracoscopic lobectomy for patients with small stage I lung cancerJ Thorac Cardiovasc Surg200913761388139319464454
- HamadaAOizumiHKatoHLearning curve for port-access thoracoscopic anatomic lung segmentectomyJ Thorac Cardiovasc Surg201815651995200330121137
- YuCGGrantCAIzattMTChange in lung volume following thoracoscopic anterior spinal fusion surgery: a 3-dimensional computed tomography investigationSpine2017421290991628609321