Abstract
Ticagrelor is a novel P2Y12 receptor antagonist which, like clopidogrel and prasugrel, functions by blocking adenosine diphosphate-mediated platelet aggregation. However, unlike the aforementioned agents, the binding of ticagrelor to this receptor is reversible. Ticagrelor is also believed to mediate some of its beneficial effects by augmenting the effects of adenosine, which is another unique pharmacologic property of this drug. In terms of antiplatelet effect, ticagrelor is more potent than clopidogrel and produces a faster and stronger inhibition of platelet aggregation. This may also be an advantage of ticagrelor over prasugrel, but this has not been adequately studied. Due to the reversible nature of the binding of ticagrelor to the platelet receptor, ticagrelor has a relatively fast offset of effect, with platelet aggregation approaching pretreatment levels about 3 days after discontinuation of therapy. This has advantages in patients requiring invasive procedures, but also makes medication adherence very important in order to be able to maintain an effective antiplatelet effect. Ticagrelor has been shown to be clinically superior to clopidogrel when given to patients with an acute coronary syndrome, resulting in significantly lower rates of myocardial infarction and vascular death. However, ticagrelor is indicated to be administered with aspirin, and the clinical benefits of ticagrelor may be less when daily dosages of aspirin exceed 100 mg. As expected, bleeding is the most common adverse effect with ticagrelor, although it occurs at rates comparable with those seen for clopidogrel with the exception of noncoronary artery bypass graft-related major bleeding and fatal intracranial bleeds, the latter of which occurs only rarely. Dyspnea is another common adverse effect with ticagrelor, although this is usually not severe and resolves with drug discontinuation. Unlike clopidogrel, there are no known pharmacogenomic concerns with ticagrelor, and emerging data suggest ticagrelor to be effective in patients resistant to clopidogrel, although more study is needed on this topic. While preliminary data suggest ticagrelor to be cost effective when compared with generic clopidogrel, the acquisition cost of ticagrelor is not insignificant and this will likely be an issue for many health care organizations. Currently, ticagrelor is well positioned to assume an active role in the treatment of coronary artery disease due to an impressive efficacy profile and reasonable safety. Its ultimate role in therapy will continue to evolve as studies on this drug continue eg, (Prevention of Cardiovascular Events in Patients with Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin, PEGASUS) and more information hopefully becomes available on its use in clopidogrel nonresponders and relative safety and efficacy compared with prasugrel.
Introduction
Patients with acute coronary syndromes (ACS) have high platelet activation which leads to thrombus formation and myocardial ischemia.Citation1 Platelets have numerous ways of becoming activated, so there are numerous targets for drugs which inhibit platelet function. Current antiplatelet agents available to clinicians for treating and preventing ACS include aspirin, glycoprotein IIb/IIIa receptor antagonists (tirofiban, abciximab, and eptifibatide), thienopyridines (clopidogrel and prasugrel), and ticagrelor. Aspirin inhibits cyclo-oxygenase-1 in platelets, which decreases thromboxane A2 concentrations. While aspirin reduces thrombotic events, stroke, and myocardial infarction by approximately 20%–25% through decreased platelet activation, this drug results in only partial inhibition of platelet aggregation (IPA).Citation2,Citation3 This has led to the use of other antiplatelet drugs with different mechanisms of action in addition to aspirin. Secondary prevention of recurrent thrombotic episodes sometimes requires the use of two antiplatelet agents with different mechanisms of action, ie, dual antiplatelet therapy. Dual antiplatelet therapy is now considered standard of care for patients with ACS with or without stenting.Citation4,Citation5
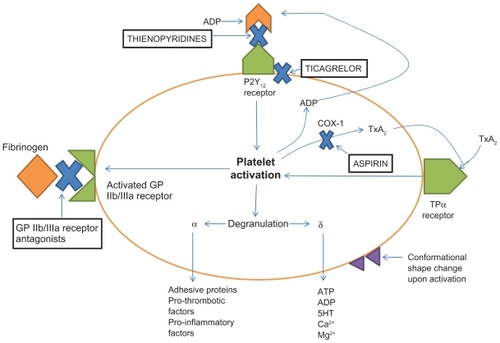
Thienopyridines (clopidogrel and prasugrel) work by covalently and irreversibly binding to the adenosine diphosphate (ADP) site on the P2Y12 receptor, thereby preventing ADP-mediated platelet aggregation for the life of the platelet (). The most utilized drug in this class is clopidogrel which has been shown to be beneficial in patients with ACS regardless of whether or not percutaneous coronary intervention is performed.Citation6,Citation7 Clopidogrel is a prodrug which needs to be converted to an active metabolite via hepatic activation mainly through cytochrome P450 (CYP) 2C19 and 3A4. This leads to delayed antiplatelet activity and potential interpatient variability because of genetic polymorphisms in CYP2C19, leading to increased or decreased activity of this enzyme. Prasugrel is also a prodrug which requires hepatic activation but, unlike clopidogrel, this drug is activated much faster and has better IPA. Studies have shown greater efficacy as well as more bleeding risk with prasugrel compared with clopidogrel.Citation8
Figure 1 Adenosine diphosphate (ADP) binds to the P2Y12 receptor. This binding results in activation of the platelet which causes a conformational shape change, activation of the glycoprotein (GP) IIb/IIa receptors, and platelet aggregation. Ticagrelor binds at a site on the P2Y12 receptor that is separate from the ADP binding site and produces a non-competitive inhibition, while the thienopyridines, clopidogrel and prasugrel, bind directly to the ADP binding site on the P2Y12 receptor permanently blocking this site. Antiplatelet agents that block the P2Y12 receptor have complementary effects with aspirin in terms of platelet inhibition because their mechanism of action is different than that of aspirin, which blocks cyclo-oxygenase-1 causing a decrease in thromboxane A2.
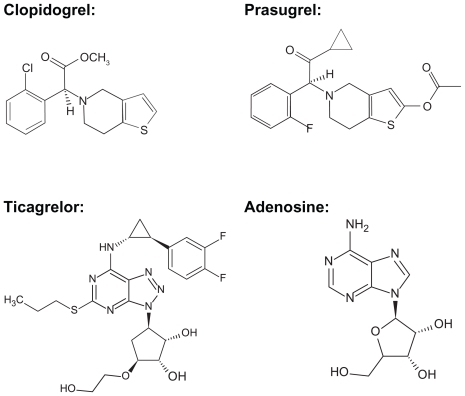
Ticagrelor is chemically, pharmacologically, and pharmacokinetically different to the thienopyridines (). This article will review the current literature surrounding the pharmacology, pharmacokinetics, clinical outcomes, adverse events, and practical therapeutic considerations with ticagrelor.
A literature search was conducted as recently as September 2011 using PubMed with the following search terms: “ticagrelor,” “Brilinta,” “PLATO trial,” “pharmacokinetics,” “pharmacodynamics,” “platelet aggregation,” “platelet activation”, and “antiplatelet drugs.” These terms were cross-referenced with each other. The literature search was only limited to articles published in English. There were no other limits so that a larger amount of studies could be collected and analyzed for utility. Additional articles were found by reviewing and cross-checking the references of relevant articles. Product package inserts and some other product-specific information were obtained from the manufacturers’ individual websites. The United States Food and Drug Administration website (fda.gov) was also reviewed for pertinent information.
Pharmacology
ADP is a potent chemical stimulator of platelet activation. ADP binds to and activates two different G-protein coupled receptors, P2Y1 and P2Y12. Activation of the P2Y1 receptor leads to reversible platelet aggregation and shape change, while activation of the P2Y12 receptor leads to a slower platelet aggregation that is more sustained and is not involved in any shape change. Both of these receptors need to be activated by ADP at the same time to elicit a typical platelet aggregation response.Citation9 Of the two receptors, P2Y12 is more tissue-selective, which makes this receptor an ideal target for drug therapy.Citation9
Both the thienopyridines and ticagrelor are P2Y12 receptor antagonists. Ticagrelor is not a thienopyridine, but rather belongs to a new class of P2Y12 receptor inhibitors known as cyclopentyl-triazolo-pyrimides. This drug is a very selective antagonist for the P2Y12 receptor on platelets and, unlike the thienopyridines, binds reversibly to this receptor. Also, unlike clopidogrel or prasugrel, ticagrelor allows ADP to bind to the P2Y12 receptor because this drug binds on a site that is distant from the ADP site on the P2Y12 receptor (). This noncompetitive inhibition blocks the conformational change of this receptor which is responsible for downstream activation of platelet aggregation.Citation10 When ticagrelor leaves the site on the P2Y12 receptor it does not alter the receptor which allows it to engage in conformational changes according to the appropriate stimuli, such as ADP binding. Ticagrelor has additional benefits beyond the inhibition of P2Y12 receptors on platelets. These P2Y12 receptors are also found in vascular smooth muscle which mediates vasoconstriction.
Ticagrelor has the ability to enter the smooth muscle of the vasculature and inhibit local P2Y12 receptors, thereby causing vasodilation. This ability to inhibit P2Y12 receptors in the vascular smooth muscle is unique to ticagrelor. Citation10 Another additional benefit of ticagrelor has to do with the ability of this drug to augment the effects of endogenous adenosine. During ACS, adenosine is released, which serves several beneficial functions, one of which is to increase coronary blood flow. There is typically an accumulation of adenosine in response to tissue ischemia and injury which occurs during ACS. Adenosine also acts as an anti-inflammatory and cardioprotective agent by improving preconditioning and postconditioning and preventing sudden death.Citation11 The chemical structure of ticagrelor is very similar to that of adenosine (). It is theorized that ticagrelor loses two key structures after oral administration, ie, propyl sulfate and benzodifluoride, by either oxygenase and/or radical fragmentation pathways. In this sense, ticagrelor is effectively a precursor of adenosine because, after the loss of these structures, the resulting compound is essentially adenosine.Citation11 Ticagrelor also inhibits uptake of adenosine by erythrocytes during ACS. The mechanism for this is thought to be inhibition of the sodium-independent equilibrative nucleoside transporters subtype 1. This transporter regulates the influx and efflux of adenosine particularly in cardiac myocytes.Citation12 This adenosine uptake inhibition appears to be dose-dependent and is 30–50-fold less potent compared with its ability to inhibit platelets.Citation10,Citation13 This results in augmentation of the coronary blood flow induced by adenosine by prolonging its half-life and increasing systemic exposure to this agent.Citation10,Citation13
Pharmacokinetics
Findings from pharmacokinetic studies in healthy volunteers and patients with atherosclerosis have provided most of the pharmacokinetic information available for ticagrelor.Citation14–Citation17 Ticagrelor is only available by the oral route and, at doses ranging from 100 to 400 mg per day, has rapid absorption from the gastrointestinal tract with peak plasma concentrations being achieved in a median of 1.5 hours and 3 hours for the parent compound and active metabolite (AR-C124910XX), respectively.Citation17 Inhibition of platelet aggregation of 80%–90% is seen within 2–4 hours after a single oral dose of ticagrelor of 180 mg.Citation15 The IPA is dose-dependent; however there is very little increase in IPA with doses greater than 100 mg twice daily.Citation16,Citation17 The half-lives of the parent drug and active metabolite are 8 and 12 hours, respectively. This, along with the reversible effects of the drug on platelet aggregation, necessitates twice daily dosing for ticagrelor. At 24 hours after a single ticagrelor dose of 300 mg and 400 mg, the mean IPAs were 75% and 85%, respectively.Citation16 The IPAs achieved by ticagrelor were higher than the IPA typically seen with clopidogrel, which is around 50%.Citation18 Pharmacokinetic studies have shown that ticagrelor is mainly restricted to the plasma space, which is where it will interact with platelets and the smooth muscle of the vasculature.Citation16 Ticagrelor is primarily metabolized hepatically by CYP3A4 and CYP3A5. Therefore, ticagrelor metabolism, blood levels, and ultimately pharmacologic effects, may be affected by other medications (eg, ketoconazole) or substances (eg, St John’s wort) that inhibit or induce CYP3A4 and/or 3A5 enzymes. While ticagrelor does not need to be bioactivated to inhibit platelet aggregation there is one active metabolite, ie, AR-C124910XX. The two enzymes most responsible for the production of this sole active metabolite are CYP3A4 and CYP3A5.Citation19 This active metabolite has plasma concentrations that are approximately one third that of its parent compound and is considered to be equipotent in terms of antiplatelet effects. Ticagrelor appears to inhibit CYP2C9 moderately and induce CYP2B6 weakly.Citation19 Ticagrelor is mainly excreted in the feces with very little of the parent drug and active metabolite (<0.05%) excreted unchanged in the urine. Therefore, no dosage adjustment is needed in patients with kidney dysfunction.Citation20 Ticagrelor has also been studied in volunteers with mild hepatic impairment. Citation21 The mean maximum concentration, area under the curve, and half-live of ticagrelor and its active metabolite were higher in volunteers with Child-Pugh class A compared with healthy volunteers but these differences are unlikely to be clinically significant and therefore no dosage adjustment is necessary in patients with mild liver dysfunction. There have been no studies looking at moderate (Child-Pugh B) or severe (Child-Pugh C) hepatic dysfunction. The prescribing information for ticagrelor does not provide specific dosing recommendations in patients with moderate hepatic impairment and states that the drug is contraindicated in patients with severe hepatic impairment.Citation20
Comparative antiplatelet effects of ticagrelor and clopidogrel
The Dose confIrmation Study assessing antiPlatelet Effects of AZD6140 versus clopidogRel in non-ST-segment Elevation myocardial infarction (DISPERSE) evaluated the pharmacodynamics, pharmacokinetics, safety, and tolerability of various dosages of ticagrelor (AZD6140) versus clopidogrel in patients receiving aspirin therapy.Citation17 This was a randomized, double-blind, parallel-group study conducted in patients with known atherosclerotic disease. The dosages of ticagrelor were 50 mg twice daily (n = 41), 100 mg twice daily (n = 39), 200 mg twice daily (n = 37), or 400 mg once daily (n = 46) and the dose of clopidogrel was 75 mg once daily (n = 37). Treatments were given for 28 days and all patients received aspirin 75–100 mg once daily. Platelet aggregation was analyzed via optical aggregometry of platelet-rich plasma taken from blood samples of the patients at times 0 (predose), 2, 4, 8, and 12 hours (post-dose) on days 1, 14, and 28 and post-dose at 24 hours on days 14 and 28. The IPA was measured using 20 μM ADP as the agonist. The safety of the trial medication was assessed by reports of adverse events, including bleeding. The results showed ticagrelor at dosages of 100 mg twice daily, 200 mg twice daily, and 400 mg once daily inhibited ADP-induced platelet aggregation to a greater extent compared with either clopidogrel or ticagrelor 50 mg twice daily. The three higher dosages of ticagrelor did not differ from each other in terms of mean IPA. With this increase in IPA, there was also an increase in bleeding with ticagrelor compared with clopidogrel. Most of the bleeding events were considered to be mild to moderate in severity. There was one major bleed in the ticagrelor group (400 mg once daily) that was gastrointestinal in nature. There was also an increased incidence of dyspnea in the ticagrelor group compared with the clopidogrel group which appeared to be dose-dependent, occurring with greatest severity in the patients receiving 400 mg once daily. The severity of dyspnea varied from mild to moderate, with a total of 29 reported instances of dyspnea, 21 of which were considered mild and eight were considered to be moderate.
Clopidogrel is the classic thienopyridine and the most popular nonaspirin antiplatelet agent used today. However, there are several limitations of clopidogrel therapy, mostly related to interpatient variability in pharmacologic effects. A substudy of the PLATelet inhibition and patient Outcomes (PLATO) trial (ie, PLATO PLATELET) showed ticagrelor to have greater and more consistent platelet inhibition than clopidogrel and did not increase the risk of bleeding.Citation22 The two cohorts that were evaluated in this substudy were 69 patients who had received either clopidogrel (300–600 mg loading dose followed by 75 mg per day) or ticagrelor (180 mg loading dose followed by 90 mg twice a day) for at least 28 days and 24 patients who had not received the study medication and have not received clopidogrel treatment within the previous 14 days. The three methods that were used to study platelet aggregation were light transmittance aggregometry (LTA), the VerifyNow P2Y12 assay (Accumetrics, Inc, San Diego, CA), and vasodilator-stimulated phosphoprotein. The LTA method uses ADP to stimulate platelet aggregation in ex vivo blood samples. A higher response to ADP (measured in μM) indicates more platelet aggregation. The VerifyNow P2Y12 assay evaluates baseline platelet function through the thrombin receptor-activating peptide channel and the combination of ADP and prostaglandin E1. The ADP is used to activate platelets through interaction with P2Y1 and P2Y12 receptors, while prostaglandin E1 is used to suppress ADP platelet activation via the P2Y1 receptor. A percent change is calculated from the baseline platelet function and results reported using P2Y12 reaction units. The threshold of >235 P2Y12 reaction units has been determined to be associated with an increased ischemic risk. Vasodilator-stimulated phosphoprotein measures the platelet reactivity index in which a threshold of >50% was determined to be associated with an increased ischemic risk. In all three studies of platelet aggregation, ticagrelor showed more suppression of platelets than clopidogrel in both peak and trough plasma concentrations. Loading doses of ticagrelor showed more platelet inhibition at one hour compared with clopidogrel.
Ticagrelor is not a prodrug and does not have to be metabolically activated to have an antiplatelet effect, while clopidogrel is a prodrug which needs to be activated before eliciting a pharmacologic effect. The bioactivation of clopidogrel occurs in two sequential steps in the liver, with one pathway going through the CYP system, particularly CYP2C19. Once clopidogrel is activated by this enzyme, the active metabolite can inhibit platelets. This bioactivation of clopidogrel takes time and is evident even with loading doses of clopidogrel. A loading dose of clopidogrel significantly shortens the time to achieve maximal IPA; without a loading dose, it takes approximately 5 days to reach maximal IPA with clopidogrel 75 mg once daily.Citation23 With 300 mg and 600 mg loading doses of clopidogrel, it takes about 4–8 hours to reach the final extent of platelet aggregation inhibition (ie, IPA observed at the end of the platelet aggregation response), which is about 30% and 45%–50%, respectively, for the 300 mg and 600 mg doses of clopidogrel.Citation15,Citation24 This is in comparison with ticagrelor in which the final IPA of 80%–90% is reached approximately 2–4 hours after a 180 mg loading dose ().Citation15,Citation24
Table 1 Comparison of P2Y12 receptor inhibitorsCitation15,Citation20,Citation24,Citation26,Citation58
The ONSET/OFFSET study was a randomized, multicenter, double-blind trial to evaluate the time to onset and offset of antiplatelet effects of ticagrelor 90 mg given twice daily compared with placebo and clopidogrel 75 mg once daily.Citation15 This study included patients with stable coronary artery disease who were receiving low-dose (75–100 mg per day) aspirin therapy. Patients were divided into one of three groups, ie, ticagrelor (n = 57), clopidogrel (n = 54), or placebo (n = 12). The ticagrelor and clopidogrel groups received loading doses (180 mg and 600 mg, respectively) before receiving the maintenance dosages. Fifty patients in each of the treatment arms were necessary for a 91% power to detect mean differences in IPA of 15% or more in the two treatment groups. Platelet function was determined by the use of three tests, ie, LTA, the VerifyNow P2Y12 assay, and vasodilator-stimulated phosphoprotein-P. The primary outcome for onset was IPA (20 μmol/L ADP) at 2 hours post initial dose, and offset was assessed by the slope of the IPA between 4 and 72 hours after the final study dose. The primary outcome was much greater in the ticagrelor group compared with the clopidogrel group (88% versus 38%, P < 0.0001). There was no difference in IPA in the ticagrelor group at 2 hours and 8 hours post loading dose, while IPA was greater in the clopidogrel group at 8 hours compared with 2 hours post loading dose (P = 0.02). The maximum IPA was much higher in the ticagrelor group (93%) when compared with the clopidogrel group (58%) after the loading dose (P value not reported). The time to reach maximum IPA was faster in the ticagrelor group (2.0 hours) compared with the clopidogrel group (7.8 hours; P value not reported). Ticagrelor also had a faster offset of antiplatelet action compared with clopidogrel. The primary outcome for offset was higher in the ticagrelor group than in the clopidogrel group (P < 0.0001).
A subanalysis of the ONSET/OFFSET data focused on the offset of antiplatelet action of both ticagrelor and clopidogrel with a high antiplatelet drug response.Citation25 Platelet activity was evaluated in this study in a similar fashion to the other studies discussed in this section. All three tests, LTA, VerifyNow, and vasodilator-stimulated phosphoprotein-P, showed significant differences in platelet function for ticagrelor compared with clopidogrel at 48 hours after the last dose was given. The IPA at 48 hours for clopidogrel was approximately 60% compared with less than 40% for ticagrelor (P < 0.01). There was good recovery of platelet function by 72 hours in patients treated with ticagrelor, with IPAs of about 20% in patients with high platelet reactivity and about 10% in patients without high platelet reactivity. This is in comparison with values of about 45% and 20%, respectively, for clopidogrel. The IPA for ticagrelor after 2 days (36%) was similar to the IPA for clopidogrel after 5 days (33%) from the last treatment dose. Since it is recommended that clopidogrel be withheld 5 days prior to surgery,Citation26 these data can be useful for gauging how long a clinician should withhold ticagrelor before an invasive procedure. While the prescribing information for ticagrelor recommends discontinuation of ticagrelor 5 days prior to surgery,Citation20 one can argue for a shorter window of about 3 days based on the ONSET/OFFSET data.
Clinical trials
The clinical trial program for ticagrelor is weighted heavily on two studies, ie, DISPERSE-2Citation27 and the PLATelet inhibition and patient Outcomes (PLATO) trial.Citation28 Based on the original DISPERSE trial, which demonstrated good safety and tolerability and superior antiplatelet efficacy with 100 mg and 200 mg twice daily dosages of ticagrelor compared with clopidogrel,Citation17 the multicenter DISPERSE-2 trial was performed to analyze the safety and efficacy of ticagrelor in 984 patients with non-ST elevation ACS. These patients were randomized in a double-blind fashion to receive ticagrelor 90 mg twice daily (n = 334), ticagrelor 180 mg twice daily (n = 323), or clopidogrel 75 mg once daily following a 300 mg loading dose (n = 327) in addition to aspirin and other adjunctive therapies. It should be noted that the 90 mg and 180 mg doses of ticagrelor used in DISPERSE-2 were reformulations of the 100 mg and 200 mg doses used in DISPERSE. The primary endpoint of major or minor bleeding at 4 weeks was seen with similar frequencies in each group (P = not statistically significant [NS]): 8.1% (clopidogrel), 9.8% (ticagrelor 90 mg), and 8.0% (ticagrelor 180 mg). When major and minor bleeds were analyzed separately, the only statistically significant difference was that minor bleeds were seen more frequently with 180 mg twice daily of ticagrelor compared with clopidogrel at 4 weeks (1.3% versus 3.8%; P = 0.05) and at 12 weeks (1.3% versus 6.1%; P = 0.01). With regards to the secondary endpoint of major adverse cardiovascular events (myocardial infarction [MI], death, stroke, severe recurrent ischemia), a favorable trend was seen only with the ticagrelor 180 mg group compared with clopidogrel in terms of fewer MIs at 4 weeks (3.5% versus 1.0%; P = 0.047) and 12 weeks (5.6% versus 2.5%; P = 0.06). Nonhemorrhagic adverse events were comparable between the clopidogrel, 90 mg ticagrelor, and 180 mg ticagrelor groups except for dyspnea (6.4%, 10.5% [P = 0.07 versus clopidogrel] and 15.8% [P < 0.0002 versus clopidogrel], respectively), nausea (3.4%, 6.6% and 6.5% [both P = 0.07 versus clopidogrel]), diarrhea (3.4%, 3.0% [P = NS versus clopidogrel] and 7.4% (P = 0.02 versus clopidogrel), hypotension (0.6%, 4.2% [P = 0.004 versus clopidogrel] and 3.7% [P = 0.01 versus clopidogrel]), and asymptomatic ventricular pauses > 2.5 seconds (4.3%, 5.5% [P = NS versus clopidogrel] and 9.9% [P = 0.014 versus clopidogrel]). A subgroup of patients (n = 330) received a 270 mg loading dose of ticagrelor prior to their maintenance dosage, but this was found to have no impact on the rate of the primary endpoint. In summary, DISPERSE-2 demonstrated 90 mg ticagrelor twice daily to possess similar safety and efficacy compared with clopidogrel, and ticagrelor 180 mg twice daily to have poorer safety compared with clopidogrel. Accordingly, the 90 mg twice daily dose of ticagrelor was pursued further in clinical development with the PLATO trial.
The multicenter PLATO trial randomized 18,624 patients with ACS (symptom onset within 24 hours) to receive double- blind treatment with either ticagrelor 90 mg twice daily following a 180 mg loading dose (n = 9333) or clopidogrel 75 mg once daily following a 300–600 mg loading dose (n = 9291).Citation28 All patients also received aspirin 75–100 mg daily (325 mg daily was permitted for 6 months after stent placement). The final diagnosis was ST elevation MI in 38% of patients, non-ST elevation MI in 43%, and unstable angina in 17%; 64% underwent percutaneous intervention during the study period and 10% underwent coronary artery bypass grafting (CABG). The primary endpoint of vascular death, MI, or stroke at 12 months (median duration of treatment was 277 days) occurred in 9.8% of patients receiving ticagrelor and 11.7% of patients receiving clopidogrel (hazard ratio [HR] 0.84; 95% confidence interval [CI] 0.77–0.92; P < 0.001). As individual endpoints, ticagrelor resulted in significant reductions in vascular death (4.0% versus 5.1%; P = 0.001) and MI (5.8% versus 6.9%; P = 0.005), but not stroke (1.5 versus 1.3%; P = 0.22). Total mortality (4.5% versus 5.9%; P < 0.001) and stent thrombosis (1.3% versus 1.9%; P = 0.009) were also lower with ticagrelor. Major bleeding rates were comparable between ticagrelor and clopidogrel (11.6% versus 11.2%; P = 0.43), although combined major and minor bleeding was greater with ticagrelor (16.1% versus 14.6%; P = 0.008), as was non-CABG-related major bleeding (4.5% versus 3.8%; P = 0.03) and fatal intracranial bleeding (0.1 versus 0.01%; P = 0.02), although fatal nonintracranial bleeding was higher with clopidogrel (0.3% versus 0.1%; P = 0.03). Despite the low event rates, intracranial bleeding is enough of a concern that prior intracranial hemorrhage is a contraindication to ticagrelor therapy.Citation20 As with the DISPERSE-2 trial, dyspnea was the major nonhemorrhagic side effect of ticagrelor, occurring in 13.8% of patients (7.8% clopidogrel; P < 0.001) and leading to discontinuation of study treatment in 0.9% of patients (0.1% clopidogrel; P < 0.001). While ventricular pauses of at least 3 seconds were more common with ticagrelor during the first week of treatment (5.8% of patients versus 3.6% with clopidogrel; P = 0.01), these had subsided by 30 days of treatment (2.1% versus 1.7% clopidogrel; P = 0.52) and were rarely symptomatic. Increases in serum uric acid (15% versus 7%) and serum creatinine (11% versus 9%) at 12 months were also more common with ticagrelor compared with clopidogrel (P < 0.001 for both).
Several subanalyses of the PLATO trial have been published, which corroborate the overall result of the parent trial. Specifically, patients with ST elevation MI intended for reperfusion with primary percutaneous coronary intervention (n = 7544),Citation29 patients intended for noninvasive management of ACS (n = 5216),Citation30 patients intended for invasive management of ACS (n = 13,408),Citation31 patients undergoing CABG (n = 1899),Citation32 patients with diabetes (n = 4662),Citation33 and patients with chronic kidney disease (creatinine clearance < 60 mL/min; n = 3237)Citation34 all demonstrated efficacy and safety with ticagrelor similar to that seen in the original PLATO trial.
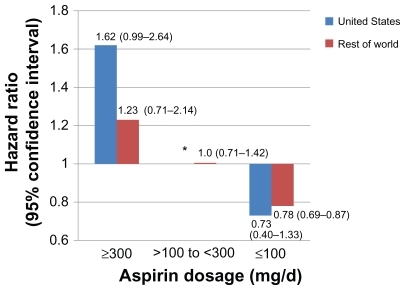
Sixty-six different predefined subgroups were analyzed in PLATO, 33 looking at the primary efficacy endpoint (eight of which were defined post hoc rather than predefined) and 33 looking at major bleeding. With regards to efficacy, 30 of 33 subgroups showed ticagrelor to be more efficacious than clopidogrel; the three subgroups that showed no efficacy benefit with ticagrelor compared with clopidogrel were: patients enrolled in North American sites (HR 1.25, 95% CI 0.93–1.67); males <82 kg or females <71 kg (HR 0.93, 95% CI 0.82–1.05); and patients not taking a lipid-lowering drug (HR 1.02, 95% CI 0.83–1.24).Citation28 For major bleeding, ticagrelor was similar to clopidogrel in all subgroups except those patients with a body mass index of 30 kg/m2 or greater, who experienced more major bleeding with ticagrelor (HR 1.21, 95% CI 1.02–1.45; P = 0.05). Of particular concern was the North American subgroup, which not only demonstrated a lack of benefit from ticagrelor compared with clopidogrel, but actually suggested that perhaps clopidogrel was a more efficacious drug in this population.Citation28 This finding initially led the United States Food and Drug Administration not to approve ticagrelor in the United States in 2010 pending further investigation of this geographic phenomenon. After several months of deliberation and data analysis by two independent statistical groups, it was concluded that the trial conduct was appropriate and that this phenomenon might have been a play of chance.Citation35 However, of 37 different factors studied in effect modifier analyses, aspirin dosage stood out as the most likely explanation for the regional differences in treatment effect. Specifically, 53.6% of patients in the United States took a median daily aspirin dosage of at least 300 mg compared with 1.7% in the rest of the world. In the United States, patients taking at least 300 mg per day of aspirin, the HR for the primary efficacy endpoint was 1.62 (95% CI 0.99–2.64), suggesting benefit with clopidogrel over ticagrelor. When lower daily dosages of aspirin were analyzed (≤100 mg), ticagrelor actually demonstrated a benefit in the United States population similar to that seen in the rest of the world ().Citation35 For this reason, the United States labeling for ticagrelor clearly states that maintenance dosages of aspirin should not exceed 100 mg daily for patients taking ticagrelor.Citation20
Figure 3 Hazard ratios and 95% confidence intervals (in parentheses) for the PLATO study population with regards to the primary efficacy endpoint subdivided by daily aspirin dosage and geographic region. Daily dosages of aspirin > 300 mg were associated with a blunting of the benefit of ticagrelor, whereas dosages < 100 mg were associated with benefit of ticagrelor compared to clopidogrel. This finding was seen regardless of geographic region, but was most pronounced in the United States population which tended to use higher daily dosages of aspirin compared to the rest of the world.Citation35
The clinical trial program with ticagrelor continues with the ongoing PEGASUS-TIMI 54 trial being conducted by the Thrombolysis in Myocardial Infarction (TIMI) investigators. PEGASUS (Prevention of Cardiovascular Events in Patients with Prior Heart Attack Using Ticagrelor Compared with Placebo on a Background of Aspirin) is expected to enroll 21,000 post-MI patients with stable coronary artery disease from 30 countries, with 25% of subjects expected to be from the United States. The primary endpoint is cardiovascular death, nonfatal MI, or nonfatal stroke. The three treatments being studied are ticagrelor 90 mg twice daily, ticagrelor 60 mg twice daily, and placebo. This study is scheduled to be completed in February 2014.Citation36
Adverse effects
As mentioned above, the PLATO study demonstrated, not unexpectedly, that bleeding was the most common adverse effect seen with ticagrelor therapy, with major or minor bleeds occurring in 16.1% of patients (versus 14.6% with clopidogrel; P = 0.008).Citation28 However, ticagrelor also has several off-target adverse effects (), arguably one of the more concerning being dyspnea. The DISPERSE-2 trial demonstrated a dose-response relationship with ticagrelor and dyspnea, with a 10.5% incidence of this adverse effect with ticagrelor 90 mg twice daily and a 15.8% incidence with 180 mg twice daily (versus 6.4% with clopidogrel).Citation27 A subgroup analysis of the ONSET/OFFSET trial assessed cardiopulmonary function in 123 patients receiving ticagrelor 90 mg twice daily, clopidogrel 75 mg once daily, or placebo. Citation37 The investigators were mainly interested in dyspnea, defined as shortness of breath either during exercise or at rest and was graded as mild, moderate, or severe. Cardiopulmonary evaluations were evaluated at baseline and at the end of 6 weeks of treatment or sooner if dyspnea or other adverse events were recorded. Dyspnea occurred in 38.6%, 9.3%, and 8.3% of patients in the ticagrelor group, clopidogrel group, and placebo group, respectively (ticagrelor versus clopidogrel, P < 0.001; ticagrelor versus placebo, P < 0.05; clopidogrel versus placebo, not statistically significant). Most of the cases of dyspnea were considered mild in nature with only three cases in the ticagrelor group deemed to be of moderate severity. Three patients stopped taking the study treatment (all were taking ticagrelor) due to dyspnea; two were categorized as moderate dyspnea and one as mild. No cases of dyspnea were deemed to be severe. In the ticagrelor group, 17 of the 22 patients who experienced dyspnea had this occur within the first week of starting medication. While many cases of dyspnea with ticagrelor lasted less than 24 hours, several patients experienced dyspnea over the duration of the study. Most cases of dyspnea resolved following drug discontinuation. Electrocardiography, echocardiography, and pulmonary function tests were also performed at baseline and after 6 weeks of treatment, none of which were significantly affected by ticagrelor.
Table 2 Nonhemorrhagic side effects observed with ticagrelor in PLATOCitation20,Citation28
Dyspnea and pulmonary function were also extensively analyzed in the PLATO trial, which demonstrated dyspnea rates of 13.8% with ticagrelor (versus 7.8% with clopidogrel; P < 0.001) and resulted in discontinuation of study treatment in 0.9% of patients (versus 0.1% clopidogrel; P < 0.001).Citation28 Overall, 14.5% of patients randomized to ticagrelor reported dyspnea at any point during the study (both on and off study treatment) compared with 8.7% of patients taking clopidogrel (P < 0.001).Citation38 Of these cases, only 0.4% (ticagrelor) and 0.3% (clopidogrel) were deemed by the study investigator to be of severe intensity. The median onset of dyspnea symptoms was 23 days from the onset of therapy with ticagrelor versus 43 days with clopidogrel (P < 0.0001). The majority of cases of dyspnea resolved either spontaneously or upon discontinuation of medication. The overall efficacy and safety of ticagrelor with respect to major adverse cardiovascular events and bleeding did not appear to be affected by the presence of dyspnea.Citation38 Pulmonary function studies were also performed in 199 patients in PLATO (101 patients receiving ticagrelor, 98 patients receiving clopidogrel), with no significant changes in pulmonary function demonstrated with ticagrelor administration in this subgroup.Citation39 While the mechanism for ticagrelor-induced dyspnea had not been clearly elucidated, a popular hypothesis is that ticagrelor inhibits adenosine reuptake and thereby increases extracellular adenosine concentrations, and exogenous adenosine administration is known to cause dyspnea, perhaps through activation of A1 receptors and resulting stimulation of pulmonary vagal C fibers.Citation38–Citation40
Another concerning adverse effect with ticagrelor in clinical trials was a dose-related increase in the incidence of ventricular pauses. This was initially seen in the DISPERSE-2 trial and was one factor in the abandonment of the 180 mg dosage of ticagrelor in late-phase clinical development.Citation27 A subgroup of 2908 patients in the PLATO trial underwent continuous ECG monitoring to document the frequency of ventricular pauses and clinical bradycardic events in patients taking ticagrelor (n = 1472) or clopidogrel (n = 1436).Citation41 Ventricular pauses lasting at least 3 seconds occurred more frequently with ticagrelor (5.8%) compared with clopidogrel (3.6%; P = 0.006) during the first week of therapy, but this difference was not significant after 30 days of treatment (2.1% versus 1.7% for ticagrelor and clopidogrel, respectively, P = 0.52). These pauses were largely asymptomatic, transient, nocturnal, and emanated from the sinus node. The incidence of clinically reported bradycardic adverse events did not differ between ticagrelor and clopidogrel through 12 months of follow- up. As with ticagrelor-induced dyspnea, the mechanism(s) responsible for ventricular pauses with ticagrelor remain to be elucidated but may involve inhibition of adenosine reuptake by erythrocytes with resulting increases in adenosine concentrations at the sinoatrial and atrioventicular nodes.Citation41
In the PLATO trial, elevations in serum creatinine and uric acid were seen more frequently in patients taking ticagrelor compared with clopidogrel ().Citation28 Concentrations of these compounds returned to baseline following drug discontinuation. The mechanism(s) for this is unknown, but may be related to impaired purine catabolism due to increased adenosine concentrations.Citation42 These side effects have not been attributed to either prasugrel or clopidogrel.
Drug interactions
As mentioned earlier, ticagrelor has the advantage over the thienopyridines of not requiring metabolic activation for activity. This would theoretically reduce the potential for drug-drug interactions with ticagrelor. Both CYP3A4 and, to a lesser extent, CYP3A5 have been identified as the isozymes responsible for the formation of the active and inactive metabolites of ticagrelor.Citation19 As such, strong inhibitors (ie, ketoconazole, itraconazole, clarithromycin, nefazodone, ritonavir, saquinavir, nelfinavir, indinavir, atazanavir, telithromycin) or inducers (ie, rifampin, dexamethasone, phenytoin, carbamazepine, phenobarbital) of these enzymes may interfere with the activity of ticagrelor, and concomitant administration should be avoided.Citation20 Ticagrelor is also an inhibitor of CYP3A4 and CYP3A5 and may increase serum concentrations of simvastatin and lovastatin which rely on CYP3A4 for metabolism. The daily dosages of simvastatin and lovastatin should not exceed 40 mg when given in combination with ticagrelor.Citation20 Ticagrelor is an inhibitor of the P-glycoprotein transporter and may therefore increase serum digoxin concentrations; these should be monitored with initiation or change in ticagrelor therapy.Citation20 As mentioned above, aspirin dosage should not exceed 100 mg per day when given with ticagrelor due to a potential loss in ticagrelor efficacy. Ticagrelor can be safely administered with unfractionated or low-molecular-weight heparin, glycoprotein IIb/IIIa inhibitors, beta-blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor antagonists, and proton pump inhibitors.Citation20 Proton pump inhibitors have mechanistically been shown to inhibit CYP2C19, which may interfere with the activation of clopidogrel. However, it is debatable as to whether or not giving clopidogrel in combination with a proton pump inhibitor is detrimental in terms of clinical outcomes.Citation43,Citation44 Because CYP2C19 is not a major metabolic pathway for ticagrelor metabolism, a potential pharmacokinetic interaction with proton pump inhibitors is not concerning.
Other practical considerations with ticagrelor therapy
The major efficacy and safety issues involving ticagrelor therapy have been discussed up to this point, but much more needs to be considered when deciding on how to integrate ticagrelor into clinical practice. Some of these issues are discussed below and summarized in .
Table 3 Practical considerations when comparing clopidogrel, prasugrel, and ticagrelor
Aspirin dosage
The prescribing information for ticagrelor contains a boxed warning regarding a potential loss of efficacy with ticagrelor when given concomitantly with daily aspirin dosages exceeding 100 mg per day.Citation20 The genesis of this warning is discussed earlier in this article, and clinicians and patients need to be aware of this and prescribe and consume aspirin in accordance with this warning. While a biological explanation has not been clearly defined to explain why higher dosages of aspirin may blunt the effectiveness of ticagrelor, it has been hypothesized that some of the benefits of ticagrelor may be dependent on prostacyclin, the production of which may be blocked by daily doses of aspirin exceeding 80 mg.Citation45 The reliance on prostacyclin for an antiplatelet effect may relate directly to the degree of P2Y12 inhibition, which would explain why the more potent ticagrelor would be more susceptible to an aspirin interaction than clopidogrel.Citation45 In the absence of a clear explanation for an aspirin-ticagrelor interaction, some experts are reluctant to accept that such an interaction even exists.Citation46 Even though the European countries in PLATO all used the same low daily dosage of aspirin, there were differences in outcomes among those countries, with some results favoring clopidogrel, some favoring ticagrelor, and some neutral, arguing that aspirin dosage did not impact the study results.Citation46
Others have looked at the issue of an interaction between aspirin and ticagrelor from a different perspective, namely questioning whether or not aspirin is even needed when ticagrelor is being administered. This is a difficult question to answer because aspirin is the standard of care for treatment and prevention of atherothrombotic disease and the thienopyridines and ticagrelor are added to, rather than given in place of, aspirin in clinical trials for ethical reasons. From a pharmacologic perspective, potent P2Y12 receptor blockade leads to a reduction in the ability of platelets to produce thromboxane A2, the primary target for the antiplatelet effect of aspirin.Citation45,Citation47 Accordingly, it has been questioned whether or not aspirin is having much, if any, antiplatelet effect in this setting because the potent P2YCitation12 antagonists are essentially serving an aspirin-like function in suppressing thromboxane A2 production. In this scenario, aspirin may paradoxically be detrimental, because it may then function primarily to suppress production of beneficial prostanoids such as prostaglandin I2.Citation45 While prostanoid inhibition is also seen with aspirin monotherapy, the beneficial antiplatelet effect may outweigh any detrimental effects on prostanoid production, resulting in a net clinical benefit.Citation45 The determination of how much incremental benefit aspirin adds to thienopyridine or ticagrelor therapy needs to be investigated in clinical trials before any conclusions can be made in this regard. For now, published clinical studies support the combination of a P2Y12 receptor antagonist with aspirin and clinical practice should reflect this.
Efficacy in clopidogrel nonresponders
It has been fairly well established that there is interpatient variability in the antiplatelet effects of clopidogrel, which is largely believed to be due to genetic polymorphisms of the CYP2C19 enzyme responsible for converting clopidogrel to its active form.Citation48,Citation49 It has been documented that the frequencies of poor metabolizers of CYP2C19 are 2% in White, 4% in Black, and 14% in Chinese racial groups.Citation26 The two polymorphisms that account for nonfunctional CYP2C19 enzymes are CYP2C19*2 and *3 which are seen in 85% of White and 99% of Asian poor metabolizers.Citation26 Major adverse cardiovascular events have been shown to occur more often in patients with reduced CYP2C19 function taking clopidogrel compared with those having good CYP2C19 function.Citation50 Genetic testing for CYP2C19 polymorphisms is now commercially available and is beginning to gain traction as a means of screening patients to predict the antiplatelet efficacy of clopidogrel. Patients who have a diminished antiplatelet effect in response to clopidogrel are deemed “nonresponders” or “poor responders”, and a proven alternative antiplatelet therapy for these patients would be very desirable.
The Response to Ticagrelor in Clopidogrel Nonresponders and Responders and Effect of Switching Therapies (RESPOND) study was designed to investigate the antiplatelet effect of ticagrelor in clopidogrel nonresponders.Citation51 Clopidogrel response was judged in 98 patients with stable coronary artery disease based on ADP-induced platelet aggregation following administration of a 300 mg loading dose of clopidogrel. Inhibition of platelet aggregation using LTA of >10% defined clopidogrel response and ≤10% clopidogrel nonresponse. After being categorized as a clopidogrel responder (n = 57) or nonresponder (n = 41), patients were randomly assigned to receive one of two double-blind treatments, ie, a 600 mg clopidogrel load followed by 75 mg once daily for 14 ± 2 days or a 180 mg ticagrelor load followed by 90 mg twice daily for 14 ± 2 days. After this initial study phase, all of the nonresponders switched study treatments, while only half of the responders switched treatments (the other half remained on the same treatment), which were also given for 14 ± 2 days. All patients also received aspirin 75–100 mg once daily. Of the patients initially identified as nonresponders to clopidogrel, 100% responded to ticagrelor after 2 weeks of treatment, defined as a >10% decrease in platelet aggregation from baseline. This is in comparison with a 75% response rate for clopidogrel (P = 0.005). Decreases in platelet aggregation of >30% and >50% were seen in 75% and 13% of ticagrelor patients, respectively, compared with 13% (P < 0.001) and 0% (P = 0.046) of patients receiving clopidogrel. Mean platelet aggregation increased from 36% to 56% (P < 0.0001) in the patients who crossed over to receive clopidogrel (initially on ticagrelor) in the second phase of the study, whereas those patients who were initially on clopidogrel and crossed over to receive ticagrelor demonstrated a decrease in mean platelet aggregation from 59% to 35% (P < 0.0001). In patients initially identified as clopidogrel responders, ticagrelor consistently demonstrated greater inhibition of platelet aggregation both before and after treatment crossover. These results not only demonstrated that ticagrelor was superior to clopidogrel in terms of inhibiting platelet aggregation both in clopidogrel responders and nonresponders, but also that ticagrelor is effective in patients who do not demonstrate an antiplatelet effect in response to clopidogrel, producing inhibition of platelet aggregation in these patients that is comparable with that seen in clopidogrel responders.
Genetic substudies were performed in the RESPOND, ONSET/OFFSET, and PLATO trials in an attempt to determine the effect of CYP2C19 metabolizer status on the efficacy of ticagrelor and clopidogrel.Citation52,Citation53 The combined data from RESPOND and ONSET/OFFSET demonstrated the antiplatelet effects of clopidogrel to be dependent on the CYP2C19 genotype, with loss-of-function carriers (ie, poor metabolizers) and intermediate metabolizers having a higher degree of platelet function. No such relationship was seen with ticagrelor.Citation52 In the PLATO genetic substudy, patients with a loss-of-function CYP2C19 allele receiving clopidogrel had a higher incidence of the primary endpoint of major adverse cardiovascular events compared with patients receiving clopidogrel who did not have any loss-of-function alleles. The event rate with ticagrelor was similar regardless of CYP2C19 polymorphisms.Citation53
Together, these studies demonstrate ticagrelor to have comparable efficacy regardless of CYP2C19 metabolizer status, unlike clopidogrel, and to be a viable alternative to clopidogrel in those patients deemed to be nonresponders to clopidogrel.
Economics
As with many new medications, drug cost promises to be a critical factor in determining the current place in therapy for ticagrelor, particularly with the pending generic availability of clopidogrel (expected May 2012 in the United States). While the acquisition cost of ticagrelor will in all probability be higher than generic clopidogrel, economic factors other than drug cost itself need to be considered when deciding on the status of clopidogrel by institutional and health plan formularies. Cost effectiveness data would represent one of these factors, and data with ticagrelor have just recently started to emerge in this regard.
An unpublished health economics substudy (available in abstract form only) from PLATO compared quality-adjusted like years (QALY) for ticagrelor and clopidogrel.Citation54 Based on PLATO event rates and drug costs of €0.17 ($0.23 United States dollars [USD]) per day for generic clopidogrel and €2.25 ($3.00) to €3.50 ($4.65) per day for ticagrelor, health care costs (based on Swedish base-case analysis) and QALYs were estimated. The results showed that patients receiving ticagrelor would be expected to gain an additional 0.13 QALYs at a cost of €2350 ($3110) to €5700 ($7550) per QALY compared with clopidogrel. This would indicate ticagrelor to be cost effective compared with clopidogrel since a threshold of €38,000 ($50,000) per QALY is typically used to determine cost effectiveness in the United States. Another cost effectiveness analysis focused on comparing ticagrelor with genotype-driven therapy with clopidogrel in a cohort of Medicare patients with ACS.Citation55 That study demonstrated an incremental cost effectiveness ratio of $10,059 USD per QALY with ticagrelor therapy compared with genotype-driven clopidogrel therapy over a 5-year period. Even in a simulation in which clopidogrel was $4 USD per month, the cost effectiveness ratio would be $11,927 USD per QALY with ticagrelor. It is estimated that ticagrelor would remain cost effective compared with genotype-driven clopidogrel therapy as long as the monthly cost of ticagrelor did not exceed $693 USD per month. The average wholesale price at the time of writing for one month of ticagrelor therapy is $265 USD ().
Medication adherence
As mentioned above, ticagrelor not only has a rapid onset in terms of antiplatelet activity, but it also has a faster offset than the thienopyridines. This may be beneficial, as mentioned earlier, in potentially decreasing the waiting time to surgery, especially if a patient needs CABG. However, there is also some concern that the relatively fast offset of antiplatelet effect may make medication adherence that much more important with ticagrelor. It has been shown that the antiplatelet effects of ticagrelor drop more precipitously than clopidogrel between 8 and 24 hours post-dose, although the IPA with ticagrelor seems to be maintained at a level comparable to or greater than clopidogrel over this time period. However, by 48 hours post dose, the IPA with ticagrelor is less than that seen with clopidogrel; by 72 hours post dose, the IPA with ticagrelor is about 20%, which is comparable with that seen 5–7 days after discontinuation of clopidogrel.Citation15,Citation25 While there are no studies that have looked at clinical outcomes related to the offset of action of ticagrelor, measures should be taken to ensure that patients will be adherent with ticagrelor, and/or support needs to be given to those patients who have a history of poor adherence to other drugs. This is of added concern because ticagrelor needs to be taken twice daily, and medication adherence is inversely related to the number of daily doses.Citation56 Patients need to be aware of the importance of not missing a dose and have a plan of action in case circumstances arise which would jeopardize adherence; for example, carrying extra tablets with them on trips in the event of an airplane flight cancellation which would extend their time away from home.
Clinical comparison versus prasugrel
There are no head-to-head comparisons with ticagrelor and prasugrel in terms of clinical outcomes. Prasugrel is a thienopyridine prodrug so, like clopidogrel requires bioactivation in order to produce a pharmacologic effect. Unlike clopidogrel, the bioactivation of prasugrel requires only one step instead of two. This drug is initially hydrolyzed in the intestines by esterases and converted to the active form primarily by CYP3A4 and CYP2B6.Citation57 The time to maximal IPA is slightly faster than clopidogrel with a loading dose, and prasugrel does have a higher maximal IPA compared with clopidogrel.Citation58 The typical loading dose of prasugrel is 60 mg, which will lead to a maximal IPA of about 80% in approximately 2–4 hours ().Citation57 A head-to-head comparison of ticagrelor and prasugrel demonstrated more potent inhibition of platelet aggregation with ticagrelor.Citation47
Because prasugrel has the same mechanism of action as clopidogrel, it irreversibly inhibits platelets and needs approximately 7 days for the antiplatelet effects to wear off completely, given that new platelets will need to be synthesized. While more efficacious than clopidogrel, clinical trials have shown an increase in life-threatening, major, and fatal bleeds with prasugrel compared with clopidogrel.Citation8 The bleeding risk was higher in specific subgroups of patients, including those who were 75 years of age or older, those who weighed less than 60 kilograms, and those who had a history of stroke or transient ischemic attacks.Citation8 Prasugrel also showed no efficacy advantage over clopidogrel in terms of reducing major adverse cardiovascular events in these subgroups of patients. Restrictions have therefore been placed on prasugrel, and this drug carries a boxed warning because of the bleeding risks. Prasugrel should not be used in patients with a history of stroke or TIA. Extreme caution should be used in patients who are at least 75 years of age or weigh less than 60 kilograms.Citation57 A meta-analysis indirectly comparing bleeding with ticagrelor and prasugrel was in favor of less bleeding with ticagrelor.Citation59 While bleeding rates were higher with prasugrel than clopidogrel in TRITON-TIMI 38Citation8 and comparable between ticagrelor and clopidogrel in PLATO,Citation28 when one compares bleeding rates according to the standardized TIMI criteria, one finds that, on a percentage-by-percentage basis, a higher percentage of patients taking ticagrelor in PLATO experienced non-CABG-related major bleeding as well as major or minor bleeding, both defined by TIMI criteria, compared with patients taking prasugrel in TRITON-TIMI 38 (). However, a greater percentage of patients taking prasugrel in TRITON-TIMI 38 reported major CABG-related bleeding compared with patients taking ticagrelor in PLATO. Bleeding rates with clopidogrel were higher in the PLATO trial compared with TRITONTIMI 38, even though both studies used similar clopidogrel doses, with the exception that 20% of patients in PLATO received a 600 mg loading dose of clopidogrel instead of the standard 300 mg loading dose (). Another difference is that the PLATO trial enrolled patients even if they had received clopidogrel before randomization, while patients in the TRITON-TIMI 38 study had to have discontinued clopidogrel for at least 5 days before randomization. It is unknown whether or not these differences in clopidogrel treatment contributed in any way to the higher bleeding rates with clopidogrel in PLATO versus TRITON-TIMI 38, thereby narrowing the window of difference between ticagrelor and clopidogrel in terms of bleeding rates, but these cannot be dismissed as contributing factors. It is also noteworthy that the major safety endpoint in PLATO was study-defined major bleeding, and it was non-CABG-related major TIMI bleeding in TRITON TIMI 38. This is not to say that prasugrel and ticagrelor are comparable in any way with regards to bleeding, because this was an indirect comparison and absolute numbers of bleeds were substantially lower in the TRITONTIMI 38 trial by virtue of an overall smaller sample size in relation to the PLATO study. However, it does argue for a standardized definition of bleeding in clinical trials in order to facilitate indirect comparisons such as these for which no direct head-to-head data exist.Citation60
Table 4 Bleeding rates in the PLATO and TRITON-TIMI 38 trials based on TIMI criteriaCitation8,Citation28
Both prasugrel and ticagrelor have shown superiority in efficacy over clopidogrel in patients with ACS.Citation8,Citation28 However, since these two new antiplatelet agents do not have any direct head-to-head comparison trials, the choice of which one to use in lieu of clopidogrel is not clear. An indirect comparative meta-analysis has been published to attempt to clarify this issue.Citation59 This meta-analysis compared these two new antiplatelet agents indirectly through their landmark trials, ie, PLATO for ticagrelor and TRITONTIMI 38 for prasugrel, and demonstrated no difference between these two drugs in terms of overall risk of death, nonfatal myocardial infarction, nonfatal stroke, or in their other composite endpoints. The only difference between the two drugs in terms of efficacy was that prasugrel was shown to have a significantly lower risk of definite or probable stent thrombosis (odds ratio = 0.64; 95% CI 0.43–0.93; P = 0.020). The cost of this benefit was an increased risk of any major TIMI bleeding, major bleeding associated with cardiac surgery, and major or minor TIMI bleeding with prasugrel.Citation59 There have been conflicting views in terms of the differences between ticagrelor and prasugrel when the clinical efficacy of these drugs is indirectly compared.Citation42 Looking more closely at these two trials in terms of mortality, ticagrelor reduced mortality in PLATO (HR = 0.78; 95% CI 0.69–0.89; P < 0.001) whereas prasugrel did not in TRITON-TIMI 38 (HR 0.95; 95% CI 0.78–1.16; P = 0.64). The PLATO trial allowed patients into the trial if they had received clopidogrel before randomization while patients in the TRITON-TIMI 38 study had to be off clopidogrel for at least 5 days before randomization. This helps illustrate that the PLATO trial gives a more realistic view of what to expect when treating with these antiplatelet agents.
In terms of off-target adverse effects, ticagrelor may cause dyspnea and cardiac conduction abnormalities as mentioned above, which is not much of a concern with prasugrel therapy. Based on the premise that these side effects are due to ticagrelor-induced elevations in adenosine concentrations, one would not expect these side effects to occur with prasugrel. Citation38,Citation41 Additionally, ticagrelor may elevate serum creatinine and uric acid concentrations, which is not something that has been attributed to prasugrel. However, there is concern regarding cancer risk with prasugrel. Specifically, a 27% increased risk in colorectal, lung, and breast malignancies was seen in the TRITON-TIMI 38 trial with prasugrel compared with clopidogrel, primarily in women.Citation42,Citation61 However, this may very well be a chance finding, given that the absolute numbers of cases was low. In the PLATO study, the incidence of either any neoplasm or a malignant neoplasm arising during treatment was comparable between ticagrelor and clopidogrel, while the incidence of a benign neoplasm occurring was actually less with ticagrelor (0.2%) compared with clopidogrel (0.4%; P = 0.02).Citation28
The potential efficacy of ticagrelor in clopidogrel nonresponders was discussed earlier, indicating that ticagrelor seems to be effective in these patients.Citation52,Citation53 Prasugrel has also been studied in patients with functional variants in clopidogrel-metabolizing enzymes and has shown potential in these patients as well.Citation62,Citation63 Because no head-to-head trials exist comparing ticagrelor with prasugrel in clopidogrel nonresponders, no conclusions can be made at this time regarding which agent to choose in this setting. Nonetheless, both prasugrel and ticagrelor have promise as alternative antiplatelet drugs for patients not responding adequately to clopidogrel.
Summary
Ticagrelor is the first reversible P2Y12 receptor antagonist to be available for clinical use. Compared with clopidogrel, ticagrelor elicits a faster and stronger antiplatelet effect and also displays greater clinical efficacy with a comparable rate of bleeding. Ticagrelor is indicated to be used with aspirin therapy, but only at aspirin dosages not exceeding 100 mg daily, because the clinical data suggest that ticagrelor may lose efficacy when combined with higher dosages of aspirin, although this is a topic of debate. The relatively fast offset of effect of ticagrelor is desirable in the sense that invasive procedures will not need to be delayed as long for fear of bleeding. However, the quicker offset of effect makes medication adherence that much more important, because missing consecutive doses may increase the risk for thrombotic events. This is even more concerning given that ticagrelor is administered twice daily, making adherence more challenging compared with medications that are administered once daily (eg, clopidogrel, prasugrel). Bleeding is the most common side effect with ticagrelor, although dyspnea, ventricular pauses, and elevations in serum creatinine and uric acid are also associated with ticagrelor therapy. Ticagrelor has been shown to inhibit platelet aggregation effectively in patients nonresponsive to clopidogrel, and there are currently no known pharmacogenetic issues with ticagrelor. Ticagrelor is currently competitively priced against clopidogrel and prasugrel, although with clopidogrel becoming available as a generic very soon, drug costs will likely be a consideration when delineating the role of ticagrelor in the treatment of coronary artery disease. Direct comparisons with prasugrel are unfortunately lacking, and the relative merits of ticagrelor and prasugrel will have to be made based on indirect comparisons. Ticagrelor is a very promising new antiplatelet drug with impressive efficacy and reasonable safety. While ticagrelor has currently established itself for the treatment of acute coronary syndromes, ongoing and future trials in broader clinical scenarios will hopefully establish more clearly its ultimate place in therapy.
Disclosure
The authors report no conflicts of interest in this work.
References
- DibCHannaEAbu-FadelMA new era for antiplatelet therapy in patients with acute coronary syndromeAm J Med Sci201034040741120818228
- MarczewskiMMPostulaMDariuszKNovel antiplatelet agents in the prevention of cardiovascular complications – focus on ticagrelorVasc Health Risk Manag2010641942920539844
- Antiplatelet Trialists’ CollaborationCollaborative overview of randomized trials of antiplatelet therapy-I: prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patientsBMJ1994308811068298418
- KushnerFGHandMSmithSC2009 focused updates: ACC/ AHA Guidelines for the Management of Patients With ST-Elevation Myocardial InfarctionJ Am Coll Cardiol2009542205224119942100
- WrightRSAndersonJLAdamsCD2011ACCF/AHA focused update of the guidelines for the management of patients with unstable angina/non ST-elevation myocardial infarctionJ Am Coll Cardiol2011571920195921450428
- The Clopidogrel In Unstable Angina To Prevent Recurrent Events Trial InvestigatorsEffects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevationN Engl J Med200134549450211519503
- MehtaSRYusufSPetersRJGEffects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE studyLancet200135852753311520521
- WiviottSDBraunwaldEMcCabeCHPrasugrel versus clopidogrel in patients with acute coronary syndromesN Engl J Med20073572001201517982182
- CattaneoMP2Y12 receptor antagonists: a rapidly expanding group of antiplatelet agentsEur Heart J2006271010101216569650
- HustedSvan GiezenJJJTicagrelor: The first reversibly binding oral P2Y12 receptor antagonistCardiovasc Ther20092725927419604248
- SerebruanyVLAdenosine release: A potential explanation for the benefits of ticagrelor in the PLATelet Inhibition and Clinical Outcomes trialAm Heart J20111611421167333
- HammondJRStolkMArcherRGPharmacological analysis and molecular cloning of the canine equilibrative nucleoside transporter 1Eur J Pharmacol200449191915102528
- Van GiezenJJSidawayJGlavesPTicagrelor inhibits adenosine uptake in vitro and enhances adenosine-mediated hyperemia responses in a canine modelJ Cardiovasc Pharmacol Ther6222011 [Epub ahead of print.]
- TengROliverSHayesMAbsorption, distribution, metabolism, and excretion of ticagrelor in healthy subjectsDrug Metab Dispos2010381514152120551239
- GurbelPABlidenKPButlerKRandomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease. The ONSET/OFFSET StudyCirculation20091202577258519923168
- TengRButlerKPharmacokinetics, pharmacodynamics, tolerability and safety of single ascending doses of ticagrelor, a reversibly binding oral P2Y12 receptor antagonist, in healthy subjectsEur J Clin Pharmacol20106648749620091161
- HustedSEmanuelssonHHeptinstallSPharmacodynamics, pharmacokinetics, and safety of the oral reversible P2Y12 antagonist AZD6140 with aspirin in patients with atherosclerosis: a double-blind comparison to clopidogrel with aspirinEur Heart J2006271038104716476694
- ThebaultJJKeifferGLoweGDRepeated-dose pharmacodynamics of clopidogrel in healthy subjectsSemin Thromb Hemost199925Suppl 291410440416
- ZhouDAnderssonTBGrimmSWIn vitro evaluation of potential drug-drug interactions with ticagrelor: cytochrome P450 reaction phenotyping, inhibition, induction, and differential kineticsDrug Metab Dispos20113970371021177984
- Brilinta prescribing informationWilmington, DEAstraZeneca2011
- ButlerKTengRPharmacokinetics, pharmacodynamics, and safety of ticagrelor in volunteers with mild hepatic impairmentJ Clin Pharmacol20115197898720926753
- StoreyRFAngiolilloDJPatilSBInhibitory effects of ticagrelor compared with clopidogrel on platelet function in patients with acute coronary syndromesJ Am Coll Cardiol2010561456146220832963
- GurbelPACummingsCCBellCRAlfordABMeisterAFSerebruanyVLOnset and extent of platelet inhibition by clopidogrel loading in patients undergoing elective coronary artery stenting: The Plavix Reduction Of New Thrombus Occurrence (PRONTO) trialAm Heart J200314523924712595840
- StoreyRFHustedSHarringtonRAInhibition of platelet aggregation by AZD6140, a reversible oral P2Y12 receptor antagonist, compared with clopidogrel in patients with acute coronary syndromesJ Am Coll Cardiol2007501852185617980251
- StoreyRFBlidenKPEcobREarlier recovery of platelet function after discontinuation of treatment with ticagrelor compared with clopidogrel in patients with high antiplatelet responsesJ Thromb Haemost201191730173721707911
- Plavix prescribing informationBridgewater, NJBristol-Myers Squibb/ Sanofi Pharmaceuticals Partnership2011
- CannonCPHustedSHarringtonRASafety, tolerability, and initial efficacy of AZD6140, the first reversible oral adenosine diphosphate receptor antagonist, compared with clopidogrel, in patients with non-ST-elevation acute coronary syndromeJ Am Coll Cardiol20075018441851 Erratum inJ Am Coll Cardiol. 2007;50:219617980250
- WallentinLBeckerRCBudajATicagrelor versus clopidogrel in patients with acute coronary syndromesN Engl J Med20093611045105719717846
- StegPGJamesSHarringtonRATicagrelor versus clopidogrel in patients with ST-elevation acute coronary syndromes intended for reperfusion with primary percutaneous coronary intervention. A Platelet Inhibition and Patient Outcomes (PLATO) Trial subgroup analysisCirculation20101222131214121060072
- JamesSKRoeMTCannonCPTicagrelor versus clopidogrel in patients with acute coronary syndromes intended for non-invasive management: substudy from prospective randomised PLATelet inhibition and patient Outcomes (PLATO) trialBMJ2011342d352721685437
- CannonCPHarringtonRAJamesSComparison of ticagrelor with clopidogrel in patients with a planned invasive strategy for acute coronary syndromes (PLATO): a randomised double-blind studyLancet201037528329320079528
- HeldCÅsenbladNBassandJPTicagrelor versus clopidogrel in patients with acute coronary syndromes undergoing coronary artery bypass surgery. Results from the PLATO Platelet Inhibition and Patient Outcomes TrialJ Am Coll Cardiol20115767268421194870
- JamesSAngiolilloDJCornelJHTicagrelor versus clopidogrel in patients with acute coronary syndromes and diabetes: a substudy from the PLATelet inhibition and patient Outcomes (PLATO) trialEur Heart J2010313006301620802246
- JamesSBudajAAylwardPTicagrelor versus clopidogrel in acute coronary syndromes in relation to renal function: results from the platelet inhibition and patient outcomes (PLATO) trialCirculation20101221056106720805430
- MahaffeyKWWojdylaDMCarrollKTicagrelor compared with clopidogrel by geographic region in the Platelet Inhibition and Patient Outcomes (PLATO) TrialCirculation201112454455421709065
- Prevention of Cardiovascular Events (eg, Death From Heart or Vascular Disease, Heart Attack, or Stroke) in Patients With Prior Heart Attack Using Ticagrelor Compared with Placebo on a Background of Aspirin (PEGASUS) Available at: http://clinicaltrials.gov/ct2/show/ NCT01225562Accessed September 23, 2011
- StoreyRFBlidenKPPatilSBIncidence of dyspnea and assessment of cardiac and pulmonary function in patients with stable coronary artery disease receiving ticagrelor, clopidogrel, or placebo in the ONSET/OFFSET studyJ Am Coll Cardiol20105618519320620737
- StoreyRFBeckerRCHarringtonRACharacterization of dyspnoea in PLATO study patients treated with ticagrelor or clopidogrel and its association with clinical outcomesEur Heart J7302011 [Epub ahead of print.]
- StoreyRFBeckerRCHarringtonRAPulmonary function in patients with acute coronary syndrome treated with ticagrelor or clopidogrel (from the Platelet Inhibition and Patient Outcomes [PLATO] Pulmonary Function Substudy)Am J Cardiol932011 [Epub ahead of print.]
- BurkiNKDaleWJLeeLYIntravenous adenosine and dyspnea in humansJ Appl Physiol20059818018515377651
- SciricaBMCannonCPEmanuelssonHThe incidence of bradyarrhythmias and clinical bradyarrhythmic events in patients with acute coronary syndromes treated with ticagrelor or clopidogrel in the PLATO (Platelet Inhibition and Patient Outcomes) Trial. Results of the continuous electrocardiographic assessment substudyJ Am Coll Cardiol2011571908191621545948
- SerebruanyVLThe TRITON versus PLATO trials: Differences beyond platelet inhibitionThromb Haemost201010325926120024505
- KreutzRPStanekEJAubertRImpact of proton pump inhibitors on the effectiveness of clopidogrel after coronary stent placement: the Clopidogrel Medco Outcomes StudyPharmacotherapy20103078779620653354
- BhattDLCryerBLContantCFClopidogrel with or without omeprazole in coronary artery diseaseN Engl J Med20103631909191720925534
- WarnerTDArmstrongPCJCurzenNPMitchellJADual antiplatelet therapy in cardiovascular disease: does aspirin increase clinical risk in the presence of potent P2Y12 receptor antagonists?Heart2010961693169420956485
- SerebruanyVLAspirin dose and ticagrelor benefit in PLATO: fact or fictionCardiology201011728028321304245
- KirkbyNSLeadbeaterPDMChanMVNylanderSMitchellJAWarnerTDAnti-platelet effects of aspirin vary with level of P2Y12 receptor blockade supplied by either ticagrelor or prasugrelJ Thromb Haemost832011 [Epub ahead of print.]
- MomaryKMDorschMPBatesERGenetic causes of clopidogrel nonresponsiveness: which ones really countPharmacotherapy20103026527420180610
- MalekLAKisielBSpiewakMCoexisting polymorphisms of P2Y12 and CYP2C19 genes as a risk factor for persistent platelet activation with clopidogrelCirc J2008721165116918577829
- HulotJ-SColletJ-PSilvainJCardiovascular risk in clopidogrel-treated patients according to cytochrome P450 2C19*2 loss-of-function allele or proton pump inhibitor coadministration. A systematic metaanalysisJ Am Coll Cardiol20105613414320620727
- GurbelPABlidenKPButlerKResponse to ticagrelor in clopidogrel nonresponders and responders and effect of switching therapies. The RESPOND studyCirculation20101211188119920194878
- TantryUSBlidenKPWeiCFirst analysis of the relation between CYP2C19 genotype and pharmacodynamics in patients treated with ticagrelor versus clopidogrel. The ONSET/OFFSET and RESPOND genotype studiesCirc Cardiovasc Genet2010355656621079055
- WallentinLJamesSStoreyRFEffect of CYP2C19 and ABCB1 single nucleotide polymorphisms on outcome of treatment with ticagrelor versus clopidogrel for acute coronary syndromes: a genetic substudy of the PLATO trialLancet20103761320132820801498
- HenrikssonMNikolicEJanzonMLong-term costs and health outcomes of treating acute coronary syndrome patients with ticagrelor based on the EU label – cost-effectiveness analysis based on the PLATO study [abstract]Value Health201114A40 Available from: http://www.astrazeneca.com/Media/Press-releases/Article/09052011-new-health-study-of-plato-brilique-with-clopidogrelAccessed September 23, 2011
- CrespinDJFederspielJJBiddleAKJonasDERossiJSTicagrelor versus genotype-driven antiplatelet therapy for secondary prevention after acute coronary syndrome: a cost-effectiveness analysisValue Health20111448349121669373
- ClaxtonAJCramerJPierceCA systematic review of the associations between dose regimens and medication complianceClin Ther2001231296131011558866
- Effient prescribing informationIndianapolis, INEli Lilly and Company2010
- WiviottSDTrenkDFrelingerALPRINCIPLE-TIMI 44 Investigators. Prasugrel compared with high loading- and maintenance-dose clopidogrel in patients with planned percutaneous coronary intervention: the Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation-Thrombolysis in Myocardial Infarction 44 trialCirculation20071162923293218056526
- Biondi-ZoccaiGLotrionteMAgostoniPAdjusted indirect comparison meta-analysis of prasugrel versus ticagrelor for patients with acute coronary syndromesInt J Cardiol201015032533120828843
- MehranRRaoSVBhattDLStandardized bleeding definitions for cardiovascular clinical trials. A consensus report from the Bleeding Academic Research ConsortiumCirculation20111232736274721670242
- The Cardiovascular and Renal Drugs Advisory Committee of the United States Food and Drug AdministrationPrasugrel secondary review Available from: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/CardiovascularandRenalDrugsAdvisoryCommittee/UCM181185.pdfAccessed September 23, 2011
- MegaJLCloseSLWiviottSDCytochrome P450 genetic polymorphisms and the response to prasugrel: relationship to pharmacokinetic, pharmacodynamic and clinical outcomesCirculation20091192553256019414633
- MegaJLCloseSLWiviottSDGenetic variants in ABCB1 and CYP2C19 and cardiovascular outcomes after treatment with clopidogrel and prasugrel in the TRITON–TIMI 38 trial: a pharmacogenetic analysisLancet20103761312131920801494